Abstract
The objective of this study was to compare the effects of 3 diet formulations containing different protein sources (animal, plant, and a combination of animal and plant) on the colonization of Campylobacter jejuni in the gastrointestinal tract of broiler chickens. A freshly isolated strain of C. jejuni (biotype IV, serotype HS O:21, O:29, HL untypable) from a broiler chicken was used to infect 3-day-old chicks that had been free of C. jejuni; 0.5 mL of an inoculum containing 108 colony-forming units was administered orally. Shedding of the organism was studied, and C. jejuni in the ceca, jejuni, and crop were enumerated by quantitative culture. The isolates recovered from the birds during the study period of 35 d were characterized and confirmed as C. jejuni by the use of standard methods and underwent biotyping, serotyping, antimicrobial susceptibility testing by disk diffusion and the E-test, and flagellin gene typing. A cyclical pattern of shedding of C. jejuni was observed in all the birds. Colonization was highest in the ceca. The ceca of birds receiving plant-protein-based feed had significantly less colonization then the ceca of birds receiving the other types of feed, whereas the differences in colonization of the jejuni and crops were not significant. Characterization by biotyping, serotyping, and flagellin gene typing showed that 95% of the recovered isolates were identical to the strain used for infecting the chicks. However, with the Lior-HL typing scheme, 74% of the recovered isolates were HL untypable. Antimicrobial resistance testing did not reveal significant differences between the infecting strain and the recovered isolates among the different feed groups.
Introduction
Campylobacter jejuni subsp. jejuni (referred to as C. jejuni) has emerged as the most important and leading cause of foodborne bacterial gastroenteritis in humans worldwide (1). The annual incidence exceeds 2.4 million cases in North America (2). At times the infection may lead to complications, including reactive arthritis and a postinfective polyneuropathy called Guillain–Barré syndrome (1,3). Chickens have been implicated in about 50 to 70% of human cases (4). Domestic and wild poultry are asymptomatic carriers of C. jejuni in their gastrointestinal (GI) tracts (5), although some strains associated with Guillain–Barré syndrome in humans have been shown to cause paralysis in experimentally infected chickens (6). Strains from poultry appear to have pathogenic potential, as evidenced by production of enterotoxins (7). This virulence is significant because many clinical isolates from humans have been shown to produce cholera-related enterotoxins (8). The GI colonization by C. jejuni is the most significant contributing factor in the contamination of poultry meat (9). The organisms are transferred onto the meat during mechanized processing of the birds (10). Therefore, the ideal way to reduce the incidence of human infection would be to significantly reduce the GI colonization of these organisms in broiler chickens.
The GI colonization by C. jejuni in birds is very complex and involves interaction of the host and pathogen, which is influenced by many environmental factors. Campylobacter is ecologically adapted to the avian GI tract and selects the ceca for colonization because the microenvironment is conducive to its survival and multiplication (11). The organism colonizes the cecal crypt mucus without attaching to the microvilli (11). Campylobacter jejuni exhibits chemotactic attraction to l-fucose, a component of mucin, and utilizes mucin as a sole substrate for growth (12). Therefore, changes in mucin composition are likely to influence C. jejuni colonization in the GI tract. Certain strategies, such as competitive exclusion and use of prebiotics, have been tried to take advantage of bacterial antagonism and thus reduce the colonization of pathogenic organisms in the GI tract of birds.
The impact of certain dietary factors on GI microbial ecology has also been studied. According to Klasing (13), dietary factors such as the amount and viscosity of fibre and the presence of fats refractory to digestion appear to influence the overall composition of the GI microflora in birds. Dietary factors have been found to strengthen the immune system's resistance to infection and thereby influence the microbial dynamics of the GI tract (13). In addition, physicochemical factors such as temperature, pH, water activity, oxidation reduction (OR) potential, and presence or absence of certain substrates have been found to influence GI colonization (14). The presence in the diet of certain complex carbohydrates that are not digested autoenzymatically can increase microbial activity in the GI tract (15). In birds, digestion is chiefly autoenzymatic, but some alloenzymatic digestion takes place in the lower GI tract, particularly the ceca. The alloenzymatic digestion is mainly due to fermentation of water-soluble nonstarch polysaccharides (NSPs) by microorganisms in the ceca. The products of fermentation, such as lactic acid, gases, and volatile fatty acids, alter the microenvironment of the GI tract, which may be detrimental to a wide range of nonfermenting microorganisms, including pathogens (16). Certain dietary substrates cause changes in mucin composition, thereby influencing the colonization of mucus-dwelling organisms.
From these findings we deduced that the type and nature of ingredients in the diet could alter the microenvironment of the ceca and therefore influence GI colonization by C. jejuni in birds. The main purpose of this study was to ascertain how diet formulations that differ in type or nature of ingredients (specifically, protein sources) influence the colonization of C. jejuni in the GI tract of broiler chickens. We also examined the shedding pattern of C. jejuni and characteristics of the isolates according to standard methods, serotyping, biotyping, antimicrobial sensitivity, and flagellin gene typing to determine any possible variations.
Materials and methods
Committee approvals
The study was designed in accordance with the guidelines of the Canadian Council on Animal Care (17) and was approved by the Animal Care and Biohazards/Safety committees of the University of Prince Edward Island (UPEI).
Birds and diet
Day-old broiler chicks were used. The study period was 35 d, corresponding to the present broiler production cycle. Eggs were purchased from Clark's Chick Hatchery, Moncton, New Brunswick, and were disinfected by being dipped in 2% formaldehyde for about 5 min. After being dried, the eggs were set in a disinfected incubator maintained at 37.5°C in the Animal Care Facilities of the Atlantic Veterinary College (AVC) for hatching.
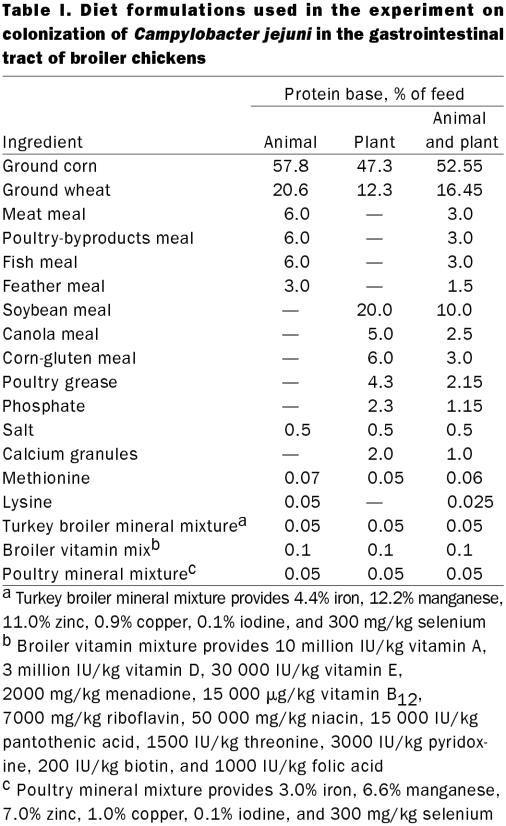
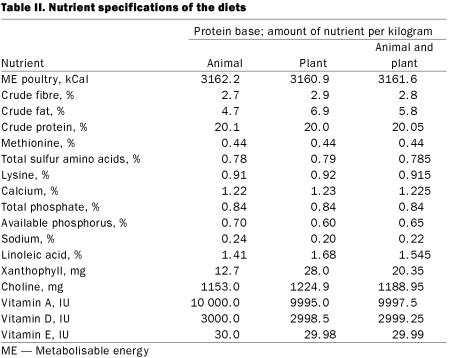
Three diet formulations were designed, with protein sources of animal origin, plant origin, and a combination of animal and plant origin. The diet formulations and nutrient specifications are given in Tables I and II. Mixed protein feed was prepared by mixing together equal weights of animal-protein-based feed and plant-protein-based feed.
Table I.
Table II.
Layout and experimental design
The birds in the control and experimental groups were housed separately in 2 rooms in the Animal Care Facilities of the AVC. The rooms were thoroughly cleaned and disinfected prior to the birds' housing. Inside each room 6 floor pens were set up. Each pen, about 1.65 × 0.86 × 0.74 m, was made of polyvinyl chloride pipe (2.5 cm in diameter) and wire mesh (with openings 2.5 cm in diameter). Hanging-type brooders (Canarm, Brockville, Ontario) fitted with infrared heat lamps (250 W; Phillips Electronics, Amsterdam, Netherlands) provided supplemental heat in each pen. The litter consisted of pine shavings, tested in advance to ensure freedom from C. jejuni. The feeders and waterers were thoroughly washed, cleaned, and disinfected before use.
We randomly assigned 112 chicks to 14 groups using computer software (Quattro Pro; Corel Corporation, Ottawa, Ontario). Six 8-bird groups were designated as control groups and 6 as experimental groups. The 2 remaining groups were necropsied to ascertain if the birds in the study group were free of Campylobacter and Salmonella. Each dietary treatment was replicated in 2 control-group pens and 2 experimental-group pens.
Animal husbandry and management
The birds were weighed and individually identified with leg bands. Litter was placed in the pens approximately 7 cm deep, and the birds were moved into the allotted pens, where the temperature was maintained according to industry standards. The birds were provided with feed and water (sterilized by autoclaving) ad libitum. Strict biosecurity protocols were observed during the daily routine of feeding, providing water, and collecting samples. The litter was changed once every week and, along with coveralls, gloves, and footwear, disposed of by incineration.
Testing of environment, feed, and birds for Campylobacter and Salmonella
Air inside the rooms, feed, and litter were sampled and cultured before the experiment to ensure that the birds did not acquire C. jejuni from these sources. Air was tested prior to the birds' housing by the settle plate method (18): 6 freshly made Campylobacter blood-free (CBF; Oxoid, Nepean, Ontario) plates were kept open in various locations in each room for 48 h. The plates had plenty of moisture and had not been dried before being placed in the open. Litter and feed samples, 1 g each, were collected from different bags and pooled, to be cultured for Campylobacter and Salmonella by enrichment procedures.
For Campylobacter testing, ground feed and litter samples, 30 g each, were placed in 100 mL of enrichment broth, prepared according to the formula of Doyle and Roman (19). The samples were incubated with vigorous agitation under microaerophilic conditions (5% oxygen, 10% carbon dioxide, 85% nitrogen) at 42°C for 18 h. Aliquots of the supernatant were plated onto CBF plates and incubated microaerophilically at 42°C for 48 h. For Salmonella testing, 30 g each of the feed and litter samples were placed in Rappaport broth and incubated at 42°C for 72 h in normal aerobic atmosphere. Aliquots of the supernatant, 25 μL each, were plated onto modified semisolid Rappaport–Vassiliadis (MSRV; Oxoid) plates, in 4 spots, and incubated for another 48 h at 42°C.
To ensure that the birds were free of Campylobacter and Salmonella, 16 birds previously randomly selected were euthanized with carbon dioxide, and their ceca, jejuni, and crops were harvested on day 1 of the experiment. Swabs were taken from the specimens with cotton-tipped applicators (Puritan Hardwood Products, Guilford, Maine, USA) and plated onto CBF plates, which were incubated microaerophilically at 42°C for 48 h. For detection of Salmonella, swabs were placed in Rappaport broth for enrichment for 72 h at 42°C, and then 25-μL aliquots were plated onto MSRV plates, in 4 spots; the plates were incubated at 42°C for 48 h.
Campylobacter challenge
A freshly isolated field strain of C. jejuni (hereafter designated as challenge strain) from a broiler chicken slaughtered in a poultry processing plant in Prince Edward Island (biotype IV, serotype HS O:21, O:29, HL untypable; biotyped and serotyped at the National Laboratory for Enteric Pathogens, Winnipeg, Manitoba) was used to infect the birds in the treatment groups on the 3rd day of the experiment. An inoculum containing approximately 108 colony-forming units (cfu) per millilitre in brain–heart infusion broth (Oxoid) was prepared, and all birds in the treatment groups were given 0.5 mL of the inoculum orally with a tuberculin syringe. The challenge dose was predetermined on the basis of a pilot study.
Sample collection and recovery of C. jejuni
Before the challenge, cloacal swabs were taken from all the birds, first from the control groups and then from the treatment groups, with calcium alginate applicators (Calgiswab, type 4; Puritan Hardwood Products). The swab was introduced into the cloaca and rotated 5 times, as described by Achen, Morishita, and Ley (20), and immediately plated onto CBF plates, which were incubated under microaerophilic conditions for 48 h. Colonies grown were subcultured onto blood agar (BA) plates after confirmation by Gram staining. The colonies grown on the BA plates were transferred into 2% sterile skim milk in cryovials (Simport Plastics, Beloeil, Québec) and stored at –76°C.
Euthanasia, necropsy, and sample collection for quantitation of C. jejuni
On day 35 of the experiment, all birds were euthanized after cloacal swabs had been taken. The birds' weights were recorded, and 4 birds were euthanized at a time by exposure to carbon dioxide gas for 5 min. At necropsy the lower abdomen was disinfected with 70% isopropanol, and the ceca, jejuni, and crops were harvested for quantitative culture of C. jejuni.
The specimens were transferred into preweighed sterile stomacher bags (Fisher Scientific, Ottawa, Ontario) and their weights determined. Peptone water (0.1%; Central Services, AVC, UPEI) was added, at 9 times the weight of the specimen, and the mixture homogenized for 1 min with a stomacher lab blender (Seward Medical, London, England). Then 100 μL of the homogenized suspension was transferred into 900 μL of 0.1% peptone water. Ten-fold dilutions were made in duplicate from the stock suspension, and 25-μL portions from each dilution were plated in 10 spots on CBF plates. The plates were incubated microaerophilically for 48 h and colonies then counted with a stereoscopic illuminator (WildM3Z; Heerbrugg, Switzerland).
Colonies typical of C. jejuni were counted in 10 spots. The mean value was used to calculate the colonization level (cfu/g) of the specimen by application of the appropriate dilution factor. The colonization level of the duplicate dilution was also determined. The mean value of the 2 dilutions was recorded for that organ. Selected colonies were tested and confirmed as C. jejuni by standard methods, as described below.
Characterization of isolates
The frozen isolates were thawed and grown microaerophilically on BA plates. The resulting colonies were characterized and confirmed as C. jejuni by means of standard tests: Oxidase (BBL oxidase; Becton Dickinson Microbiology Systems, Cockeysville, Maryland, USA), catalase (3% hydrogen peroxide), hippurate (Remel Lenexa, latex agglutination (JCL, Integrated Diagnostics, Baltimore, Maryland, USA), and susceptibility to the antibiotics nalidixic acid (30 μg; Oxoid) and cephalothin (30 μg; Oxoid).
Biotyping and serotyping
Forty isolates randomly selected with the use of computer software (Minitab, version 12; State College, Pennsylvania, USA) were biotyped and serotyped at the National Center for Enteric Pathogens. All cultures were grown microaerophilically on Mueller–Hinton agar (Oxoid) containing 5% sheep blood (Oxoid) for 48 h. They were biotyped by the detection of hippurate hydrolysis, rapid H2S production, and DNA hydrolysis (21,22). For the Penner (HS) serotyping scheme the passive hemagglutination test with soluble heat-stable (O) antigens (23) was used; this involved heated extracts, sensitized sheep erythrocytes, and antisera in microtitre plates. Serotyping with the Lior (HL) scheme was performed by slide agglutination with live bacteria and crude and absorbed antisera for the detection of heat-labile antigens (24).
Antimicrobial susceptibility patterns
The disk diffusion method (25) was used to determine the susceptibility of the isolates to 12 antibiotics (at the specified concentrations): amikacin (30 μg), ampicillin (10 μg), cephalothin (30 μg), chloramphenicol (30 μg), ciprofloxacin (30 μg), enrofloxacin (5 μg), erythromycin (15 μg), gentamicin (30 μg), kanamycin (30 μg), nalidixic acid (30 μg), streptomycin (30 μg), and tetracycline (30 μg). Mueller–Hinton agar with 5% sheep blood was used for testing. The antibiotic disks were purchased from Oxoid.
The zones of inhibition were recorded. Escherichia coli ATCC strain 25922 and C. jejuni ATCC strain 33291 were tested in parallel as controls. Owing to the lack of standards for testing and accepted breakpoints for determining resistance, 1-way analysis of variance (ANOVA) was performed (Minitab, version 12) to ascertain any differences in susceptibility patterns.
E-test for minimum inhibitory concentration (MIC)
The E-test (PDM epsilometer; AB Biodisk, Solna, Sweden) was conducted according to the manufacturer's instructions with the following antibiotics: ampicillin, chloramphenicol, ciprofloxacin, erythromycin, gentamicin, kanamycin, streptomycin, and tetracycline.
Escherichia coli ATCC strain 25922, Staphylococcus aureus ATCC strain 25923, and C. jejuni ATCC strain 33291 were tested in parallel as controls. To ascertain any differences in MIC among the isolates, the data were analyzed by 1-way ANOVA with Minitab, version 12.
Flagellin gene typing of isolates
The isolates selected for biotyping and serotyping were characterized with flagellin gene typing according to the procedure described by Nachamkin et al (26), with some modifications. The isolates were grown on BA plates microaerophilically, and their genomic DNA was extracted with the use of a commercial kit (Wizard genomic DNA purification kit; Promega, Madison, Wisconsin) according to the manufacturer's instructions. Pilot experiments revealed that pure DNA was needed for the gene to amplify; therefore, the DNA extraction kit was used.
For amplification of the flaA gene of C. jejuni, consensus primers were designed on the basis of the published primer sequences (26) and Blast search results, with “flaA gene of C. jejuni strain D1118” used as the query sequence (Genbank; PubMed access no. AF 050197) (27). Differences from the published sequences were observed in the bases at the following bracketed positions. To accommodate these differences, we decided to provide either base (consensus) at that site so that 1 of them would be available during the reaction. The forward primer (5'-3') was ATG GGA TTT CGT ATT AAC AC(A/C) AAT G, and the reverse primer (5'-3') was CTA CTG TAG (C/T)AA TCT TAA AAC ATT (Bio/Can Scientific, Mississauga, Ontario).
The polymerase chain reaction (PCR) amplification was performed in a 50-μL mixture containing the following: 37 μL of sterile double-distilled water, 5 μl of 10× PCR buffer, 1 μL of 2 mM dNTP mix (0.2 mM each), 1 μL each of the forward and reverse primers (2.5 pM), 3 μL of 25 mM magnesium chloride, and 1 μL of Taq polymerase (1U/μL; MBI Fermentas, Burlington, Ontario), to which 1 μL of the DNA template was added. The reaction was performed in a thermal cycler (Hybaid, Ashford, England) under the following conditions: stage 1, denaturation at 95°C for 5 min; stage 2 (25 to 30 cycles for all 3 steps), step 1, 95°C for 45 s; stage 2, step 2, annealing at 55°C for 45 s; stage 2, step 3, 72°C for 40 s; and stage 3, elongation for 3 min. After amplification, the presence of PCR products was detected by agarose electrophoresis in the presence of ethidium bromide. Then 40 μL of PCR product was digested with 5 units of the restriction enzyme DdeI (Promega) by incubation for 2 h at 37°C. Enzyme-digested PCR products were run on 4% Nusieve GTG agarose gel (BMA, Rockland, Maine, USA) containing ethidium bromide for 4 h at 85 V in 0.5% Tris-borote/EDTA (TBE) buffer. The gel was destained for 1 h in 0.5% TBE buffer, and photographs were taken with the computer-based gel imaging system (Gene Snap Gel documentation system; Syngene, Cambridge, England).
Data analysis
To determine whether there was any significant difference in the fecal shedding of C. jejuni between the birds fed the 3 diet formulations, we analyzed diet against percentage of birds shedding C. jejuni with 1-way ANOVA (Minitab, version 12). To determine if there was any significant difference in the intestinal colonization of C. jejuni between the birds fed the 3 diets, we analyzed the data by a mixed model procedure (SAS Institute Inc., Cary, North Carolina, USA). The model evaluated the classes (organ, feed, and bird) in 3 levels against colonization. The tests of fixed effects were on feed, organ, and organ–feed. Residual components were analyzed with univariate procedures (SAS Institute) for skewness, kurtosis, and normality. The least-squares means of colonization levels that showed significance were displayed graphically. To determine whether the antimicrobial susceptibility profiles of the C. jejuni recovered from the birds fed different diet formulations were significantly different, we again used 1-way ANOVA (Minitab, version 12). The model enabled evaluation of diet against zones of inhibition of the isolates. Significance was assumed at the 0.05 level.
Results
Dietary studies
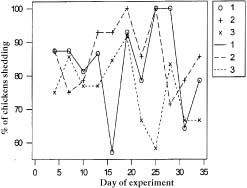
The air, litter, feed, and organs of birds randomly selected and tested by culture were negative for Campylobacter and Salmonella. The cloacal swabs taken from both experimental and control birds prior to C. jejuni challenge were also negative for these organisms. Most birds (83.3%) were shedding C. jejuni 24 h after oral inoculation. The proportions shedding on the 4th day were 87.5% in each of the animal-feed and mixed-feed groups and 75.0% in the plant-feed group. On the day of necropsy (day 35) the proportions shedding were 85.7%, 66.6%, and 78.5% in the animal-feed, plant-feed and mixed-feed groups, respectively. There was no statistically significant difference between the feed groups (P > 0.05) in the shedding of C. jejuni. Except for days 16 and 31, the proportions shedding were lower in the plant-feed group than in the animal-feed group. Figure 1 depicts the cyclical pattern of shedding from day 4 to day 35.
Figure 1. Fecal shedding of Campylobacter jejuni over 35 d among broiler chickens receiving (1) animal-protein-based feed (solid line), (2) mixed-protein-based feed (broken line), or (3) plant-protein-based feed (dotted line).
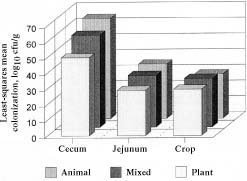
None of the birds in the control groups had C. jejuni recovered by culture. In the treatment groups, C. jejuni was recovered from the ceca of all birds, irrespective of diet, whereas it was recovered from the jejuni of 92.8% of each of the animal-feed and mixed-feed groups and 83.3% of the plant-feed group, and from the crops of 50.0%, 41.6%, and 78.5% of the animal-feed, plant-feed, and mixed-feed groups, respectively. The mean log10 colonization levels in the ceca were 6.3, 4.9, and 5.8 cfu/g in the animal-feed, plant-feed, and mixed-feed groups, respectively. The levels in the jejuni were 3.4, 2.8, and 3.2 cfu/g, respectively, and in the crops 2.9, 2.8, and 3.0 cfu/g, respectively. The differences in cecal colonization between the feed groups were statistically significant, but the differences in jejunal and crop colonization were not. Figure 2 compares the least-squares mean values of colonization between the feed groups.
Figure 2. Colonization of the birds' ceca, jejuni, and crops by C. jejuni according to diet; cfu — colony-forming units.
Characterization of isolates
All isolates recovered from the chickens had the appearance of Campylobacter and were gram-negative, curved, slender rods. All were identified as C. jejuni on the basis of positive reactions in oxidase, catalase, hippurate, latex agglutination tests, sensitivity to nalidixic acid (30 μg), and resistance to cephalothin (30 μg).
The Penner scheme showed that 95% of the isolates were biotype IV, serotype HS O:21, O:29, identical to the strain used for challenge. The remaining 5% were biotype II, serotype HS O:2; both these isolates were from the plant-feed group. When serotyping by the Lior scheme was also taken into account, only 75% of the isolates were identical to the challenge strain. Of the remaining 25% (10 isolates) 5 were from the plant-feed group (3 were HL 40 and 2 were HL 4), and the other 5 were from the animal-feed group (all were HL 40). With the Lior scheme 74% of the isolates were HL untypable.
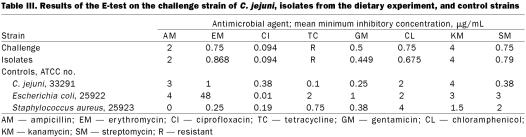
In the disk diffusion tests with 12 antibiotics, all the isolates were resistant to cephalothin and tetracycline, as indicated by the absence of an inhibition zone. Statistical analysis of the zones of inhibition of the isolates showed no significant differences between the feed groups. Table III presents the mean inhibitory concentrations (MICs) of 8 of the antibiotics obtained by E-testing the isolates, the challenge strain, and the control strains. All isolates were found to be resistant to tetracycline. Susceptibility to erythromycin was classified as intermediate, according to the National Committee on Clinical Laboratory Standards guideline breakpoints. One-way ANOVA showed no significant difference in the MICs for isolates from the various feed groups.
Table III.
Flagellin gene typing of isolates
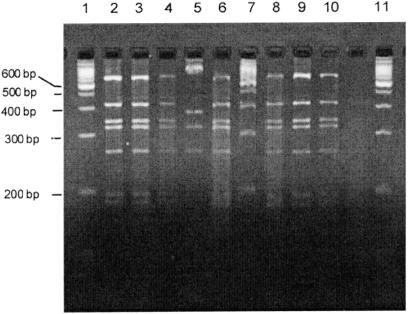
Figure 3 shows the patterns of restriction-fragment-length polymorphism of the PCR products of amplified flaA gene from the isolates. The band patterns were identical for 95% of the isolates (all biotype IV, serotype HS O:21, O:29) and the challenge strain; the other 5% exhibited slight variation in band pattern (Figure 3, lane 5).
Figure 3. Patterns of restriction-fragment-length polymorphism of the products of flaA gene amplification by polymerase chain reaction for various isolates of C. jejuni recovered from the birds. Lanes 1, 7, and 11 show 100 base-pair (bp) DNA weight markers. The patterns are identical to that of the challenge strain for all but 5% of the isolates (the last represented in lane 5).
Discussion
Preventing birds from becoming colonized with Campylobacter is difficult in farming conditions. To reduce the colonization of C. jejuni in broiler chickens, the factors that affect the colonization have to be understood well. The effects of various environmental factors that influence GI colonization of C. jejuni in broiler chickens, particularly dietary factors, have not been addressed so far. The main purpose of our study was to determine whether changes in diet composition have any effect on the GI colonization of C. jejuni in broiler chickens.
The strain of C. jejuni that we used for challenge of the birds was found to be capable of colonizing without producing any apparent adverse health effects. The challenge dose of ~ 5 × 107 cfu was adequate for colonizing 3-d-old birds, as in a previous study (28). However, in another study a dose of 108 to 1010 cfu was needed for adequate colonization in birds 8 to 9 d old (29). Colonization of birds with C. jejuni is influenced by many factors, and perhaps older birds need more organisms for adequate colonization.
Establishment of C. jejuni in the intestinal tract results in extensive fecal excretion (30). We found that about 83% of the birds excreted organisms within 24 h of challenge. However, all birds did not excrete organisms uniformly on all sampling days. A cyclical or intermittent fecal shedding pattern was observed. Similar observations were made in a previous study (20). The reason for the intermittent excretion is not understood clearly but may relate to cyclical emptying of cecal contents.
By culture, we recovered C. jejuni from the ceca of all birds, irrespective of diet. Our data on colonization of the ceca agreed with those of previous reports, in which the level ranged from 4.0 to 7.0 log10/g (20,31). Also as in previous studies (20,32), we recovered C. jejuni in higher numbers from the crops. However, it is not clear whether the presence of C. jejuni in the crops is colonization or contamination when hungry birds ingest fecal matter after feed has been withdrawn prior to slaughter. More studies are needed on this subject, because the potential for carcass contamination is high owing to possible rupture of the crop during evisceration.
Our data showed a significant difference in cecal colonization between the 3 feed groups but no significant differences in colonization of the jejuni and crops. The level of colonization was highest in the birds receiving animal feed and lowest in those receiving plant feed. This observation is important because less colonization in birds fed plant protein means less carcass contamination during processing and a reduced risk of infection in humans.
The lower level of colonization in the birds fed plant protein may be explained in terms of a combined influence of various substrates in the diet on colonization by C. jejuni. The indigenous microflora modify the colonization of pathogens (13). Although birds have a simple digestive system, some fermentation takes place in their ceca, where the microenvironmental conditions favour this process. In the plant-protein-based feed, certain complex-carbohydrate components, such as water-soluble NSPs, which are not affected by digestive enzymes, may favour fermentation. Fermentation gives rise to products such as lactic acid, volatile fatty acids (VFAs), and gases. These products would probably alter physicochemical conditions such as temperature, pH, and OR potential, thereby creating a microenvironment within the ceca that may inhibit many microorganisms. A lowering of pH or an increase in acidity is detrimental to a wide range of microorganisms (16). The changes in OR potential would affect the composition of microorganisms (14,33). High concentrations of VFAs have effects on many microorganisms (16). Distension of the ceca with products of fermentation may accelerate cecal emptying. The micronutrient iron is essential for the growth of most microorganisms, including Campylobacter; whether free iron is more available in 1 type of feed than another is worth investigation.
Characterization of the isolates by various tests showed them to be C. jejuni. Biotyping and serotyping confirmed that 95% of the isolates were identical to the challenge strain. Untypable strains are not uncommon among C. jejuni, but using the combination of the Penner and Lior serotyping schemes has been found to offer more discrimination and reliability than using a single scheme (34,35). It is difficult to compare the results of O antigen typing with those of HL antigen typing because the 2 schemes detect different antigens of the bacterium (35). Although phenotypic techniques such as serotyping are good methods for differentiating strains for epidemiologic purposes, the influence of environmental factors on the stability of most of the phenotypic properties is a weakness (36). Further studies are required to determine if changes in serotype could occur in the intestines of chickens.
Determining the patterns of resistance to antimicrobials by susceptibility testing of C. jejuni was considered important because of the possibility of acquired resistance and the role of feed-induced ecologic differences in the GI tract. The resistance patterns also served as additional means to compare the recovered isolates with the challenge strain. All recovered isolates and the challenge strain were found to be sensitive to all the antimicrobials tested except tetracycline, by both the disk diffusion method of sensitivity testing and MIC determination with the E-test. Dietary differences did not appear to influence the antimicrobial-sensitivity profile. There were no significant differences in MIC among the isolates, irrespective of diet. The E-test has been described as comparable to standard antimicrobial susceptibility tests such as agar-dilution and broth-microdilution methods (37).
The purpose of molecular characterization by flagellin gene typing was to determine whether all isolates recovered from the birds were identical to the challenge strain or whether any changes in genome had taken place. Flagellin gene typing has recently been described as a means to differentiate between Campylobacter strains, and this method has the discriminatory power of analyzing strains (26). We found that 95% of the isolates exhibited band patterns identical to that of the challenge strain. However, 5% of the isolates showed some variation in band pattern, which suggests the potential for genomic rearrangements in the intestinal tract of birds. Indeed, some workers have pointed out that recombinations within or between flagellin loci occur in Campylobacter strains, suggesting instability of the genome (38,39).
The strain-discriminating potential of flagellin gene typing agreed with that of serotyping with the Penner scheme in this study, though Lior typing did not fully agree. However, the combination of the discrimination potential of flagellin gene typing, the results of Penner serotyping, and the antimicrobial susceptibility patterns appears to be adequate to conclude that 95% of the isolates recovered during the experiment were the same as the challenge strain. Whether changes affecting serotype that are due to environmental influences on phenotypic stability could occur among a small percentage of a densely packed population of C. jejuni in the ceca and large intestine of chickens is worth further study.
In conclusion, this experiment revealed that a plant-protein-based feed caused a significant reduction in cecal colonization of C. jejuni in broiler chickens. Further research, with the use of additional strains of C. jejuni, may strengthen the validity of our findings.
Footnotes
Acknowledgments
This study was supported by a grant from the Poultry Industry Council, Guelph, Ontario, to Harry Hariharan and Ted Van Lunen. Additional funds were provided by Agriculture Canada and the Atlantic Veterinary College.
Address all correspondence and reprint requests to Dr. Harry Hariharan; telephone: (902) 566-0934; fax: (902) 566-0851; e-mail: hhariharan@upei.ca
Received August 21, 2002. Accepted January 10, 2003.
References
- 1.Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. Campylobacter jejuni — an emerging foodborne pathogen. Emerg Infect Dis 1999;5:28–35. [DOI] [PMC free article] [PubMed]
- 2.Friedman CR, Neimann J, Wegener HC, Tauxe RV. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser MJ, eds. Campylobacter, 2nd ed.: ASM Press, Washington D.C., 2000:121–125.
- 3.Allos BM. Association between Campylobacter infection and Guillain–Barré syndrome. J Infect Dis 1997;176:125–128. [DOI] [PubMed]
- 4.Stern NJ, Hernandez MP, Blankenship L, et al. Prevalence and distribution of Campylobacter jejuni and Campylobacter coli in retail meats. J Food Prot 1985;48:427–430. [DOI] [PubMed]
- 5.Skirrow MB. Campylobacter enteritis — the first five years. J Hyg 1982;89:175–184. [DOI] [PMC free article] [PubMed]
- 6.Hariharan H, Murphy G, Shanmugham J. Natural and experimental models of Guillain–Barré syndrome and Campylobacter jejuni. Biomed 1999;19:87–97.
- 7.Ahmed H, Hariharan H, Yason C. Drug resistance and toxigenic properties of Campylobacter jejuni from broiler chickens [abstract]. Canadian Veterinary Medical Association 44th Annual Convention, St- John's, Newfoundland July 5–8, 1992:469.
- 8.Hariharan H, Panigrahi D. Cholera like enterotoxin in certain Campylobacter jejuni strains: some observations. Microbiologica 1990;13:7–9. [PubMed]
- 9.Grant IH, Richardson NJ, Bokenheuser VD. Broiler chickens as potential source of Campylobacter infections in humans. J Clin Microbiol 1980;11:508–510. [DOI] [PMC free article] [PubMed]
- 10.Genigeorgis C, Hassuney M, Collins P. Campylobacter jejuni infection on poultry farms and its effect on poultry meat contamination during slaughtering. J Food Prot 1986;49:895–903. [DOI] [PubMed]
- 11.Beery JT, Hugdahl MB, Doyle MP. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microbiol 1988;54:2365–2370. [DOI] [PMC free article] [PubMed]
- 12.Hugdahl MB, Beery JT, Doyle MP. Chemotactic behavior of Campylobacter jejuni. Infect Immun 1988;56:1560–1566. [DOI] [PMC free article] [PubMed]
- 13.Klasing KC. Nutritional modulation of resistance to infectious diseases. Poult Sci 1998;77:1119–1125. [DOI] [PubMed]
- 14.Sinell HJ. Interacting factors affecting mixed populations. In: Sillker JH, ed. Microbial ecology of foods, vol.1. Factors affecting life and death of microorganisms. Acad Press, London, England, 1980:215–231.
- 15.Hofshagen M, Kaldhusdal M. Barley inclusion and avoparcin supplementation in broiler diets. Effects on small intestinal flora and performance. Poult Sci 1992;71:959–969. [DOI] [PubMed]
- 16.Chambers JR, Spencer JL, Modler HW. The influence of complex carbohydrates on Salmonella typhimurium colonization pH and density of broiler ceca. Poult Sci 1997;76:445–451. [DOI] [PubMed]
- 17.Canadian Council on Animal Care. Guide to the care and use of experimental animals. Ottawa: CCAC, 1993.
- 18.Collins CH, Lyne PM, Grange JM. Collin's and Lyne's Microbiological methods. Butterworths, London, England, 1992.
- 19.Doyle MP, Roman DJ. Recovery of Campylobacter jejuni and Campylobacter coli from inoculated foods by selective enrichment. Appl Environ Microbiol 1982;43:1343–1353. [DOI] [PMC free article] [PubMed]
- 20.Achen M, Morishita TY, Ley EC. Shedding and colonization of Campylobacter jejuni in broilers from day of hatch to slaughter age. Avian Dis 1998;42:732–737. [PubMed]
- 21.Lior H. New, extended biotyping scheme for Campylobacter jejuni, Campylobacter coli and Campylobacter laridis. J Clin Microbiol 1984;20:636–640. [DOI] [PMC free article] [PubMed]
- 22.Lior H, Patel A. Improved toluidine blue–DNA agar for detection of DNA hydrolysis by campylobacters. J Clin Microbiol 1987;25:2030–2031. [DOI] [PMC free article] [PubMed]
- 23.Penner JL, Hennessy JN. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol 1980;12:732–737. [DOI] [PMC free article] [PubMed]
- 24.Lior H, Woodward DL, Edgar JA, Laroche LJ, Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol 1982;15:761–768. [DOI] [PMC free article] [PubMed]
- 25.Vanhoof R, Dierickz R, Coignau H, Hubereachta JMH, Kaufman L, Butzler JP. Disk sensitivity testing for Campylobacter jejuni. Eur J Clin Microbiol 1984;3:160–162. [DOI] [PubMed]
- 26.Nachamkin I, Ung H, Patton CM. Analysis of HL and O serotypes Campylobacter strains by the flagellin gene typing system. J Clin Microbiol 1996;34:277–281. [DOI] [PMC free article] [PubMed]
- 27.Atschull SF, Maden TL, Schaffer AA, Zhang Z, Mills W, Lipman. DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997;25:3389–3402. [DOI] [PMC free article] [PubMed]
- 28.Sanyal SC, Islam KMN, Neogi PKB, Islam M, Spielman P, Huq MI. Campylobacter jejuni diarrhea models in infant chickens. Infect Immun 1984;43:931–936. [DOI] [PMC free article] [PubMed]
- 29.Shoeni JL, Doyle MP. Reduction of Campylobacter jejuni colonization of chicks by cecum colonizing bacteria producing anti C. jejuni metabolites. Appl Environ Microbiol 1992;58:664–670. [DOI] [PMC free article] [PubMed]
- 30.Welkos SL. Experimental gastroenteritis in newly hatched chicks infected with Campylobacter jejuni. J Med Microbiol 1984;18:233–248. [DOI] [PubMed]
- 31.Doyle MP. Colonization of chicks by Campylobacter jejuni. In: Blankenship LC, ed. Colonization control of human bacterial enteropathogens in poultry. Academic Press, San Diego, California, USA 1991:121–129.
- 32.Byrd JA, Corrier DE, Hume ME, Bailey RH, Stanker LH, Hargis BM. Incidence of Campylobacter in crops of preharvest market age broiler chickens. Poult Sci 1998;77:1303–1305. [DOI] [PubMed]
- 33.Hinton A, Corrier DE, Spates GE, et al. Biological control of Salmonella typhimurium in young chickens. Avian Dis 1990;34:626–633. [PubMed]
- 34.Frost JA, Oza AN, Thwaitei RT, Rowe B. Serotyping scheme for Campylobacter jejuni and Campylobacter coli based on direct agglutination of HS antigens. J Clin Microbiol 1998;36:335–339. [DOI] [PMC free article] [PubMed]
- 35.Woodward DL, Rodgers FG. Identification of Campylobacter heat stable and heat labile antigens by combining Penner and Lior serotyping schemes. J Clin Microbiol 2002;40:741–745. [DOI] [PMC free article] [PubMed]
- 36.Yan W, Chang N, Taylor DE. Pulsed field gel electrophoresis of Campylobacter jejuni and Campylobacter coli genomic DNA and its epidemiologic application. J Infect Dis 1991;163:1068–1072. [DOI] [PubMed]
- 37.Baker CN. The E-test and Campylobacter jejuni. Diagn Microbiol Infect Dis 1992;15:469–472. [DOI] [PubMed]
- 38.Harrington CS, Carter FMT, Carter PE. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol 1997;35:2386–2392. [DOI] [PMC free article] [PubMed]
- 39.Hanninen ML, Makela PP, Pitkala A, Rautelin H. A three year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from Helsinki area. J Clin Microbiol 2000;38:1998–2000. [DOI] [PMC free article] [PubMed]