Abstract
Thirteen pygmy goats (Capra hircus) from a herd naturally infected with Mycobacterium avium ss. paratuberculosis (MPTB) were monitored with 4 diagnostic assays for 2 to 15 mo. Cellular and humoral immune responses to the infection were assessed with assays of gamma interferon (IFNγ), serum antibody [enzyme-linked immunosorbent assay (ELISA) and agar gel diffusion (AGID)], and radiometric fecal culture. Microscopic examination and radiometric culture of tissue from 12 sites were performed at necropsy. Goats were considered infected if MPTB was isolated from any tissue sample collected at necropsy. Mycobacterial isolates were confirmed as MPTB with an IS900 polymerase chain reaction assay. Ten goats whose antemortem tests indicated infection carried heavy organism burdens at necropsy, both within and beyond the gastrointestinal system. False-negative ELISA, AGID, and/or culture results were obtained in 5 of the 10 confirmed cases during the study period. In 3 goats with sporadic fecal shedding of MPTB or detectable IFNγ response, or both, no abnormalities were detected at necropsy and no MPTB was isolated from the tissue samples; the antemortem fecal-culture and IFNγ results were thus considered false-positive. Diagnosticians should be alert to the possibility of both false-positive and false-negative test results for Johne's disease in goats. False-positive fecal-culture results may occur when a high prevalence of infection exists in the herd and the premises are likely to be heavily contaminated. The diverse antemortem testing patterns seen in these goats underscore the importance of using varied diagnostic assays serially or in parallel to increase the likelihood of identifying all infected goats.
Introduction
Johne's disease (paratuberculosis) is a contagious and emaciating gastrointestinal disease of ruminants caused by infection with Mycobacterium avium ss. paratuberculosis (MPTB). Troublesome for the cattle and sheep industries, the disease is also of concern for breeders of other ruminants, such as elk, deer, and goats, as well as for managers of captive wildlife. This infection has been reported in rabbits, fox, horses, pigs, humans, and primates (1,2). Transmission of MPTB has been shown to occur by multiple routes in cattle, including fecal–oral, in utero, and via milk or colostrum (3). This organism is thought to infect animals in the first months of life and elicits a slowly progressive inflammatory response in the gastrointestinal tract that is not clinically evident until months to years later. Clinical signs of this infection are the same as for many other diseases: chronic weight loss and, in some species, unremitting diarrhea. Diarrhea is usually absent in infected sheep and infrequent in infected goats and deer (4). The organism can disseminate beyond the gastrointestinal tract to other organ systems in advanced cases (5).
Materials and methods
The source pygmy goat (Capra hircus) herd had been managed as the only hoofstock on a farm for 5 y and averaged 30 adult animals. Kids were born into the herd and raised on the premises. Additions to the herd were made from outside sources as well.
Several goats in the herd were extremely thin, and the owner had noted periodic diarrhea in these animals. One debilitated goat was diagnosed with Johne's disease by histopathologic study [acid-fast bacilli (AFB) were observed in lesions consistent with those of paratuberculosis] and isolation of MPTB by radiometric culture of tissue. Subsequently, the entire herd (30 adults and juveniles) was screened for MPTB infection by radiometric bacterial culture of feces and by enzyme-linked immunosorbent assay (ELISA) for serum antibodies. Thirteen adult goats with test results indicative of infection by either means were moved to an animal research facility at the University of Wisconsin School of Veterinary Medicine and housed indoors, following husbandry guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. Sawdust bedding in their 2 rooms (each 119 ft2) was replaced twice a week, and the rooms were pressure-hosed once a week. On average, each room contained 6 goats. On a monthly basis, fecal and blood samples were collected from each goat until the goat was humanely euthanized. Since the goats were euthanized when it was clinically necessary or for economic reasons after 15 mo of study, the number of samples that could be collected per goat varied, from 2 to 15.
Fecal samples were collected per rectum. The procedure for detection of MPTB in ruminant fecal samples has been reported previously (6). Tissue samples were collected from 12 sites (ileum, jejunum, cecum, colon, lymph nodes (ileocecal, mesenteric, and mediastinal), liver, uterus or testes, lung, muscle, and kidney). They were processed for culture similarly to fecal samples after 3 g of the tissue had been homogenized in 5 mL of sterile phosphate-buffered saline (PBS) with a Stomacher (Tekmar, Cincinnati, Ohio, USA). Mycobacterial isolates were confirmed as MPTB with an IS900 polymerase chain reaction assay.
The cellular immune response to infection with MPTB was assessed by measuring the level of IFNγ produced by peripheral blood mononuclear cells in response to stimulation by purified protein derivative (PPD) antigen from M. avium ss. avium (PPD-A), a mycobacterial antigen proxy for MPTB. The assay has been shown to detect IFNγ from caprine lymphocytes (CSL Ltd., Melbourne, Australia) (7). Blood was collected from the jugular vein into heparin-containing tubes, and aliquots were placed in 4 vials within 8 h. One aliquot was stimulated with PPD-A [0.003 mg/mL; National Veterinary Services Laboratories (NVSL), Ames, Iowa, USA], one with PPD from M. bovis (PPD-B, 0.003 mg/mL; NVSL), and one with phytohemagglutinin antigen (0.0010 mg/mL; Sigma Chemical Company, St. Louis, Missouri, USA) as a positive mitogen control to assess the viability of the lymphocytes. The remaining aliquot served as an unstimulated control; PBS, 10 μL/mL, was added to it. If the positive mitogen control demonstrated that the goat's lymphocytes were viable, and if the optical density (OD) resulting from PPD-A stimulation minus the OD for the kit control was greater than 0.1 OD units, the result was considered elevated.
The caprine serum antibody ELISA was an adaptation of the MPTB Antibody Test Kit licensed by the US Department of Agriculture for use in cattle (IDEXX Laboratories, Inc., Westbrook, Maine, USA). An anti-goat/sheep immunoglobulin (Sigma) conjugated with horseradish peroxidase according to the instructions of the supplier (Zymed Laboratories, South San Francisco, California, USA) was substituted for the conjugate provided in the kit. The assay was performed according to the manufacturer's instructions, the substitute conjugate being used at a dilution of approximately 1:12 000. The positive control was ELISA positive serum from a goat confirmed to have been infected by MPTB by isolation of the organism from multiple tissues. The ELISA OD630nm data were reported as an S/P ratio (sample — negative control/positive control — negative control).
The agar gel immunodiffusion (AGID) assay (ImmuCell, Portland, Maine, USA) was performed according to the manufacturer's specifications, with the use of serum that had been frozen at −70°C.
A complete necropsy was performed for each goat. Sections of the same tissues collected for radiometric culture were also processed and stained with Ziehl–Neelsen's method and with hematoxylin and eosin for microscopic examination. A scoring system (0–4) was used to express the relative number of AFB in the tissue samples.
Results
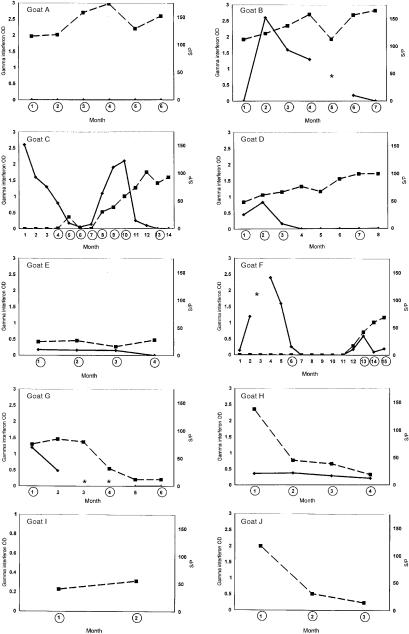
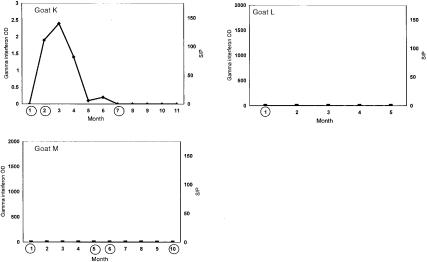
Goats were considered infected if MPTB was isolated by radiometric culture from any tissue collected at necropsy. Of the 13 goats, 10 (goats A–J) met the case definition for infection; Figure 1 shows the temporal patterns of test results for these goats. Figure 2 shows the temporal patterns for the 3 goats (K–M) that did not meet the case definition.
Figure 1. Assay results for the 10 goats meeting the case definition of infection with Mycobacterium avium ss. paratuberculosis. OD — optical density; S/P — for results of the enzyme-linked immunosorbent assay (ELISA) (see text for explanation); squares — ELISA values; diamonds — values for gamma interferon; asterisks — data not available. The circled months are those in which fecal shedding was detected.
Figure 2. Assay results for the 3 goats not meeting the case definition.
The typical clinical presentation was progressive weight loss despite a good appetite and adequate rations, rough hair coat with mild alopecia distinct from normal seasonal hair loss, plus weakness and an inability to rise in the goats with advanced disease. Diarrhea was rarely observed. At necropsy there was neither gross nor histopathologic evidence of other health factors that might have contributed to the goats' condition, such as secondary malnutrition, gastrointestinal parasites, caprine arthritis-encephalitis (CAE), or chronic infection with other bacteria, such as Corynebacterium pseudotuberculosis.
All 10 infected goats had elevated ELISA results at some point during the study period. Three (G, H, and J) showed a declining S/P value in the months before necropsy. ELISA values neared those of the kit negative control for 5 infected goats (C, F, G, H, and J) during the study. All serum samples for the 3 noninfected goats produced ELISA results bordering the S/P values for the kit negative control. AGID assays performed on frozen serum samples first produced a visible precipitation line either with the first monthly sample that had an S/P value above 0.25 (6 goats) or with the sample obtained 1 mo after the first sample that had an S/P value above S/P 0.25 (4 goats). If subsequent ELISA values were above 0.25, AGID precipitation lines were visible for the same sample.
Of the 10 infected goats, 7 (B–H) produced elevated amounts of IFNγ at least once during the study period. Goat C showed 2 IFNγ peaks; antibody levels rose during the decline after the 2nd peak. This pattern was recorded for goat F as well, although less IFNγ was produced over a shorter period in the 2nd peak. Initially elevated and then slowly declining IFNγ values were recorded concurrently with elevated antibody values for goat B. Of the 3 noninfected goats, 1 (K) had elevated levels of IFNγ in 5 consecutive monthly samples; the next 5 consecutive monthly samples were negative for IFNγ. The other 2 noninfected goats were consistently IFNγ-negative.
During the study, MPTB was isolated from fecal samples by radiometric culture at least once in all 10 cases. It was the only mycobacterial species isolated from any fecal sample collected. In 8 cases (A–F, H, and J), fecal shedding of the organism was detected in 3 or more consecutive monthly samples. In 4 cases (C, D, F, and G), fecal-culture results were negative at least once. The organism was also isolated at least once from fecal pellets from the 3 goats not meeting the case definition and was recovered from a fecal sample collected at birth from a kid (not part of the study group) born to an infected doe.
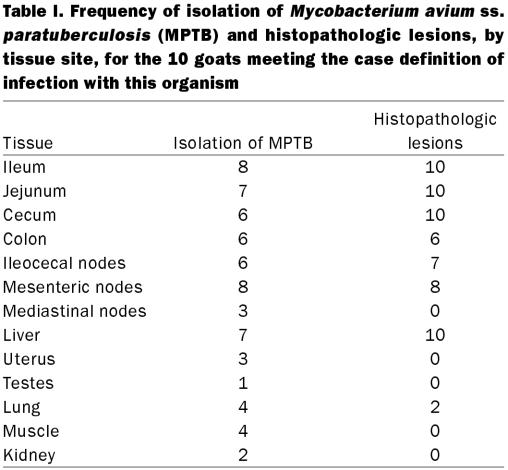
In each of the 10 cases MPTB was recovered from at least 1 tissue (Table I). It was the only mycobacterial species isolated from any tissue sample. The organism was recovered from extraintestinal tissues, such as hindlimb muscle, uterus, testes, lung, and kidney and was cultured from the hepatic tissue of 7 of the 10 infected goats. It was not isolated from any of the tissues sampled from 3 goats; thus, these animals were not considered to have true infection. The bacilli were isolated from the intestinal contents and intestinal tissue of a mid-gestation fetus sampled at necropsy of an infected doe.
Table I.
Gross pathological abnormalities in the 10 cases varied in extent and degree but were characterized by emaciation, loss of fat stores, corded mesenteric lymphatics, and mildly to moderately enlarged mesenteric lymph nodes with pale, nodular foci. Corrugation and thickening was noted in the distal gastrointestinal tract, but to a lesser extent than is usually seen in a comparably clinically affected bovid. Gross findings in the 3 noninfected goats were within normal limits.
The frequency, by tissue, of histopathologic findings consistent with Johne's disease is shown in Table I. Each of the 10 goats with histopathologic evidence of paratuberculosis in the gastrointestinal tract also exhibited granulomatous hepatitis with rare AFB. In the 10 cases, the presence of AFB in at least 1 tissue was scored as 3. These 10 goats had a primarily lymphocytic and plasmacytic, and to a lesser extent histiocytic, inflammatory infiltrate in the ileum, jejunum, cecum, and liver. Although primarily observed within the superficial (villar) lamina propria, the infiltrate occasionally extended into an edematous submucosa. Six of the 10 goats exhibited a similar infiltrate in the colon. Focal accumulations of macrophages contained rare to numerous intracytoplasmic AFB, depending on the tissue site and animal. Granulomatous lymphadenitis was present in mesenteric or colic lymph nodes or both tissues. Giant cells were rarely identified in any of the tissues examined histologically. Granulomatous lesions were noted in the liver of all 10 goats. Microscopic findings in the 3 noninfected goats were within normal limits.
Discussion
Paratuberculosis is a slowly developing disease that elicits cellular and humoral immunologic responses at different phases of the infection. The purpose of this study was to track the progress of naturally occurring MPTB infection in pygmy goats using serologic, IFNγ and fecal-culture assays. The test results presented diverse diagnostic patterns, demonstrating the possibility of both false-positive and false-negative antemortem results during surveillance for Johne's disease in a heavily infected herd.
Since the goats were naturally rather than experimentally infected, each goat was likely at a different phase of MPTB infection during the study period, as would occur in any goat herd seen in veterinary practice. This factor may have contributed to the variety in diagnostic patterns, from what may be considered typical (as in goat F: early IFNγ spike; subsequently climbing antibody values; and multiple, although intermittent, positive fecal-culture results) to a more unusual pattern (as in goat M: no detectable IFNγ or antibody but multiple positive fecal-culture results). Other factors likely to have contributed to the diversity of diagnostic pattern include differences in age at infection, MPTB dose received and age at receipt, immunologic status, and perhaps individual genetic susceptibility, as has been seen in other mycobacterial infections (8).
The 10 goats meeting the case definition displayed pathological findings in keeping with what is termed a TH2 or lepromatous pattern of disease: frequent fecal shedding of the organism plus a heavy MPTB tissue burden. Elevated antibody values were recorded for 7 of these goats for a number of months, another facet of the TH2 pattern. Based on the finding in this study of evidence of infection (AFB plus tissue lesions and/or isolation of the organism) in hepatic tissue in all 10 cases, it may be useful to sample the liver postmortem for evidence of Johne's disease in goats in addition to the sites usually recommended (ileum and mesenteric lymph nodes).
The 3 goats not meeting the case definition either were never infected, or were infected but cleared the infection, or were infected but had such slight and focal evidence at necropsy that it could not be found with the methods employed. One of these goats (K) displayed some characteristics of the tuberculoid form of MPTB infection: repeatedly elevated IFNγ values in response to PPD-A stimulation and sporadic low-level shedding of MPTB. These findings are in keeping with those for 2 experimentally infected goats with antemortem IFNγ production in response to PPD-A stimulation that were free of signs of active infection at necropsy (9). Positive IFNγ test results have also been reported in sheep without histopathologic evidence of infection that came from infected flocks (10). It is possible that the cell-mediated response, as indicated by the detectable IFNγ released from peripheral blood mononuclear cells, may have arrested the progress of the infection during the study period.
It was interesting to note the relationship between the IFNγ and antibody responses in the 2 goats not producing antibody at the start of the study (C and F). In both cases, a 2-peak IFNγ production pattern was seen. During the 2nd peak, antibody production began, and it continued as IFNγ production declined. This overlap does not mirror what has been reported in cattle: that the cellular immune response precedes the humoral reaction to infection, described as the TH1–TH2 shift (11). Even less similar were the findings for goat B: 4 mo of concurrently elevated values for IFNγ and antibody.
The lack of specificity frequently reported with assays using mycobacterial PPD preparations (12) was found in this study as well. For virtually all samples, the same interpretation of the IFNγ assay was reached whether the peripheral blood mononuclear cells were stimulated by PPD-B (data not shown) or by PPD-A. Since MPTB was the only mycobacterial organism isolated from any fecal or tissue sample, however, we believe that the cellular and humoral immune responses seen in these goats were stimulated by that particular mycobacterium.
The antemortem isolation of MPTB from fecal pellets taken from the goats with no postmortem evidence of infection despite intensive scrutiny raises the possibility that positive fecal-culture results may not always indicate true infection. If these 3 goats were truly free of infection, the antemortem fecal-culture results must be considered false-positive. Goats can be coprophagic, and since these adult animals were living among infected animals (first in a large outdoor pen and then in an indoor enclosure), it is possible that the positive fecal-culture results could be explained by “pass-through”, as opposed to true infection. Herds with a high prevalence of Johne's disease are likely to be managed on highly contaminated premises; the diagnostic specificity of fecal culture in this situation may be less than 100%.
Isolation of MPTB from neonatal feces as well as from the intestinal contents and intestinal tissue of a mid-gestation fetus supports the hypothesis that in utero infection by MPTB can occur in goats. Thus, accurate record-keeping is important in goat husbandry to enable culling or close monitoring of potentially infected offspring of any doe with a diagnosis of the disease. Even if the kid escapes in utero infection, transmission of the organism through milk from an infected doe or through contact with contaminated manure may occur.
Although the ELISA is reported to be a more sensitive method of antibody detection than the AGID in cattle (13), the 2 assays performed similarly during this study. Perhaps once these goats began to produce antibody the amount rose so precipitously that it swamped any difference in detection sensitivity between the 2 tests. Serum samples would need to be collected more often than monthly to test this hypothesis.
The pygmy goat species represents an instructive naturally susceptible animal model for further study of the immunopathological events occurring during the course of MPTB infection. The species displays immunologic responses that vary in character, extent, and duration. This variability, however, can confound attempts to control the infection in a herd if a single diagnostic assay is used in a test-and-cull program. Serial or parallel testing with assays aimed at different aspects of the infection (organism shedding and cellular or humoral response) can increase the likelihood of identifying infected goats in a herd with previously confirmed cases of Johne's disease.
Footnotes
Acknowledgments
The authors thank Ms. Debra Teubert and Ms. Joely Kramsky for their valuable technical assistance. We also thank the herd owner for his support of the research and the Minnesota Diagnostic Laboratory for making the initial diagnosis. This study was funded by the Johne's Testing Center, University of Wisconsin School of Veterinary Medicine, Madison, Wisconsin, USA.
Address all correspondence and reprint requests to Dr. Elizabeth J.B. Manning; telephone: (608) 265-4958; fax: (608) 263-9754; e-mail: emanning@facstaff.wisc.edu
Received April 3, 2002. Accepted August 23, 2002.
References
- 1.Beard PM, Daniels MJ, Henderson D, et al. Paratuberculosis infection of nonruminant wildlife in Scotland. J Clin Microbiol 2001;39:1517–1521. [DOI] [PMC free article] [PubMed]
- 2.Hines ME II, Kreeger JM, Herron AJ. Mycobacterial infections of animals: pathology and pathogenesis. Lab Anim Sci 1995;45:334–351. [PubMed]
- 3.Sweeney RW, Whitlock RH, Rosenberger AE. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J Clin Microbiol 1992;30:166–171. [DOI] [PMC free article] [PubMed]
- 4.Stehman SM. Paratuberculosis in small ruminants, deer, and South American camelids. Vet Clin North Am Food Anim Pract 1996;12:441–455. [DOI] [PubMed]
- 5.Whitlock RH, Rosenberger AE, Sweeney RW, Spencer PA. Distribution of M. paratuberculosis in tissues from cattle herds infected with Johne's disease. In: Chiodini RJ, Hines ME II, Collins MT, eds. Proceedings of the 5th International Colloquium on Paratuberculosis. Rehoboth, Massachussetts: International Association for Paratuberculosis, 1997:200–210.
- 6.Collins MT, Kenefick KB, Sockett DC, Lambrecht RS, McDonald J, Jorgensen JB. Enhanced radiometric detection of Mycobacterium paratuberculosis using filter concentrated fecal specimens. J Clin Microbiol 1990;28:2514–2519. [DOI] [PMC free article] [PubMed]
- 7.Rothel JS, Jones SL, Corner LA, Cox JC, Wood PR. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust Vet J 1990;67:134–137. [DOI] [PubMed]
- 8.Mackintosh CG, Qureshi T, Waldrup K, Labes RE, Dodds KG, Griffin JF. Genetic resistance to experimental infection with Mycobacterium bovis in red deer (Cervus elaphus). Infect Immun 2000;68:1620–1625. [DOI] [PMC free article] [PubMed]
- 9.Storset AK, Hasvold HJ, Valheim M, et al. Subclinical paratuberculosis in goats following experimental infection: an immunological and microbiological study. Vet Immun Immunopathol 2001;80:271–287. [DOI] [PubMed]
- 10.Perez V, Tellechea J, Corpa JM, Gutierrez M, Marin JFG. Relation between pathologic findings and cellular immune responses in sheep with naturally acquired paratuberculosis. Am J Vet Res 1999;60:123–127. [PubMed]
- 11.Koets AP, Rutten VP, Hoek A, et al. Heat-shock protein-specific T-cell responses in various stages of bovine paratuberculosis. Vet Immun Immunopathol 1999;70:105–115. [DOI] [PubMed]
- 12.Jungersen G, Huda A, Hansen JJ, Lind P. Interpretation of the gamma interferon test for diagnosis of subclinical paratuberculosis in cattle. Clin Diagn Lab Immunol 2002;9:453–460. [DOI] [PMC free article] [PubMed]
- 13.Sockett DC, Conrad TA, Thomas CB, Collin MT. Evaluation of 4 serological tests for bovine paratuberculosis. J Clin Microbiol 1992;30:1134–1139. [DOI] [PMC free article] [PubMed]