Abstract
During infection, nutrient deprivation can alter bacterial phenotype. This, in turn, may have implications for pathogenesis and prophylaxis. Actinobacillus pleuropneumoniae (biotype 1) and Haemophilus parasuis, respiratory tract pathogens of swine, are both V-factor-dependent. The concentrations of V factor in the extracellular fluids of pigs are unknown and may limit the growth of these bacteria in vivo. The aim of this study was to determine the concentrations of nicotinamide adenine dinucleotide (NAD) in select porcine body fluids and to compare the availability of NAD in vivo with the affinities of the organisms for this compound. Levels in plasma, tissue fluids (peritoneal, pleural, synovial, and cerebrospinal), and laryngeal, tracheal, and lung washings were determined with an enzymatic cycling assay. We concluded that, although the NAD supply in the respiratory tract is probably not growth-limiting, it may become limiting if the organisms are disseminated.
Actinobacillus pleuropneumoniae (biotype 1) and Haemophilus parasuis are important pathogens of swine, the former causing porcine pleuropneumonia (1,2) and the latter Glässer's disease (a disseminated infection characterized by polyserositis, polyarthritis, and meningitis) (3). These bacteria are fastidious in their nutritional requirements and characteristically require culture media supplemented with specific pyridine nucleotides or their precursors (V factor). Although V factor is usually provided as nicotinamide adenine dinucleotide (NAD), this nutritional requirement can be met by several pyridine compounds, including nicotinamide mononucleotide (NMN) and nicotinamide riboside, but not by nicotinamide or nicotinic acid (4). This specificity reflects the limited capacities of these organisms for pyridine compound metabolism (5,6).
Nutrient deprivation is a key environmental factor affecting bacterial phenotype, including the expression of virulence factors during the course of infection (7,8); the affected virulence factors may include antigens that may be useful as vaccine components. Although A. pleuropneumoniae and H. parasuis possess efficient mechanisms for the acquisition of pyridine nucleotide precursors, their growth rates can be limited by the supply of these compounds (4); with the type strains (A. pleuropneumoniae ATCC 27088; H. parasuis ATCC 19417), growth limitation begins at concentrations of NAD below 2 μM. Limiting in vitro growth by reducing the concentration of exogenous NAD or NMN from 5 to 0.1 μM can result in a change in the outer membrane protein profile of A. pleuropneumoniae (9). Furthermore, with A. pleuropneumoniae, reducing the initial NAD concentration from about 450 to about 15 μM would appear to be associated with increased adhesiveness (without a change in hydrophobicity), thinner and irregular capsule structure, and, in some strains, increased production of fimbriae and altered outer membrane protein profiles (10).
Whether in vivo growth is limited by pyridine nucleotide supply is unknown. Information on the concentrations of pyridine nucleotides and their precursors in extracellular fluids of mammals is scant. Serum contains nicotinamide (11), but this precursor is not utilized by A. pleuropneumoniae or H. parasuis. In addition, although intracellular NAD(P)(H) concentrations are high [for example, in human erythrocytes, approximately 30–60 μM for NAD, 10–40 μM for NADP, 10–30 μM for NADH, and 20–40 μM for NADPH (12,13)], mammalian cells possess extracellular NAD(P) nucleosidases that catabolize NAD(P) to nicotinamide (14,15). Although low concentrations of pyridine nucleotide precursors can support the growth of A. pleuropneumoniae and H. parasuis (4), given the potential for NAD-dependent growth limitation to induce phenotypic changes in these bacteria, their capacity to compete with host tissues for utilizable NAD(P) precursors may influence the phenotypes of these bacteria during infection. Therefore, we undertook a study to determine the NAD contents of porcine body fluids at sites of bacterial growth during infection.
During experimental infections to characterize the pathogenicity of porcine Actinobacillus and Haemophilus organisms (16), samples of tissue fluids and lavage washings of the respiratory tract were collected for assay of NAD. Blood was collected in tubes containing ethylenediaminetetraacetic acid, and the plasma was recovered after centrifugation. All other samples were collected as rapidly as possible after euthanasia. Peritoneal, pleural, synovial, and cerebrospinal fluids were collected by aspiration. Lung lavage fluid was obtained by the bronchial instillation of 6 mL of phosphate-buffered saline (PBS), pH 7.4. Sections of larynx and trachea 3 to 4 cm long and 1.5 cm in diameter were excised and clamped off with the use of hemostats, then the inner surfaces were rinsed with 3 mL of PBS. In all cases, fluid recovery was about 50%. Samples were centrifuged (at 16 000 × g for 5 min) and the supernatants removed and stored in aliquots at −70°C (or below) until assayed for NAD content (within 31 d). Thawed samples were centrifuged (at 16 000 × g for 2 min), and 200 μL of each supernatant was added to 200 μL of 0.3 M HCl. Samples were incubated for 10 min at 60°C, cooled on ice, and neutralized through the dropwise addition of 200 μL of a solution of equal parts of 0.36 M triethanolamine-HCl (pH 7.4 with KOH) and 0.6 M KOH during continuous vortexing of the samples. Samples were then centrifuged (at 16 000 × g for 10 min), and 50-μL aliquots of each supernatant were assayed for NAD content by the method of internal standards (25 pmol NAD) to correct for possible interference by sample components. The NAD content was determined by means of an enzymatic cycling assay, essentially as described by Lilius et al (17) except that Hepes was the buffer system used and 12.5 units of alcohol dehydrogenase was used per assay.
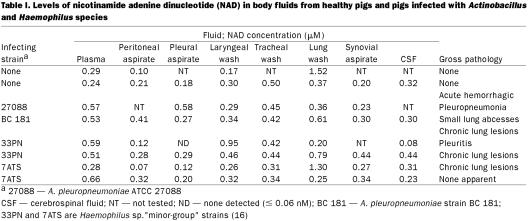
The NAD levels in plasma; lavage fluid from tracheal, laryngeal, and lung washings; and peritoneal, pleural, synovial, and cerebrospinal fluids are presented in Table I. Notably, the levels in these extracellular fluids were considerably lower than those found in human erythrocytes [approximately 30–60 μM (12,13)] and in rat tissues [approximately 0.3 μmol/mL in blood and 0.33, 0.93, 0.78, and 0.75 μmol/g in lung, heart, kidney, and liver, respectively (18)]. Infection with A. pleuropneumoniae (and "minor-group" Haemophilus) apparently had little effect on tissue-fluid NAD levels, although it appeared to increase plasma NAD levels.
Table I.
Both A. pleuropneumoniae and H. parasuis demonstrate efficient NAD utilization, with growth rate constants of 0.24 and 0.21 μM, respectively (4). Growth rates begin to be unrestricted by NAD at concentrations greater than 2 μM. Although the assays of body fluid samples (plasma, peritoneal, pleural, synovial, and cerebrospinal) are likely to yield reliable NAD concentrations, the levels of NAD in lavage fluids are likely to be underestimates owing to the dilution effect of the large buffer volume (compounded by loss of buffer volume, presumably by tissue absorption). If the dilution factor is greater than 10 (which is highly likely), then the mucosal surfaces are probably covered by fluids that contain NAD at levels in excess of those needed for growth rates to be unimpaired by pyridine nucleotide supply. However, assuming that extracellular V factor is present primarily as NAD, comparison of tissue-fluid levels with the growth rate constants suggests that NAD levels are sufficiently low to at least partially inhibit growth rates after dissemination of the bacteria from the mucosal surfaces.
Other nutrients (such as, iron) are also either in low concentration or are sequestered from bacteria colonizing and invading mammals (7,8). Competition for many nutrients at growth-rate-limiting concentrations has complex physiological effects on bacteria (19), and combined stresses can increase virulence profoundly (20). Accordingly, the NAD levels encountered at different body sites may result in changes in the phenotype of A. pleuropneumoniae, and possibly H. parasuis, during an infection, and in particular during spread from the respiratory mucosa (likely not NAD-limiting) to the deeper tissues (possibly NAD-limiting). Although it is clear that NAD restriction can modify the phenotype of A. pleuropneumoniae (9,10), the combined effects of this and other nutrient limitations on overall pathogenesis clearly merit further attention.
Footnotes
Acknowledgments
The authors thank the late Dr. S. Rosendal for his help in acquisition of the fluid samples and the Natural Sciences and Engineering Research Council of Canada for their generous support.
Dr. O'Reilly's current address is Oncology Research, Novartis Pharma AG, CH-4002 Basel, Switzerland.
Address all correspondence and reprint requests to Dr. Donald F. Niven; telephone: (514) 398-7886; fax: (514) 398-7990; e-mail: niven@nrs.mcgill.ca
Received September 26, 2002. Accepted November 28, 2002.
References
- 1.Rycroft AN, Garside LH. Actinobacillus species and their role in animal disease. Vet J 2000;159:18–36. [DOI] [PubMed]
- 2.Bossé JT, Hanson T, Sheehan BJ, et al. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect 2002;4:225–235. [DOI] [PubMed]
- 3.MacInnes JI, Desrosiers R. Agents of the "suis-ide diseases" of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can J Vet Res 1999;63:83–89. [PMC free article] [PubMed]
- 4.O'Reilly T, Niven DF. Defining the metabolic and growth responses of porcine haemophili to exogenous pyridine nucleotides and precursors. J Gen Microbiol 1986;132:807–818. [DOI] [PubMed]
- 5.O'Reilly T, Niven DF. Pyridine nucleotide metabolism by extracts derived from Haemophilus parasuis and H. pleuropneumoniae. Can J Microbiol 1986;32:733–737. [DOI] [PubMed]
- 6.Martin PR, Shea RJ, Mulks MH. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J Bacteriol 2001;183:1168–1174. [DOI] [PMC free article] [PubMed]
- 7.Brown MRW, Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol 1985;39:527–556. [DOI] [PubMed]
- 8.Ferenci T. Regulation by nutrient limitation. Curr Opin Microbiol 1999;2:208–213. [DOI] [PubMed]
- 9.O'Reilly T, Niven DF, Brown MRW. Phenotypic variation in the outer membrane protein composition of Actinobacillus (Haemophilus) pleuropneumoniae: non-specific effect of exogenous pyridine nucleotide supply. Vet Microbiol 1991;29:159–172. [DOI] [PubMed]
- 10.Van Overbeke I, Chiers K, Charlier G, et al. Characterization of the in vitro adhesion of Actinobacillus pleuropneumoniae to swine alveolar epithelial cells. Vet Microbiol 2002;88:59–74. [DOI] [PubMed]
- 11.Bernofsky C. Physiologic aspects of pyridine nucleotide regulation in mammals. Mol Cell Biochem 1980;33:135–143. [DOI] [PubMed]
- 12.Stocchi V, Cucchiarini L, Magnani M, Chiarantini L, Palma P, Crescentini G. Simultaneous extraction and reverse-phase high-performance liquid chromatographic determination of adenine and pyridine nucleotides in human red blood cells. Anal Biochem 1985;146:118–124. [DOI] [PubMed]
- 13.Micheli V, Sestini S. Determining NAD synthesis in erythrocytes. Methods Enzymol 1997;280:211–221. [DOI] [PubMed]
- 14.Johnson GS. A pyrophosphatase which degrades NAD+ is located on the external surface of cultured fibroblasts: evidence that NAD+ is not extruded during treatment with N-methyl-N'-nitro-N-nitrosoguanidine. Arch Biochem Biophys 1984;229:538–543. [DOI] [PubMed]
- 15.Goodman SI, Wyatt RJ, Trepel JB, Neckers LM. NAD glycohydrolase: enzyme characterization using intact mammalian erythrocytes. Comp Biochem Physiol [B] 1982;71:333–336. [DOI] [PubMed]
- 16.Rosendal S, Boyd DA, Gilbride KA. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med 1985;49:68–74. [PMC free article] [PubMed]
- 17.Lilius E-M, Multanen V-M, Toivonen V. Quantitative extraction and estimation of intracellular nicotinamide nucleotides of Escherichia coli. Anal Biochem 1979;99:22–27. [DOI] [PubMed]
- 18.Rawling JM, ApSimon MM, Kirkland JB. Lung poly(ADP-ribose) and NAD+ concentrations during hyperoxia and niacin deficiency in the Fischer-344 rat. Free Radic Biol Med 1996;20:865–871. [DOI] [PubMed]
- 19.Gottschal JC. Growth kinetics and competition — some contemporary comments. Antonie Van Leeuwenhoek 1993;63:299–313. [DOI] [PubMed]
- 20.Brener D, DeVoe IW, Holbein BE. Increased virulence of Neisseria meningitidis after in vitro iron-limited growth at low pH. Infect Immun 1981;33:59–66. [DOI] [PMC free article] [PubMed]