Abstract
Both reversible and irreversible inhibition of mitochondrial respiration have been reported following the generation of nitric oxide (NO) by cells. Using J774 cells, we have studied the effect of long-term exposure to NO on different enzymes of the respiratory chain. Our results show that, although NO inhibits complex IV in a way that is always reversible, prolonged exposure to NO results in a gradual and persistent inhibition of complex I that is concomitant with a reduction in the intracellular concentration of reduced glutathione. This inhibition appears to result from S-nitrosylation of critical thiols in the enzyme complex because it can be immediately reversed by exposing the cells to high intensity light or by replenishment of intracellular reduced glutathione. Furthermore, decreasing the concentration of reduced glutathione accelerates the process of persistent inhibition. Our results suggest that, although NO may regulate cell respiration physiologically by its action on complex IV, long-term exposure to NO leads to persistent inhibition of complex I and potentially to cell pathology.
Nitric oxide (NO), because of its close resemblance to oxygen, has been used since the 1950s as a biochemical probe for the study of oxygen binding to cytochrome c oxidase, i.e., complex IV, in the mitochondrial respiratory chain (reviewed in ref. 1). Several studies from the 1960s to the early 1980s (reviewed in ref. 2) helped to clarify the mechanism of the interaction between NO and this enzyme. However, it was only after the discovery of the l-arginine:NO pathway (3) that studies on the inhibitory control by NO of cell respiration acquired a definite biological significance. It has now been established that short-term exposure to physiological concentrations of NO rapidly inhibits complex IV in a reversible manner which is competitive with oxygen (4, 5).
Long-term exposure to NO has previously only been studied by using cells activated with cytokines and bacterial products to produce NO. In these experiments the effect of NO was investigated either on the generating cells themselves or on cells coincubated with them. In both cases cell respiration was found to be inhibited in a persistent manner due to inhibition of various mitochondrial enzymes, i.e., complexes I to IV in the respiratory chain, as well as aconitase in the citric acid cycle (6–9).
It has not been clear, however, whether inhibition of the enzymes is due to NO or to the action of other substances produced during cell activation. Indeed, superoxide, which is also generated under these conditions, can interact with NO to form peroxynitrite (ONOO−) (10). ONOO− inhibits these enzymes in an irreversible manner both in vitro and in vivo (11–15), suggesting that ONOO− may play a major role in the inhibition of respiration occurring under these conditions. NO itself may, however, have a direct long-lasting effect on respiration, as, for example, seems to occur in the cytotoxic activity of microglial cells toward oligodendrocytes and neurons (16, 17) and the self-induced inhibition of respiration in astrocytes (18).
To investigate in greater detail the mechanism(s) by which long-term exposure to NO inhibits cell respiration, we have studied the effect of a slow-releasing NO donor (Z)-1-[2-aminoethyl]-N-(2-ammonioethyl)amino]diazen-1-ium 1,2- diolate (DETA-NO) (19) on the oxygen consumption of two types of cells: the nonactivated J774 parental cell line and its derivative clone C3C, which is known to be defective in the generation of superoxide (20). Our results demonstrate that long-term exposure of cells to NO leads to inhibition of complex I and complex IV. Although the inhibition of complex IV remains reversible throughout the time of exposure, the inhibition of complex I, which seems to be due to S-nitrosylation of this enzyme, becomes progressively persistent as the concentration of reduced glutathione (GSH) in the cell decreases. Thus, GSH protects the functioning of the enzyme, probably by either preventing the nitrosylation of critical thiols or by removal of NO from already nitrosylated SH moieties.
MATERIALS AND METHODS
Materials.
Culture media and fetal calf serum were obtained from GIBCO. DETA-NO, a compound known to release NO with a half-life of 20 h at pH 7.4 (19), was purchased from Alexis Co. (Nottingham, U.K.). Mn(III) tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP) was purchased from Calbiochem. Measurements of GSH were performed using the Bioxytech GSH-400 kit from Oxis International (Portland, OR). Oxyhemoglobin (Hb) was prepared as described previously (21). Myxothiazol, rotenone, carbonyl cyanide p-trifluoromethoxy phenylhydrazone (FCCP), N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD), β-hydroxybutyrate, 3-nitropropionic acid, luminol, and all other reagents were from Sigma–Aldrich.
Cell Culture and Preparation.
Murine macrophage J774 parental cells and the C3C clone were grown in suspension using a MCS biological stirrer (Techne Laboratories, Cambridge, U.K.) in DMEM supplemented with 10% fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. Only cells in the logarithmic growth phase and with >95% viability (as judged by the trypan blue exclusion test) were used for the experiments. At the time of the experiments the cells were centrifuged, washed, and resuspended at a density of 107 cells/ml in an incubation medium consisting of (in mM): 118 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1 CaCl2, 20 glucose, and 25 Hepes (pH 7.2). Protein content in the samples was determined using the BCA procedure (Pierce). In selected experiments, cells were resuspended in glucose-free medium and respiration was sustained on pyruvate (20 mM) as described in Results. In the experiments in which the long-term effects of inhibition of respiration by NO were investigated, cells were treated in the presence or absence of DETA-NO in the incubation medium under sterile conditions and then washed twice and resuspended in fresh culture medium at a density of 2.5 × 105 cells/ml. Aliquots were taken at various time points and cell number and viability were analyzed under the inverted microscope after trypan blue staining. Cells were then centrifuged, washed, and resuspended in the incubation medium for measurement of oxygen consumption.
Measurement of Oxygen Consumption and NO Generation.
Cells suspended in the incubation medium at a density of 107 cells/ml were incubated at 37°C in the presence or absence of DETA-NO. One-milliliter samples were analyzed at the time points indicated in the various experiments in a gas-tight vessel thermostated at 37°C, equipped with both a Clark type oxygen electrode (Rank Brothers, Bottisham, U.K.) and a NO electrode (Iso-NO; World Precision Instruments, Stevenage, U.K.) connected to a chart recorder. NO release and cellular oxygen consumption were thus measured simultaneously as described (4). The oxygen electrode was calibrated assuming the concentration of oxygen in the incubation medium at 37°C to be 200 μM; the NO electrode was calibrated with NO-saturated water as described (4).
To investigate the reversibility of NO-induced inhibition of respiration, Hb (8 μM) was added to the cells to remove the NO instantaneously. Removal of NO was always carried out at identical oxygen concentrations (60 μM) to avoid interference in the measurements due to the competition between different concentrations of oxygen and NO at the level of cytochrome c oxidase (4). We define persistent inhibition of respiration as the proportion of this inhibition which is not immediately (<30 s) reversible by the addition of Hb.
In those experiments in which blockers of the citric acid cycle and the mitochondrial respiratory chain were used, the inhibitors and the corresponding respiratory substrates were added to the cells immediately before addition of the NO donor. The concentrations of the various compounds were selected in preliminary experiments designed as follows: oxygen consumption in untreated cell samples was followed for 4 min, and the various blockers (3-nitropropionic acid, myxothiazol, rotenone) were added at increasing concentrations to select the minimally effective concentration required to reduce oxygen consumption to <10% of control. The corresponding respiratory substrates (β-hydroxybutyrate, TMPD plus ascorbate, succinate) were then added at increasing concentrations to select the one that restored respiration to at least 90% of the level of untreated controls. The maintenance of the respiration rate thus obtained was then followed over a 6-h period and compared with that of untreated controls. In those experiments in which cells were subjected to illumination with light, cell suspensions were exposed to high intensity (≈8 Mlx) light from a cold light source (KL1500; Schott, Mainz, Germany). Data on oxygen consumption by DETA-NO-treated cells are expressed as percentage of that measured in cells subjected to the same treatment in the absence of the NO donor (controls).
Assay of Complex I Activity.
The activity of complex I was assayed as described (22). Briefly, the cells were centrifuged and the cell pellet was freeze thawed three times. Twenty microliters of cellular homogenate (0.3 mg) was added to 1 ml of 10 mM potassium phosphate buffer (pH 8.0) containing 100 μM NADH in a 1-ml cuvette at 37°C. The rate of NADH oxidation was followed at 340 nm for 2 min in a UV spectrophotometer. Then 5 μl of 10 mM ubiquinone-1 was added and the stimulated rate of NADH oxidation was taken as the complex I activity, using an extinction coefficient of 6.81 mM−1 cm−1 at 340 nm. No increase in NADH oxidation was observed in the presence of the complex I inhibitor rotenone (2 μM).
Measurement of Reactive Oxygen Intermediates.
The release of reactive oxygen intermediates from J774 and C3C cells was measured using luminol-enhanced chemiluminescence. Measurements were always performed in parallel with the measurements of oxygen consumption. One hundred microliters of the cell suspension (corresponding to 106 cells) was taken from the incubates with or without DETA-NO at the indicated time points and diluted 1:10 with incubation medium. Luminol was then added at a final concentration of 0.2 mM and cells analyzed in a Wallac-LKB 1250 luminometer equipped with a thermostatically controlled cuvette at 37°C. Photon counts generated by the chemiluminescent reaction with luminol were documented with a strip chart recorder. As a positive control, the protein kinase C activator, phorbol 13-myristate 12-acetate, known to stimulate the oxidative burst (23), was added at the end of each experiment and its addition always yielded a large amount of reactive oxygen species both in control and DETA-NO-treated cells.
Statistical Analysis.
The results are expressed as means ± SEM; n represents the number of individual experiments. Statistical analysis was performed using Student’s t test for unpaired variables (two tailed).
RESULTS
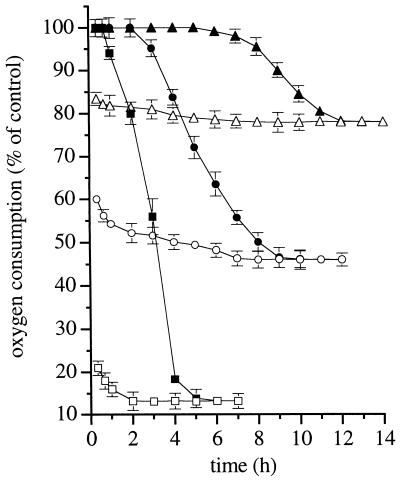
Addition of DETA-NO to J774 cells suspended in the incubation medium resulted in a gradual generation of NO, as detected by the NO electrode. This reached a plateau after approximately 15 min and remained constant for the duration of the experiment. At the maximum concentration of DETA-NO used (0.5 mM), a concentration of ≈1.5 μM NO was achieved. Addition of Hb immediately abolished the NO signal. The inhibition by DETA-NO of cell respiration was always immediate and, in the early phases, fully reversible with Hb, consistent with previous reports (4, 5, 18). The extent of inhibition was dependent on the concentration of DETA-NO (Fig. 1). After 1 h of incubation, however, the ability of Hb to reverse the inhibition decreased progressively until it became ineffective between 5 and 12 h, depending on the concentration of the NO donor. The larger the initial concentration of DETA-NO the shorter the time for Hb to become ineffective (Fig. 1). Persistent inhibition of respiration by DETA-NO was due neither to an action of the corresponding amine, DETA, nor to the stable decomposition product of NO, nitrite, since if the compound was allowed to decompose fully under acidic conditions, no effect was observed on the rate of oxygen consumption (not shown). The effect of DETA-NO was not the consequence of a nonspecific toxic effect of NO since the cells were viable at the end of the experiment (6 h), as assessed by trypan blue exclusion: the viability was 82 ± 4.7 and 85 ± 3.9% (P > 0.05, n = 6) in controls and in cells exposed to 0.5 mM DETA-NO, respectively. Opening of the mitochondrial permeability transition pore, an event that can be facilitated by NO and may eventually lead to cell death (24), was also excluded, since treatment of cells with the inhibitor of pore opening, cyclosporin A (1 μM) (23), did not affect the onset of persistent inhibition of respiration (not shown, n = 2).
Figure 1.
Effects of exposure to DETA-NO on cell respiration in J774 cells. Cells were incubated with different concentrations of DETA-NO (0.03 mM, triangles; 0.1 mM, circles; 0.5 mM, squares) and oxygen consumption was measured in samples taken at the indicated time points: before (open symbols) or after (filled symbols) addition of Hb (8 μM; n = 5). Oxygen consumption in DETA-NO-treated cells, here and in the subsequent figures, is reported as percentage of that observed in samples treated in the same way but without DETA-NO (controls). The rate of oxygen consumption in control cells was 14.3 ± 0.78 μM min−1 (n = 5) and did not vary significantly throughout the experiment.
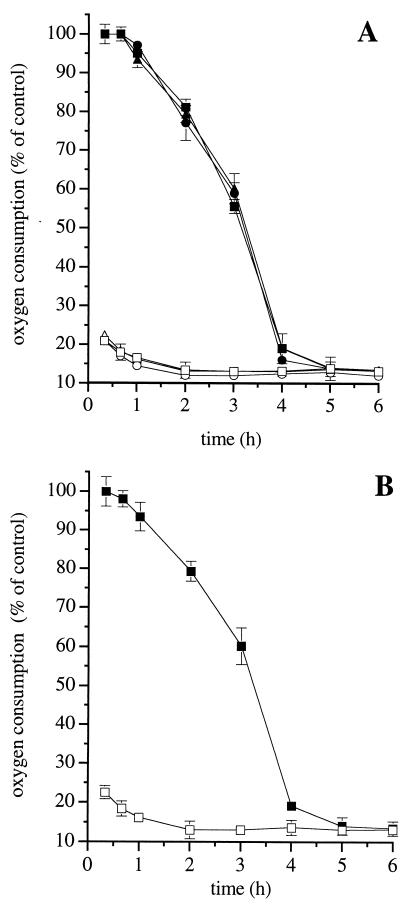
DETA-NO (0.5 mM) administered alone to the cells did not induce any detectable generation of reactive oxygen intermediates, as assessed by luminol-enhanced chemiluminescence (not shown, n = 2). In addition, the ONOO− scavengers methionine (20 mM) and uric acid (1 mM), as well as the membrane-permeant superoxide dismutase mimetic Mn-TBAP (0.3 mM), did not affect the time course of onset of persistent inhibition of respiration (Fig. 2A). Further experiments were carried out using the J774 C3C mutant clone, which does not release superoxide even when stimulated with phorbol 13-myristate 12-acetate or the mitochondrial complex III inhibitor antimycin A (20). Treatment of these cells with DETA-NO (0.5 mM) led to a time-dependent persistent inhibition of respiration, which was similar in both kinetics and extent to that obtained with parental J774 cells (Fig. 2B).
Figure 2.
Role of ONOO− and superoxide in the irreversible inhibition of respiration induced by DETA-NO. (A) J774 cells were treated with DETA-NO (0.5 mM) alone (squares) or in combination with the ONOO− scavenger methionine (20 mM, circles) or the superoxide dismutase mimetic MnTBAP (0.3 mM, triangles), and oxygen consumption was measured before (open symbols) or after (filled symbols) addition of Hb (8 μM, n = 4). (B) Cells of the J774 mutant C3C clone were treated with DETA-NO (0.5 mM) and oxygen consumption was measured before (open symbols) or after (filled symbols) addition of Hb (8 μM). The rate of oxygen consumption in control C3C cells was 11.8 ± 1.41 μM min−1 ( n = 3).
The site of inhibition of NO was then studied by analyzing its effects on individual steps of the metabolic pathways involved in oxidative phosphorylation. Exposure of control cells for 2 min to the protonophore FCCP (1 μM), which uncouples the flow of electrons from the synthesis of ATP, led to an increase in oxygen consumption of 59.4 ± 4.3% (n = 5). In contrast, in cells exposed to DETA-NO (0.5 mM) for 6 h FCCP did not have any effect, indicating that the target of the persistent action of NO was located upstream of ATP synthesis, transport, or consumption.
In cells resuspended in glucose-free medium, in which respiration was maintained by the presence of pyruvate (20 mM), neither the degree nor the time of onset of persistent inhibition by DETA-NO (0.5 mM) was affected, showing that impairment of glycolysis did not play a relevant role either (Table 1). Inhibition of the citric acid cycle with 3-nitropropionic acid (0.6 mM), an inhibitor of succinate dehydrogenase (25), in the presence of β-hydroxybutyrate (6 mM), which supplies mitochondria with NADH via its conversion to acetoacetate, did not change the kinetics of onset of persistent inhibition by DETA-NO (0.5 mM), ruling out any major role for inhibition of the citric acid cycle (Table 1). Oxygen consumption carried out by the activity of complex IV was tested in the presence of the complex III inhibitor myxothiazol (0.5 μM), whose action does not lead to superoxide production (26). In this situation, TMPD (80 μM) plus ascorbate (4 mM) were used to supply electrons to complex IV. Oxygen consumption dependent on complex II activity was tested using the respiratory substrate succinate (6 mM) in the presence of the complex I inhibitor rotenone (2 μM). As shown in Table 1, DETA-NO-induced inhibition of respiration via complex IV was always fully reversible by Hb even after prolonged treatment (up to 6 h) with NO. Respiration occurring via complex II (and complexes III and IV) was likewise fully reversible by Hb. Complex I activity in broken cells was then assayed by measuring oxidation of NADH. Treatment with DETA-NO (0.5 mM) for 6 h reduced NADH oxidation from 7.4 ± 1.4 nmol mg−1 min−1 to 2.2 ± 1.0 (P < 0.05, n = 6).
Table 1.
Effects of DETA-NO on oxygen consumption using different inhibitors and substrates
| Treatment | Pathways | Oxygen consumption (% of controls) at time of analysis after administration of DETA-NO
|
|||||
|---|---|---|---|---|---|---|---|
| 20 min | 20 min + Hb | 3 h | 3 h + Hb | 6 h | 6 h + Hb | ||
| None | 19.4 ± 0.81 | 100 ± 1.11 | 13.7 ± 0.73 | 32.5 ± 0.87 | 13.3 ± 0.56 | 13.4 ± 0.76 | |
| Pyruvate | Citric acid cycle/ complexes I-III-IV | 18.0 ± 0.98 | 102 ± 1.12 | 15.4 ± 0.67 | 36.4 ± 0.13 | 14.6 ± 0.06 | 14.3 ± 0.71 |
| 3-Nitropropionic acid + | |||||||
| β-hydroxybutyrate | Complexes I-III-IV | 20.6 ± 1.22 | 101 ± 2.76 | 13.5 ± 0.67 | 36.3 ± 0.25 | 13.1 ± 0.78 | 13.2 ± 0.21 |
| Myxothiazol + | |||||||
| TMPD/ascorbate | Complex IV | 21.3 ± 1.34 | 104 ± 0.89 | 15.3 ± 1.26 | 105 ± 0.97 | 14.1 ± 0.84 | 106 ± 1.02 |
| Rotenone + succinate | Complexes II-III-IV | 19.7 ± 0.67 | 108 ± 1.22 | 13.2 ± 0.27 | 103 ± 1.43 | 13.5 ± 1.65 | 107 ± 1.12 |
Cells were resuspended in the incubation medium and then treated with various blockers of the mitochondrial respiratory complexes in the presence of specific substrates as indicated. DETA-NO (0.5 mM) was then added and oxygen consumption was analyzed before and after addition of Hb (8 μM) at the indicated time point. Values are expressed as percentage of that in cells treated with the same inhibitors and substrates but incubated in the absence of DETA-NO. The concentrations of the various inhibitors of the mitochondrial respiratory chain, as well as of the appropriate substrates, were selected in preliminary experiments as described in Materials and Methods. n = 4.
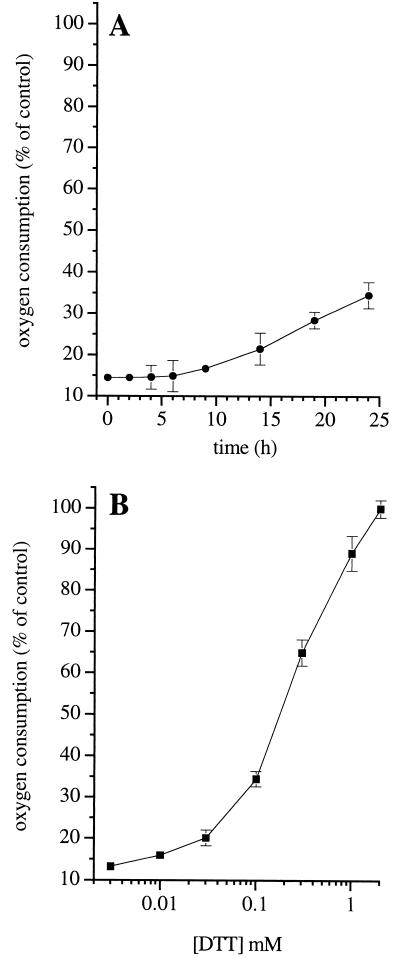
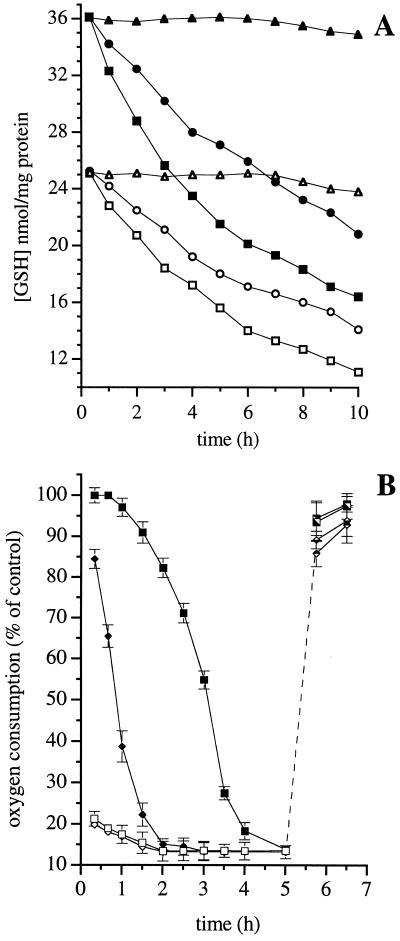
In cells in which respiration was persistently inhibited by the action of NO (DETA-NO, 0.5 mM for 6 h) on complex I, removal of the NO donor did not result in an appreciable increase in oxygen consumption within the first 6 h, and only a small recovery was observed (≤25%) during the next 18 h (Fig. 3A). Addition of the reducing agent DTT rapidly restored the ability of Hb to reverse inhibition of respiration in a concentration-dependent manner (Fig. 3B). Exposure of the cells to DETA-NO (0.1 and 0.5 mM) resulted in a concentration- and time-dependent reduction of intracellular GSH (Fig. 4A). Furthermore, when cells were incubated for 18 h in culture medium with l-buthionine (S,R)-sulfoximine (l-BSO; 0.3 mM), an inhibitor of the γ-glutamylcysteine synthetase (27), the intracellular concentration of GSH fell by 40% (Fig. 4A) and the time required for the onset of persistent inhibition of respiration by NO was reduced (Fig. 4B). Similar results were obtained when cells were preincubated with diamide (0.5 mM; 1 h), an agent known to oxidize intracellular GSH (28) (not shown, n = 2). When DETA-NO was removed by extensive washing and the cells were treated with the membrane-permeant GSH analogue, GSH methyl ester (1 mM), or when cells were exposed to high intensity light, there was a complete recovery of respiration, both in the control and in the l-BSO-treated cells (Fig. 4B). Consistent with these observations, the activity of complex I, measured directly after 45-min treatment with light or DTT (1 mM), returned to levels similar to those observed before DETA-NO treatment (93.2 ± 12.9 and 83.2 ± 16.0% of recovery, respectively; n = 3).
Figure 3.
Spontaneous and DTT-induced recovery of respiratory function in J774 cells after long-term exposure to NO. (A) Cells were treated with DETA-NO (0.5 mM) for 6 h in the incubation medium under sterile conditions, then washed, resuspended in culture medium, and maintained in the cell incubator. Oxygen consumption by cell samples was determined at the indicated time points (n = 3). (B) Cells were treated with DETA-NO (0.5 mM) for 6 h in the incubation medium. Increasing concentrations of DTT were added 5 min before addition of Hb (8 μM), after which oxygen consumption was measured (n = 6).
Figure 4.
Mechanism of inhibition of respiration at complex I by DETA-NO. (A) Changes in GSH concentration were measured in control cells (triangles) or in cells treated with DETA-NO (0.1 or 0.5 mM, circles and squares, respectively) after an 18-h incubation in culture medium in the absence (filled symbols) or presence (open symbols) of l-BSO (0.3 mM). Results shown are representative of two experiments. (B) J774 cells were incubated for 18 h in the culture medium in the absence (squares) or presence of l-BSO (0.3 mM, diamonds) and then treated with DETA-NO (0.5 mM). Oxygen consumption was measured before (open symbols) or after (filled symbols) addition of Hb (8 μM). After 5 h of incubation, DETA-NO was removed by washing and the cells were incubated with GSH–methylester (1 mM; filled symbols, Lower) or exposed to a high intensity light (filled symbols, Upper; n = 3).
DISCUSSION
Our results show that prolonged exposure of J774 cells to NO results in inhibition of cell respiration that is initially reversible by Hb. With time, the effect of NO becomes progressively persistent. The degree of this inhibition is a function of the concentration of NO whereas its onset is a function of the concentration and the time of exposure. Once established, this persistent inhibition of respiration spontaneously recovers only partially over the subsequent 24 h.
Irreversible inhibition of respiration has previously been ascribed to the generation of ONOO− (6–9). Those studies were all performed in activated cells in which NO and superoxide are cogenerated and widespread formation of ONOO− is likely to occur (reviewed in ref. 2). The action of ONOO− is in all cases immediate, irreversible, not confined to a specific target, and results from as yet undefined chemical reactions which may include oxidation, nitration, and nitrosation of cellular targets (29). Our experiments now demonstrate that NO itself can cause persistent inhibition of respiration. Involvement of generalized formation of ONOO− was excluded by the lack of any detectable production of reactive oxygen species during exposure to NO and by the lack of effect of scavengers of ONOO− and superoxide. In addition, a generalized formation of ONOO− would have led to inhibition of other mitochondrial enzymes, which we did not observe. Furthermore, treatment with NO of the C3C clone deficient in superoxide production resulted in persistent inhibition similar to that observed in the parental cells.
Using drugs known to block selectively specific complexes within the mitochondrial electron transport chain and appropriate substrates to maintain cellular respiration, we excluded the possibility that glycolysis or events occurring downstream of ATP synthesis were contributing to the persistent inhibition of respiration. Using a similar approach we also excluded any involvement of complexes II-IV, the citric acid cycle, and aconitase. This latter observation is in agreement with previous results showing that inhibition of aconitase only occurs at very high concentrations of NO (50–100 μM, see refs. 11 and 12). Thus, the persistent effect of NO on respiration seems largely confined to complex I, a fact that was confirmed when the direct activity of this enzyme was specifically investigated.
At a time when inhibition by NO was no longer reversible by Hb, addition of the reducing agent DTT was able to restore cellular respiration fully. This indicated that the inhibition is sensitive to changes in the redox status of the cell. In addition, we found that prolonged exposure of the cells to NO resulted in a concentration- and time-dependent decrease in intracellular GSH, an observation that confirms recently published results (30). Moreover, we observed that depletion of GSH with either l-BSO or diamide accelerated the onset of the persistent inhibition, whereas replenishment of GSH at a time when respiration was persistently inhibited restored the ability of Hb to reverse this inhibition. The fact that light also restores cellular respiration fully suggests that NO is acting through a chemical modification that is photolabile, such as the formation of nitrosothiols or complexes of NO with transition metals. The ability of GSH and other reducing agents such as DTT to reverse the inhibition is consistent with the involvement of a nitrosothiol. Thus, NO seems to inhibit complex I through nitrosylation of critical available thiols. The effect we observe, however, is not likely to be the same as the immediate inhibition of complex I by NO previously shown to occur under conditions of anoxia (31, 32).
Nitrosylation of critical thiols by NO has been described before and is important in the control of the activity of certain enzymes such as glyceraldehyde-3-phosphate dehydrogenase, caspases, and transglutaminase (33–35), or proteins involved in intracellular signal transduction, such as the ryanodine receptor (36). Interestingly, in the case of complex I, the protection of thiols seems to be a process by which GSH continuously counteracts the effects of NO. The mechanism by which GSH does this is not clear at present; however, it may be through the scavenging of nitrosating species or by direct removal of NO from already nitrosylated protein thiols, i.e., via transnitrosylation and formation of S-nitrosoglutathione. This requires further investigation. We do not know whether these processes occur physiologically; however, when increased generation of NO takes place during, for example, defence against invading microorganisms, this may represent an in-built protective mechanism for the mammalian cells against nitrosative stress leading to host cell damage only when intracellular thiols or other redox constituents have decreased below a critical concentration.
The mechanism by which NO nitrosylates thiols in complex I remains to be understood. This could be after interaction with oxygen or by direct reaction of NO with thiols and subsequent one-electron reduction. Alternatively, an increased generation of superoxide within (37) or near complex I may also lead to formation of ONOO− and immediate interaction with thiols. If this latter possibility were correct then a localized generation of superoxide and consequent formation of ONOO− in a specific location restricts its biological action to a defined target. This would differ from the current assumption that superoxide and NO are generally cogenerated over a wide area, leading to formation of ONOO− throughout the cell and to nonspecific effects.
Unlike previous observations, our present findings suggest a stepwise process leading from the physiological inhibition of complex IV to the pathophysiological inhibition of complex I. In this context it is interesting that inhibition of complex I and reduction of intracellular GSH have been described in certain pathological conditions of the central nervous system, including Huntington’s, Alzheimer’s, and Parkinson’s diseases (38).
Acknowledgments
We thank V. Borutaite for her help with the in vitro assay of complex I activity, N. Foxwell for supplying the J774 cells, and A. Higgs for her critical revision of the manuscript. This work was supported in part by grants from the European Economic Community (to E.C.), Biotechnology and Biological Sciences Research Council (to G.C.B.), Lacer (to M.F.), and Glaxo Wellcome (to S.M.).
ABBREVIATIONS
- NO
nitric oxide
- ONOO−
peroxynitrite
- GSH
reduced glutathione
- DETA-NO
(Z)-1-[2-aminoethyl]-N-(2-ammonioethyl)amino]diazen-1-ium 1,2-diolate
- MnTBAP
Mn(III) tetrakis (4-benzoic acid)porphyrin chloride
- FCCP
carbonyl cyanide p-trifluoromethoxy phenylhydrazone
- TMPD
N,N,N′,N′-tetramethyl-p-phenylenediamine
- Hb
oxyhemoglobin
- l-BSO
l-buthionine (S,R)-sulfoximine
References
- 1.Malmström B G. Q Rev Biophys. 1973;6:389–431. doi: 10.1017/s0033583500001578. [DOI] [PubMed] [Google Scholar]
- 2.Bolaños J P, Almeida A, Stewart V, Peuchen S, Land J M, Clark J B, Heales S J R. J Neurochem. 1997;68:2227–2241. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- 3.Palmer R M, Ashton D S, Moncada S. Nature (London) 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 4.Brown G C, Cooper C E. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 5.Cleeter M W J, Cooper J M, Darley-Usmar V M, Moncada S, Schapira A H V. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 6.Granger D L, Lehninger A L. J Cell Biol. 1982;95:527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drapier J-C, Hibbs J B., Jr J Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 8.Geng Y, Hansson G K, Holme E. Circ Res. 1992;71:1268–1276. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- 9.Bolaños J P, Peuchen S, Heales S J R, Land J M, Clark J B. J Neurochem. 1994;63:910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- 10.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro L, Rodriguez M, Radi R. J Biol Chem. 1994;269:29409–29415. [PubMed] [Google Scholar]
- 12.Hausladen A, Fridovich I. J Biol Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- 13.Radi R, Rodriguez M, Castro L, Telleri R. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 14.Bolaños J P, Heales S J R, Land J M, Clark J B. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 15.Lizasoain I, Moro M A, Knowles R G, Darley-Usmar V, Moncada S. Biochem J. 1996;314:877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boje K M, Arora P K. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 17.Merrill J, Ignarro L J, Sherman M P, Melinek J, Lane T E. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 18.Brown G C, Bolaños J P, Heales S J R, Clark J B. Neurosci Lett. 1995;193:201–204. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- 19.Hrabie J A, Klose J R, Wink D A, Keefer L K. J Org Chem. 1993;58:1472–1476. [Google Scholar]
- 20.Kiyotaki C, Peisach J, Bloom B R. J Immunol. 1984;132:857–865. [PubMed] [Google Scholar]
- 21.Feelisch M, Kubitzek D, Werringloer J. In: Methods in Nitric Oxide Research. Feelisch M, Stamler J S, editors. U.K.: Wiley; 1996. pp. 455–478. [Google Scholar]
- 22.Ragan C I, Wilson M T, Darley-Usmar V M, Lowe P N. In: Mitochondria: A Practical Approach. Darley-Usmar V M, Rickwood D, Wilson M T, editors. Oxford: IRL Press; 1987. pp. 79–112. [Google Scholar]
- 23.Baggiolini M, Wymann M P. Trends Biochem Sci. 1990;15:69–72. doi: 10.1016/0968-0004(90)90179-f. [DOI] [PubMed] [Google Scholar]
- 24.Kroemer G, Zamzami M, Susin S A. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 25.Coles C J, Edmonson D E, Singer T P. J Biol Chem. 1979;254:5161–5167. [PubMed] [Google Scholar]
- 26.Turrens J F, Alexandre A, Lehninger A L. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 27.Griffith O W, Meister A. J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 28.Kosower N S, Kosower E M, Wertheim B, Correa W. Biochem Biophys Res Commun. 1969;37:593–598. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- 29.Pryor W A, Squadrito G L. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 30.Bolaños J P, Heales S J R, Peuchen S, Barker J E, Land J M, Clark J B. Free Radical Biol Med. 1996;21:995–1001. doi: 10.1016/s0891-5849(96)00240-7. [DOI] [PubMed] [Google Scholar]
- 31.Stuehr D J, Nathan C F. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stadler J, Billiar T R, Curran R, Stuehr D J, Ochoa J B, Simmons R L. Am J Physiol. 1991;29:C910–C916. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- 33.Mohr S, Stamler J S, Brüne B. J Biol Chem. 1996;271:4209–4214. doi: 10.1074/jbc.271.8.4209. [DOI] [PubMed] [Google Scholar]
- 34.Dimmler S E, Haendeler J, Nehls M, Zeiher A M. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melino G, Bernassola F, Knight R A, Corasaniti M T, Nisticò G, Finazzi-Agrò A. Nature (London) 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Eu J P, Meissner G, Stamler J S. Nature (London) 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 37.Cadenas E, Boveris A, Ragan C I, Stoppani A O M. Arch Biochem Biophys. 1977;180:248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 38.Walker J E. Q Rev Biophys. 1992;25:253–324. doi: 10.1017/s003358350000425x. [DOI] [PubMed] [Google Scholar]