Abstract
The hypothesis that an altered expression of CD11/CD18 on bovine circulating monocytes, polymorphonuclear leukocytes (PMN), or both, contributes to an increased mastitis susceptibility in periparturient cows was tested. Expression of CD18 and CD11a, -b, -c on bovine monocytes and PMN were assessed in 8 Friesian-Holstein cows by flow cytometry from 2 wk before calving to 5 wk after calving. Minor changes in adhesion molecule expression levels were detected throughout the experimental period. Compared with PMN, monocytes exhibited an expression level that was similar for CD18, higher for CD11a and CD11c, but lower for CD11b. Differences in density may reflect the relative importance of these adhesion molecules on both leukocyte types. In this study, the decreased number of milk resident macrophages and PMN observed during the periparturient period could not be attributed to changes of CD11/CD18 levels on circulating leukocytes.
The monocyte-macrophage lineage and the polymorphonuclear leukocytes (PMN) constitute a major non-specific defense of the cow udder against invading microorganisms. Macrophages are the predominant cell type in milk from uninfected bovine mammary glands. However, in the early postpartum period, the absolute number of milk-resident macrophages and PMN is strongly diminished (1,2). This decrease in somatic cell counts is related to increased milk volume and high milk production. In addition, impaired PMN recruitment, which has been directly implicated in a high incidence of Escherichia coli mastitis among periparturient cows (3,4), may potentially contribute to this decrease. Monocytes are influenced by the same hormonal and metabolic factors that elicit PMN immunosuppression. Therefore, we hypothesized that the migratory capacity of monocytes from the blood into the mammary gland during the periparturient period might also be affected. Indeed, recent studies have pointed out that certain functions of blood monocytes in early lactating cows are clearly disturbed (5,6).
The successive steps during the migration of leukocytes to sites of acute inflammation involve the combined action of cytokines, chemoattractants, and multiple families of adhesion molecules. Among the latter, the leukocyte β2-integrins (CD11/CD18) are of critical importance. The CD18 (β) common chain is a 95-kDa glycoprotein non-covalently coupled to a specific CD11 (α) chain (CD11a, -b, or -c) to form active transmembrane molecules in bovine bone marrow derived cells. These molecules mediate firm adhesion to endothelial adhesion molecules, contribute to leukocyte diapedesis, and enable leukocytes to leave the blood circulation in order to function as phagocytes at the site of inflammation (7,8,9).
Currently, there is a paucity of knowledge regarding the possibility of a dysfunction of monocyte migratory capacity leading to increased susceptibility to coliform mastitis in the peripartum period. Expression of β2-integrin on blood monocytes during peripartum has not been examined in cattle, whereas previous studies analysing CD18 expression on PMN report controversial results (10,11,12). Therefore, our main objective was to determine whether β2-integrins on bovine blood monocytes undergo altered expression during the periparturient period. Since differences may occur in the relative abundance of the individual α subunits without a net variation in total CD11/CD18, the expression for CD11a, -b, and -c was measured separately. Additionally, CD11/CD18 expression on PMN was analyzed in parallel and differences between both leukocyte types were evaluated.
Experiments were performed on 8 healthy, high-yielding Friesian-Holstein cows in their 1st to 3rd parity from the Ghent University dairy herd (Biocentrum Agri-vet, Melle, Belgium). The average milk production by the cows was about 9000 kg fat corrected milk/lactation. All cows were clinically healthy, calved normally, and had no symptoms of typical periparturient diseases. The cows were clinically evaluated twice weekly during the experimental period which was delimitated between 2 wk prior to the expected calving date and 5 wk after calving. There was no evidence of mastitis during the whole experimental period and somatic cell counts never exceeded 50 000 mL−1. Cows were fed a balanced daily ration of hay and concentrate according to their requirements for milk production. They had free access to pasture and water. Feed was provided twice daily at 06:30 and 16:00 h. Cows were milked twice daily at 07:00 and 16:30 h. Blood samples were obtained with heparinized vacuum tubes (BD Vacutainer, Plymouth, United Kingdom) by jugular venipuncture between 08:00 and 09:00 h twice weekly during the experimental period.
The β2-integrin α subunits were detected with IL-A99, IL-A15, or IL-A46 mAb directed to bovine CD11a, -b, or -c subunits, respectively (International Livestock Research Institute, Nairobi, Kenya) (7,13). The MF14B4 is a mAb developed to detect the bovine CD18 β subunit (Immunology Unit, Facultés Universitaires Notre Dame de la Paix, Namur, Belgium) (14). The optimal binding concentration of each mAb was determined by flow cytometric titration. All primary antibodies were of mouse origin and were detected with fluorescein isothiocyanate (FITC)-labelled goat anti-mouse immunoglobulin G (IgG) (Sigma Company, Bornem, Belgium), as secondary antibody.
Blood aliquots of 100 μL were incubated in polypropylene tubes (Becton Dickinson, San José, California, USA) at 37°C for 30 min with 50 μL RPMI 1640 (Gibco BRL, Scotland, United Kingdom), 1% albumin fraction V (Merck KG&A, Darmstadt, Germany), 0.2% NaN3 (control), and saturating amounts of anti-bovine mAb recognizing the different β2-integrin subunits, as described above. After incubation, indirect immuno-fluorescence was performed, as previously reported (15).
Flow cytometry was performed on a Coulter EPICS 751 flow cytometer (Coulter Electronics Ltd., Luton, United Kingdom). Fluorescence intensity was standardized for each run using Flow-Check Fluorospheres (Coulter, Miami, Florida, USA). The fluidic system of the flow cytometer was rinsed (Isoton II; Coulter, Krefeld, Germany) between samples. Analysis was done on 10 000 cells using the 488 nm laser line of the argon-ion laser beam. Monocytes and PMN were gated and characterized by forward and side light scattering characteristics. In addition, the monocyte population was further identified with anti-ovine CD14 mAb (Serotec, Oxford, United Kingdom), which cross-reacts with bovine monocytes. The threshold was set based on the upper limit (98%) of background fluorescence. The mean fluorescence intensity (MFI) of positively FITC-labeled monocytes and PMN were measured from this level.
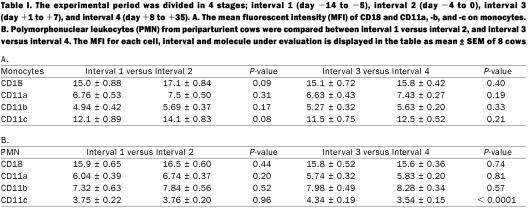
The experimental 7-week peripartum period was subdivided in 4 discrete stages. Interval 1 (day −15 to −5) was compared with interval 2 (day −4 to 0), and interval 3 (day +1 to +7) was compared with interval 4 (day +8 to +35). The MFI of CD18, CD11a, CD11b, and CD11c on monocytes and PMN were analyzed using the mixed model with cow as random effect and time as categorical fixed effect (Statistical analysis software (SAS), version 8; SAS, Cary, North Carolina, USA). Differences between monocytes and PMN were also analyzed by the mixed model with cow as the random effect and time as categorical fixed effect. Tests were performed to investigate whether MFI differed between cell types overall. The 2 comparisons, interval 1 versus interval 2, and interval 3 versus interval 4, were each tested at a significance level α = 0.025 (Bonferroni's multiple comparisons adjustment). Data are displayed as mean ± SEM of 8 cows.
To our knowledge, the present study constitutes the first report of β2-integrin expression on circulating bovine monocytes during peripartum. The population gated as monocytes by size and granularity was CD14 positive for circa 80% of the cells. The results show no significant differences in MFI of CD18 on monocytes, nor of the different CD11 subunits (Table I). This observation indicates that the decrease in milk resident macrophages during peripartum is presumably not related to variations of surface β2-integrins on blood monocytes. Within the same samples, PMN β2-integrin expression was also measured. Although a moderate increase in CD18 expression was observed during interval 2 (near calving; Table I), CD18 on PMN did not undergo significant changes during peripartum, as previously described (12). However, the group of Kehrli found a peak of CD18 expression levels at calving (10,11). The apparent discrepancy in significance might be caused by the higher blood sampling frequency during the day of calving in studies by Kehrli. Alternatively, a more pronounced PMN activation status at birth might have contributed to the dissimilarity of the results. No significant differences in MFI could be found for the CD11 subunits on the PMN population except for a higher CD11c density observed during the 3rd interval (day +1 to +7) as compared to the 4th interval (day +8 to +35) (4.34 ± 0.19 versus 3.54 ± 0.15; P < 0.0001). Since CD18 and CD11 are considered to form integrin heterodimers by covalent linkage, one could expect that the net variation in total CD11 would be accompanied by a proportional change of CD18 density. However, it is possible that the relatively small change of CD11c MFI on PMN did not cause any detectable effect on PMN CD18 levels.
Table I.
Although expression of CD11/CD18 on monocytes and PMN is essential for firm adhesion to the endothelium and for further transmigration (8,9,16), it is important to bear in mind that the expression, per se, of these molecules does not solely determine the functional capacity of leukocytes to adhere and migrate in vivo. Of equal importance for this hyperadherence is the conformational activation of the integrin complex (17). It is possible that monocytes and PMN are activated at the end of pregnancy, since this period has been associated with several changes in peripheral blood leukocyte numbers and functions (3,4,5,6). Alternatively, altered expression of other adhesion molecules involved in monocyte, PMN migration, or both, which were not evaluated in the present study, such as L-selectin, β1-integrin, or the vascular counterreceptors on endothelial cells (16), could potentially contribute to decreased monocyte and PMN recruitment during peripartum. Indeed, decreased L-selectin expression levels have been reported in bovine PMN during the periparturient period (11,18).
Compared to PMN, monocytes exhibit similar expression levels for CD18. In contrast, relative differences for the individual CD11 subunits between the 2 cell types were observed. The MFI of CD11c and, albeit less pronounced, also of CD11a were higher on monocytes than on PMN, for example, 12.4 ± 0.32 versus 3.74 ± 0.08 (P < 0.0001) and 7.19 ± 0.19 versus 5.97 ± 0.14 (P = 0.0004), respectively. The results are consistent with those obtained previously in cattle (7). Conversely, the CD11b subunit had a lower expression on monocytes than on PMN (5.49 ± 0.14 versus 7.99 ± 0.20; P < 0.0001). The present study confirms earlier observations regarding the relative expression level of CD11b on monocytes and PMN in humans and cattle (7,19,20). All antigens were present on both monocytes and PMN, suggesting a common functional relevance of these molecules in circulating myeloid cells. Differences of expression intensity could however, be detected between both leukocyte populations. These variations probably reflect the relative importance of each β2-integrin subunit on mature bovine monocytes and PMN.
Overall, only minor variations of β2-integrin expression on bovine monocytes and PMN were detected during the periparturient period. This would suggest that alterations in CD11/CD18 adhesion molecule expression in the peripartum on circulating monocytes and PMN do not substantially contribute to the decrease in milk-resident macrophages or PMN, and, subsequently, to the increased susceptibility to mastitis in this period.
Footnotes
Acknowledgments
The authors thank the European Community for the Marie Curie Research Training Grant (no FAIR-BM-974214) and the Fund for Scientific Research (grant no. 3G008699) for their generous support.
Address all correspondence and reprint requests to Dr. C. Burvenich; telephone: + 32 9 2647321; fax: + 32 9 2647499; e-mail: christian.burvenich@rug.ac.be
Received September 3, 2002. Accepted December 20, 2002.
References
- 1.Jensen DL, Eberhart RJ. Total and differential cell counts in secretions of the nonlactating bovine mammary gland. Am J Vet Res 1981;42:743–747. [PubMed]
- 2.Mcdonald JS, Anderson AJ. Total and differential somatic cell counts in secretions from noninfected bovine mammary gland: the periparturient period. Am J Vet Res 1981;42:1366–1368. [PubMed]
- 3.Burvenich C, Paape MJ, Hill AW, et al. Role of the neutrophil leucocyte in the local and systemic reactions during experimentally induced E. coli mastitis in cows immediately after calving. Vet Quart 1994;16:45–50. [DOI] [PubMed]
- 4.Vandeputte-Van Messom G, Burvenich C, Roets E, et al. Classification of newly calved cows into moderate and severe responders to experimentally induced Escherichia coli mastitis. J Dairy Res 1993;60:19–29. [DOI] [PubMed]
- 5.Preisler MT, Weber PSD, Tempelman RJ, et al. Glucocorticoid receptor expression profiles in mononuclear leukocytes of periparturient Holstein cows. J Dairy Sci 2000;83:38–47. [DOI] [PubMed]
- 6.Sordillo LM, Pighetti GM, Davis MR. Enhanced production of bovine tumor necrosis factor-α during the periparturient period. Vet Immunol Immunopathol 1995;49:263–270. [DOI] [PubMed]
- 7.Cox E, Mast J, Mac Hugh N, et al. Expression of β2-integrins on blood leukocytes of cows with or without bovine leukocyte adhesion deficiency. Vet Immunol Immunopathol 1997;58:249–263. [DOI] [PubMed]
- 8.Diez-Fraile A, Meyer E, Burvenich C. Regulation of adhesion molecules on circulating neutrophils during coliform mastitis and their possible immunomodulation with drugs. Vet Immunol Immunopathol 2002;86:1–10. [DOI] [PubMed]
- 9.González-Amaro R, Sánchez-Madrid F. Cell adhesion molecules: selectins and integrins. Crit Rev Immunol 1999;19:389–429. [PubMed]
- 10.Kimura K, Goff JP, Kehrli ME Jr. Effects of the presence of the mammary gland on expression of neutrophil adhesion molecules and myeloperoxidase activity in periparturient cows. J Dairy Sci 1999;82:2385–2392. [DOI] [PubMed]
- 11.Lee EK, Kehrli ME Jr. Expression of adhesion molecules on neutrophils of periparturient cows and neonatal calves. Am J Vet Res 1998;59:37–43. [PubMed]
- 12.Meglia GE, Johannisson A, Petersson L, Persson-Waller K. Changes in some blood micronutrients, leukocytes and neutrophil expression of adhesion molecules in periparturient dairy cows. Acta Vet Scand 2001;42:139–150. [DOI] [PMC free article] [PubMed]
- 13.Splitter G, Morrison WI. Individual antigens of cattle. Antigens expressed predominantly on monocytes and granulocytes: identification of bovine CD11b and CD11c. Vet Immunol Immunopathol 1991;27:87–90. [DOI] [PubMed]
- 14.Lettesson JJ, Delcommenne M. Production of a monoclonal antibody to the light chain of the bovine β2-integrin family (BoCD18). Vet Immunol Immunopathol 1993;39:103–108. [DOI] [PubMed]
- 15.Roets E, Burvenich C, Diez-Fraile A, Noordhuizen-Stassen EN. Evaluation of the role of endotoxin and cortisol on modulation of CD18 adhesion receptors in cows with mastitis caused by Escherichia coli. Am J Vet Res 1999;60:534–540. [PubMed]
- 16.Beekhuizen H, Van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol 1993;54:363–378. [PubMed]
- 17.Hughes BJ, Hollers JC, Crockett-Torabi E, Smith CW. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J Clin Invest 1992;90:1687–1696. [DOI] [PMC free article] [PubMed]
- 18.Monfardini E, Paape MJ, Wang Y, et al. Evaluation of L-selectin expression and assessment of protein tyrosine phosphorylation in bovine polymorphonuclear neutrophil leukocytes around parturition. Vet Res 2002;33:271–281. [DOI] [PubMed]
- 19.Hartnell A, Moqbel R, Walsh GM, et al. Fcγ and CD11/CD18 receptor expression on normal density and low density human eosinophils. Immunol 1990;69:264–270. [PMC free article] [PubMed]
- 20.Lundahl J, Halldén G, Hed J. Differences in intracellular pool and receptor-dependent mobilization of the adhesion-promoting glycoprotein Mac-1 between eosionophils and neutrophils. J Leukoc Biol 1993;53:336–341. [DOI] [PubMed]