Abstract
Effects of amikacin, gentamicin, kanamycin, and streptomycin on renal tissue superoxide dismutase, glutathione peroxidase, glutathione and malondialdehyde, serum creatinine, potassium, sodium, total protein, glucose, uric acid, and total bilirubin levels were investigated. All aminoglycoside antibiotics decreased renal tissue glutathione levels.
Aminoglycoside antibiotics are widely used as antibacterial agents for the treatment of severe aerobic gram (−) infections. However, aminoglycoside induced nephrotoxicity and ototoxicity are limiting factors for their clinical use and oxygen free radicals have been involved in aminoglycoside induced nephrotoxicity and ototoxicity (1,2).
Superoxide dismutase (SOD) and glutathione peroxidase (GPX) are antioxidant enzymes. They protect cells against oxygen free radical damage. Glutathione (GSH) has a very important role in protecting against oxygen free radical damage by providing reducing equivalents for several enzymes; GSH is also a scavenger of hydroxyl radicals and singlet oxygen (3). Free oxygen radicals can induce lipid peroxidation in cells, and malondialdehyde (MAD) is formed during oxidative degeneration and accepted an indicator of lipid peroxidation (4).
In the present study, the effects of aminoglycoside antibiotics in mice; on renal SOD; GPX; GSH; and MDA, determinants of possible renal tissue damage, were evaluated. Serum creatinine was measured as an indicator of nephrotoxicity, uric acid, and total bilirubin were measured as extracellular antioxidants. Other serum biochemical parameters total protein, glucose, potassium, and sodium were also measured.
Fifty male Balb/C mice (age 3.5 to 4 mo; weighing 27 to 34 g) were used throughout the experiment (Animal Research Institute, Konya, Turkey). The mice were fed a standard pelleted diet and tap water ad libitum as drinking water. The mice were divided into 5 groups of 10 mice. The mice were injected subcutaneously with isotonic saline solution (group 1), amikacin sulfate (100 mg/kg/d body weight (BW), Amikozit Amp; Eczacibasi, Istanbul, Turkey) (group 2), gentamicin sulfate (100 mg/kg/d BW, Gentavet Enj; Veta, Istanbul, Turkey) (group 3), kanamycin sulfate (100 mg/kg/d BW, Kanovet Enj; Veta, Istanbul, Turkey) (group 4), and streptomycin sulfate (300 mg/kg/d BW, Streptomycine Sulfate IE; Ulagay, Istanbul, Turkey) (group 5), for 4 d.
At the end of the experiment, blood samples were taken from the heart by cardiac puncture under light ether anesthesia. The mice were immediately killed by cervical dislocation after bleeding each mouse in the experimental groups, as well as in the control group.
Serum creatinine, total protein, total bilirubin, glucose, and uric acid concentrations were measured with an auto-analyzer (Technicon RA-TX; Bayer, Leverkussen, Germany) and potassium (K) and sodium (Na) levels with flame photometer (Jenway PFP7; Spectronic Analytical Instruments, Garforth, West Yorkshire, United Kingdom).
The right kidneys were immediately removed and homogenized. Renal SOD (Randox Laboratories Ltd., Crumlin, Antrim, United Kingdom) and GPX (Randox Laboratories Ltd.) activities and total protein (Sigma Chemical Company, St. Louis, Missouri, USA) levels were determined in the supernatants spectrophotometrically using commercially available kits. The SOD and GPX activities were expressed as U mg−1 tissue protein. Renal GSH and MDA levels in the supernatants were measured by previously reported methods (5,6), and were expressed as μmol/mg and nmol/mg tissue protein, respectively.
All data are expressed as mean ± SE. Tukey multiple range test (SPSS for windows, version 6.0; SAS Institute Inc., Cary, North Carolina, USA) was used to determine statistical differences between control and experimental groups.
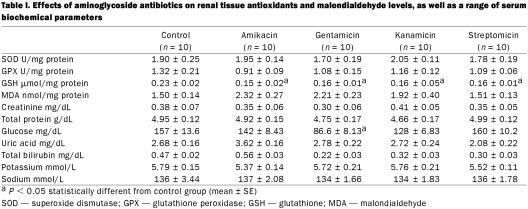
Effects of aminoglycoside antibiotics on renal tissue antioxidants, malondialdehyde level, and some serum biochemical parameters are presented in Table I. None of the aminoglycoside antibiotics had an effect on the renal tissue SOD, GPX, and MDA levels (P > 0.05). Renal GSH levels decreased in all experiment groups (P < 0.05). Statistically significant change in glucose levels was within the reference range.
Table I.
Many studies have demonstrated the ability of aminoglycoside antibiotics to facilitate the generation of oxygen free species both in vivo and in vitro, and this process plays an important role in aminoglycoside induced nephrotoxicity and ototoxicity (1,2). Oxygen free radicals are important mediators of tissue injury, and these radicals may change antioxidant and MDA levels within the tissues.
In the present study, aminoglycoside antibiotics did not change extracellular antioxidants, total bilirubin, and uric acid levels. Antibiotics caused decreases in GPX activity and increases in MDA levels, but was not statistically significant (P > 0.05). Many studies have been conducted on the relationship between gentamicin and antioxidant enzymes; however, there are a few involving other aminoglycosides. Previously, studies reported that streptomycin and gentamicin caused increases in renal MDA levels, and gentamicin depressed GPX activity in kidney and heart (7,8). It was stated that oxygen free radicals involved in aminoglycoside nephrotoxicity and singlet oxygen might directly inactivate GPX activities (9).
Decreased renal GSH level was observed in all groups in the present experiment. GSH defends the cell against the toxic effects of hydroxyl radicals and singlet oxygen. In general, when GSH levels decrease, glutathione–related enzymes decrease as well. Previous studies stated that gentamicin caused a decrease in renal glutathione levels in rats (10).
In summary, the results of the study demonstrate that aminoglycoside-induced nephrotoxicity is related to lipid peroxidation, when aminoglycoside antibiotics must be used at high dosage or for a long time, tissue injury may occur in the kidney.
Footnotes
Acknowledgment
The authors thank SUBAPK (2001/143, Selcuk University, Konya, Turkey) for their generous support.
Address correspondence and reprint requests to Dr. Enver Yazar; telephone: + 90 32 22 410 041; fax: + 90 33 22 410 063; e-mail: eyazar@selcuk.edu.tr
Received May 30, 2002. Accepted January 9, 2002.
References
- 1.Conlon BJ, Aran JM, Erre JP, Bmith DW. Attenuation of aminoglycoside induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res 1999;128:40–44. [DOI] [PubMed]
- 2.Pedraza-Chaveri J, Maldonado PD, Medina-Campos O, et al. Garlic ameliorates gentamicin nephrotoxicity: relation antioxidant enzymes. Free Radic Biol Med 2000;29:602–611. [DOI] [PubMed]
- 3.Diplock AT. Antioxidants and free radicals scavengers. In: Rice-Evans CA, Burdon RH, eds. Free radical damage and its control, Elsevier Science, Amsterdam, Netherlands, 1994.
- 4.Neilsen F, Mikkelsen BB, Neilsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chemist 1997;47:1209–1214. [PubMed]
- 5.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–77. [DOI] [PubMed]
- 6.M hara M, Uch yama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978;86:271–278. [DOI] [PubMed]
- 7.Vijayalekshmy KS, Menon V, Leelamma S. Role of antibiotics in lipid peroxidation. Ind J Biochem Biophys 1992;29:371–374. [PubMed]
- 8.Ozturk HS, Kavutcu M, Kaçmaz M, Canbolat O, Durak I. The effects of gentamicin on the activities of glutathione peroxidase and superoxide dismutase enzymes and malondialdehyde levels in heart tissues of guinea pigs. Cur Med Res Opin 1997;14:47–52. [DOI] [PubMed]
- 9.Turrens JF. The potential of antioxidant enzymes as pharmacological agents in vivo. Xenobiotica 1991;21:1033–1040. [DOI] [PubMed]
- 10.Ramsammy LS, Ling KY, Josepovitz C, Levine R, Kaloyanides GL. Effect of gentamicin on lipid peroxidation in rat renal cortex. Biochem Pharmacol 1985;34:3895–3900. [DOI] [PubMed]