Abstract
The additive genetic heritability of bipolar EEG coherence in a sample of 305 non-twin sibships comprising 690 individuals (age range 7–65) was estimated. Heritabilities were examined in 6 frequency bands for each of 15 coherence pairs, both inter-hemispheric and intrahemispheric. The heritabilities of the bipolar EEG coherence ranged from 0.22 to 0.63 in 79 of the 90 phenotypes which had coherences high enough to provide meaningful values for the estimation of heritability. Heritabilities were greatest in the low and high alpha frequency bands, while theta and beta bands had comparable heritabilities. Coherences themselves were greatest in the low and high alpha frequency bands, while theta coherences were somewhat larger than beta. Higher heritability values were not associated with higher coherences. The examination of bivariate genetic correlations suggests that there is a difference between theta and alpha bands in genetic control of interhemispheric coherence.
Keywords: coherence, bipolar, heritability, EEG
1 Introduction
Coherence is a linear synchronization measure between two signals recorded at different locations. It is a statistical measure of the average agreement in phase difference, weighted by amplitude, between two signals measured over time, and is frequency specific. Coherence values range from 0 to 1, with 1 meaning perfect agreement in phase difference and 0 meaning completely random phase differences. The authoritative introduction to the measurement of the coherence of scalp recorded EEG signals is to be found in Nunez et al. (1997); see also Srinivasan et al. (1998); Nunez et al. (1999); Grieve et al. (2003). We now briefly summarize the results of that paper. In the case of scalp recorded EEG signals, the coherence value represents, to varying degrees, the effects of synchronization of activity between sources located near the two scalp locations and the effects of electromagnetic propagation from common sources affecting the two locations, as well as the effect of the reference electrode used in the recording. This is because the signal recorded at a single electrode is a function not only of the neural activity directly beneath it, but also of neural activity at more distant locations propagated by electromagnetic fields as mediated by the intervening tissue and other matter. This latter phenomenon is called volume conduction. Volume conduction effects can contribute strongly to coherence measures of “raw” signals, inflating their values. A number of different data transformations (data preprocessing methods) if performed before the coherence calculation can reduce volume conduction and reference electrode effects. The Laplacian (or current source density) provides a reference independent transformation which eliminates most volume conduction effects. Signals transformed to bipolar form by subtractions between adjacent electrodes, which is also a reference independent transformation, fall between Laplacian and “raw” data, eliminating volume conduction effects from about twice the distance between the two electrodes forming the bipolar pair. However, bipolar coherence values are affected by the orientations of the bipolar pairs because of the intrinsic geometry of the electrode configuration and because of the anisotropy of volume conduction effects.
Since a coherence value is a statistical, rather than directly measured quantity, it has confidence intervals. The confidence intervals for coherence values are not only a function of the number of epochs used to calculate coherence but also of the coherence value itself, with smaller coherence values having wider confidence intervals as a proportion of their size (Bendat and Piersol, 1971; Wang and Tang, 2002; Wang et al., 2004). Any function of coherence values will have statistical characteristics dependent upon the statistical characteristics of the coherence values themselves. This means that the confidence intervals of heritability values must be wider for low coherence data as opposed to high coherence data, because other relevant factors, the number of epochs used in the coherence calculations plus factors involved in the genetic calculations, are equal in any given experiment. Since these other factors may vary between different experiments, careful examination of the research methodology using coherence values as inputs must be performed.
We are not aware of any studies establishing normal ranges for coherence in adult subjects using bipolar data. We expect coherence values from bipolar data to be smaller than those for monopolar data because of the inflation of monopolar coherence values by volume conduction effects as mentioned previously. A number of studies have reported monopolar coherence values for resting EEG in normal children and adults using sites with varying distances between electrode pairs (Barry et al., 2004, 2005; Srinivasan, 1999; Thatcher et al., 1986; Winterer et al., 2003). While these studies are in general agreement in finding that monopolar coherence decreases with increasing distance between pairs, one study (Srinivasan, 1999) finds alpha band coherence values in adult subjects increasing with distance in intrahemispheric electrode pairs greater than 15 cm apart. Significantly larger coherence values in the eyes closed resting state were observed in adults as compared to children in the alpha band, particularly in intrahemispheric pairs, by Srinivasan (1999), which is consistent with the increase in alpha coherence found from 8 to 12 years of age by Barry et al. (2004) in a non-longitudinal study. Since in the maturational process myelination of long connective fibres continues into adulthood (Yakovlev and Lecours, 1967; Holland et al., 1986; Courchesne, 1990), the physiological basis of EEG coherence may change from childhood to adulthood.
We are not aware of any heritability studies that have been undertaken with coherence values from bipolar electrode derivations. We review here the studies of heritability of coherence and other synchronization measures taken from monopolar data. Intrahemispheric theta coherence in young twins was investigated by van Baal et al. (1998), who found heritabilities ranging from .3 to .71 among different pairs of scalp locations. Generally speaking, they found that the heritabilities for longer distances were greater than those for shorter distances. A study with a similar methodology (van Baal et al., 2001) tracked changes in heritability from a longitudinal sample of children tested at 5 and 7 years of age. Heritabilities involving frontal channels tended to decrease slightly while those involving parietal channels tended to increase slightly during this time interval. Intrahemispheric coherence in a wider range of frequency bands in adolescent twins was investigated by van Beijsterveldt et al. (1998), who found heritabilities ranging from .40 to .82 among different pairs of scalp locations in the theta, alpha, and beta bands. No consistent relation between distance or topography and heritability was found. The heritability of synchronization likelihood, a non-linear measure of synchronization between signals, was studied in young twins by Posthuma et al. (2005). The reported heritabilities ranged from .1 to less than .7, with the highest values in the alpha bands. Since values of synchronization likelihood reported at each electrode were an average of the pairwise comparisons with each of the other electrodes, topographic specificity is diminished in contrast to values for distinct pairwise calculations.
We designed a heritablity study which differed in a number of ways from the earlier studies. Our study used coherence values calculated between bipolar derivations rather than monopolar derivations. This was to limit possible volume conduction effects on coherence values. We investigated the heritablity of interhemispheric coherences in addition to the intrahemispheric coherences used in earlier studies. This aspect we regard as espcially significant in view of the arrangement of commissural and associational pathways in the human brain. Our study covered a population of sib-pairs heterogeneous in age, rather than MZ/DZ twins with a restricted age range. In our population, about 10% were less than seventeen years old, and more than 17% greater than 45 years old. In light of the fact that coherence in children and adults may involve different neurophysiological factors and may vary in a non-linear manner with respect to age (Thatcher et al., 1986), our heritability estimates are not stricly comparable to those from studies whose populations are more homogeneous in age.
2 Methods
2.1 Participants
Subjects were participants in an IRPG (Investigator-Initiated Interactive Research Project) collaborative study examining novel phenotypes for genetic analysis in alcoholism at the following sites: SUNY Downstate Medical Center, Indiana University School of Medicine, Washington University School of Medicine, and Howard University. Probands with at least one sibling over 13 years of age were recruited from psychiatric inpatient or outpatient treatment programs for alcohol and/or chemical dependency. Detoxification was complete before the potential proband was approached. Additional family members between the ages of 7 to 70 years were also recruited for the study. The study also included control families which were randomly ascertained to be representative of the general population; they were recruited from HMOs, drivers license records, and dental clinics. Subjects for the present analysis were all sibling pairs selected from the pool of families described above with two or more siblings. The final dataset contained 690 subjects from 305 families (248 families had 2 sibs, 43 had 3 sibs, 8 had 4 sibs, 6 had 5 or greater number of sibs). The mean age range among sibs was 5.0 years with a standard deviation of 4.0 years. The institutional review board at each site approved the research procedures and written consent was obtained from each individual prior to participation. Subjects were excluded from the neurophysiological assessment if they manifested uncorrected sensory deficits, hepatic encephalopathy or cirrhosis of the liver, significant head injury or seizures, acute or chronic illness, were on medication that affects or influences brain functioning, had a positive breath analyzer test for alcohol use, had undergone neurosurgery, tested positive for HIV, or used psychoactive substances in the any of the 5 days preceding the assessment.
2.2 Data Recording
All four sites used the same experimental procedures and EEG acquisition hardware and software. Each subject wore a fitted electrode cap (Electro-Cap International Inc.; Eaton, OH) using the 19-channel montage as specified according to the 10–20 International system [FP1, FP2, F7, F3, FZ, F4, F8, T7, C3, CZ, C4, T8, P7, P3, PZ, P4, P8, O1, O2]. The nose served as reference and the ground electrode was placed on the forehead. Electrode impedences were always maintained below 5 KOhm. The electrooculogram (EOG) was recorded from electrodes placed supraorbitally at the outer canthus of the eye. Vertical and horizontal eye movements were monitored to perform ocular artifact correction. EEG was recorded with the subjects seated comfortably in a dimly lit sound-attenuated temperature-regulated booth (Industrial Acoustics Company; Bronx, NY). They were instructed to keep their eyes closed and remain relaxed. Subjects were also cautioned not to fall asleep. Electrical activity was amplified 10,000 times by Sensorium EPA-2 Electrophysiology amplifiers (Charlotte, VT), with a bandpass between 0.02 Hz to 50 Hz and recorded using the Neuroscan software system (Compumedics Limited; El Paso, TX) running on i86 PCs. The sampling rate was 256 Hz and the activity was recorded for 4.25 minutes.
2.3 Data Reduction
EEG analysis was performed at SUNY Downstate Medical Center. A continuous interval comprising 256 seconds of EEG data was used for analysis. Offline raw data were subjected to wavelet filtering and reconstruction to eliminate high and low frequencies (Bruce and Gao, 1994; Strang and Nguyen, 1996). The s12 wavelet was used to perform a 6 level analysis, and the output signal was reconstructed using levels d6 through d3. This procedure is roughly equivalent to applying a band pass filter with a range of 2–64 Hz to the data. Subsequently, eye movements were removed by use of a frequency domain method developed by Gasser (Gasser et al., 1985, 1986). This method subtracts a portion of observed ocular activity from observed EEG to obtain the true EEG, based on the difference between the cross-spectral values of trials with high ocular activity and those with low ocular activity. Visual inspection of corrected data confirmed satisfactory artifact removal characteristics.
In order to improve the localization of our signals, consideration was given to both the Laplacian and bipolar data transformations. In our case, given that much of the data was recorded with the International 10–20 system of electrode placement, which offers only 19 scalp electrodes, the use of the Laplacian was ruled out. To reduce volume conduction and reference specific effects, a bipolar transformation was used. We accepted the fact that bipolar transformations might reduce coherences when the coherent activity extended across areas which were spanned by the electrodes included in the bipolar pair (Essl and Rappelsberger, 1998). The data were software transformed into 38 bipolar derivations formed by the subtraction of adjacent electrodes in both lateral and sagittal orientations (Figure 1), and analyzed in 254 overlapping 2-second epochs by use of a Fourier transform and windowed using a Hamming function to improve the accuracy of the spectral results (Hamming, 1983). (We will use the following terminology: a derivation is a single signal obtained either as the “raw” data from a single electrode or by subtracting the signals at adjacent electrodes to obtain a bipolar derivation. A coherence pair is a pair of derivations whose coherence is estimated, and called bipolar or monopolar depending on the kind of derivation used in their estimate. A bipolar coherence pair is called sagittal or lateral depending on the orientation of the two derivations, which is the same in any pair.) The disadvantage of bipolar derivations for coherence estimates is that bipolar coherence pairs are more sparsely distributed on the scalp than monopolar pairs although this is partially compensated for by the use of both sagittally and laterally oriented pairs for interhemispheric coherence. We chose both interhemispheric and intrahemispheric pairs for examination, and for the interhemispheric pairs, pairs in both sagittal and lateral orientation, forming three cases for consideration. Only symmetrically placed derivations were used for the interhemispheric coherence pairs. Further exclusions were made on the basis of the coherence values themselves, as explained in section 3. This resulted in the inclusion of 15 of the possible 703 pairs in the analysis. The location of these pairs is displayed in figure 2.
Fig. 1.
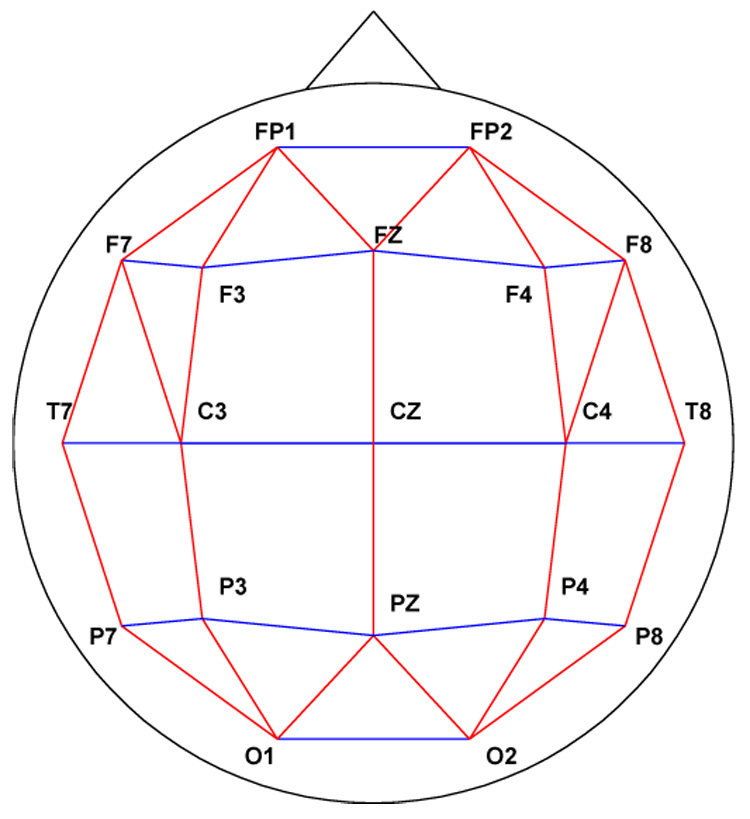
Schematic representation of the 19 electrode montage indicating the 38 channel bipolar lead configuration. Lines in red joining electrode locations indicate sagittal derivations; lines in blue indicate lateral derivations.
Fig. 2.
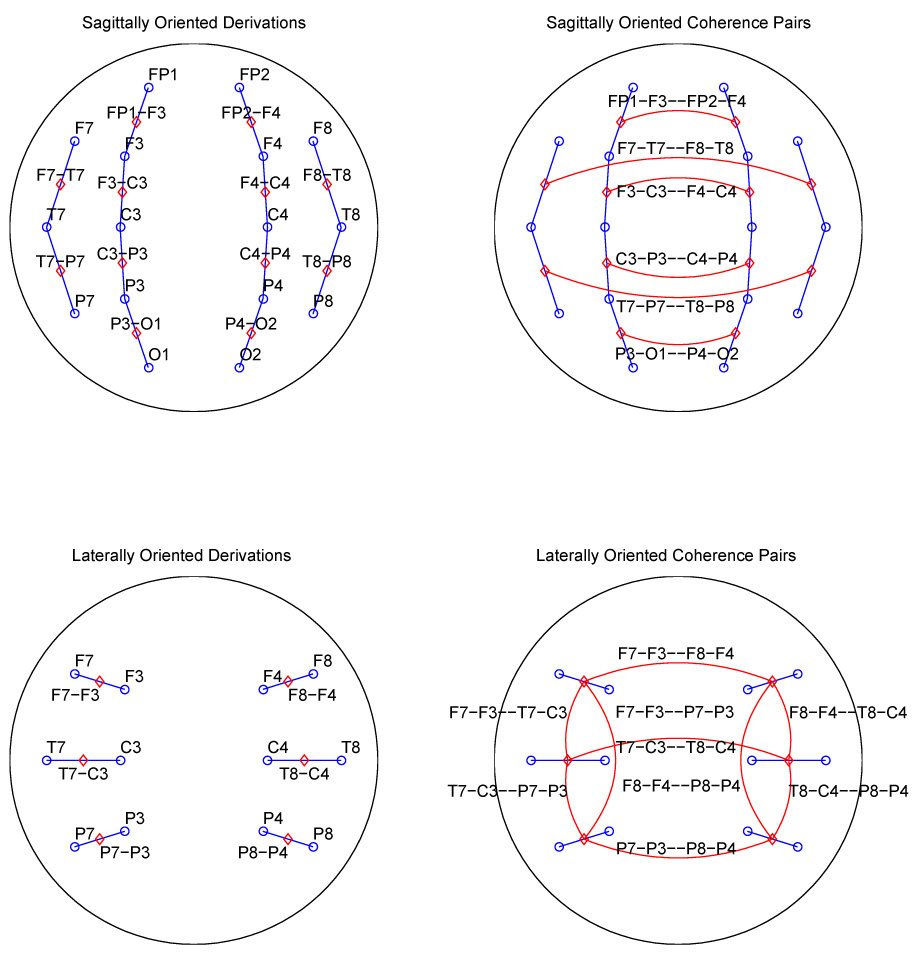
Schematic representation of the 15 bipolar coherence pairs indicated by lines in red; the bipolar derivations are indicated by lines in blue. The red diamonds indicate the midpoints of the bipolar derivations. Note that sagittally oriented pairs provide only interhemispheric coherences, while laterally oriented pairs provide both interhemispheric and intrahemispheric coherences.
The standard coherence calculation,
where
and Xin is the Fourier transform of the signal in channel i at epoch n, and ‘*’ indicates the complex conjugate, was applied to each .5 Hz frequency bin, and the results were aggregated by band and divided by bandwidth. Because of the overlapping time intervals, the number of epochs used for calculating confidence intervals for the coherence estimates was set at 128. This is conservative, since the averaging across frequency bands adds a slight additional element of independence. The bands were set as follows: theta = 3–7.5 Hz, low alpha = 8–9.5 Hz, high alpha = 10–11.5 Hz, low beta = 12–15.5 Hz, mid beta = 16–19.5 Hz, high beta = 20–27.5 Hz.
2.4 Genetic modeling
Additive genetic heritabilities and their standard errors were calculated using the robust variance component model implemented in SOLAR (Sequential Oligogenic Linkage Analysis Routines) (Almasy and Blangero, 1998). The univariate variance component method (Almasy and Blangero, 1998) was used to evaluate the heritabilities of EEG traits, where the phenotypic variance of the EEG were decomposed as the sum of its additive genetic and environmental variance components. The observed phenotypic vector yi = (yi1, …, yini)T from i-th pedigree is assumed to follow a multivariate t-distribution
where ni denotes the pedigree size, xi is a matrix of covariates including the intercept terms, β is a vector containing the covariate effects, Φi is the kinship matrix, is proportional to the variance due to additive genetic factors, is proportional to the variance resulting from individual-specific environmental effects, and Ini is an identity matrix, and tk(µ, Ψ, υ,) denotes the k-variate t-distribution (Lange et al., 1989) with location vector µ, scale matrix Ψ and υ degrees of freedom. The robust approach based on t-distributions is particularly suitable for modeling data with longer than normal tails and may effectively mute the impact of residual outliers (Lange et al., 1989). Age, age-squared, and gender were included in the analyses as covariates and retained only if the associated p-value < 0.05. To test whether this model for age effects was adequate, we analyzed several phenotypes with a local polynomial regression method for age, and found that heritability estimates differed inconsequentially from the reported values. Because our data consist of only sib-pairs, the additive genetic effect may be confounded with early common environment(Falconer and Mackay, 1996). Various studies (Smit et al., 2005; van Baal et al., 1996; van Beijsterveldt et al., 1998) indicate that the EEG power spectral traits and EEG coherence traits are more likely to be influenced by genetic factors and unique environmental factors and less by common environment factors. It is reasonable to assume that this holds also for our data. So throughout the paper, we use the word “heritability” although it may be more proper to use “familial effect”.
The bivariate variance component model (Almasy et al., 1997) was used to estimate the pairwise genetic correlations for selected coherence pairs in each frequency band. The method decomposes the covariation between two phenotypes into the portion due to shared genetic factors and that due to shared environment. Prior to the bivariate analysis, all phenotypes were adjusted for covariate effects determined in the univariate approach. The genetic correlation measures the extent to which two traits are affected by shared genetic effects. A smaller genetic correlation between the EEG measures at two scalp locations implies that the EEG measures are mainly influenced by the genetic factors specific to each location, and/or unique environment or measurement noise. On the other hand, a larger genetic correlation indicates that common genetic factors play a major role in determining the EEG measures.
3 Results
All included coherence pairs were required to have coherences greater than .1 in at least 3 of the frequency bands calculated, as smaller values are too low to provide valid data for heritability calculations following the criterion given by Nunez (Nunez et al., 1997, p. 503). In addition, heritability results are not reported when the mean coherence is less than .1 in individual frequency bands. The heritability results are presented in table 1 and the coherence results in table 2.
Table 1.
Summary of Heritability Results by Band. (‘NA’ indicates that the mean coherence was too low to provide a valid heritability estimate.)
| Channel Pairs | Theta | Low Alpha | High Alpha | Low Beta | Mid Beta | High Beta |
|---|---|---|---|---|---|---|
| Interhemispheric Sagittal | ||||||
| FP1-F3–FP2-F4 | 0.37 | 0.42 | 0.38 | 0.34 | 0.19 | 0.18 |
| F7-T7–F8-T8 | 0.50 | 0.59 | 0.63 | NA | NA | NA |
| F3-C3–F4-C4 | 0.50 | 0.59 | 0.51 | 0.46 | 0.66 | 0.57 |
| T7-P7–T8-P8 | 0.35 | 0.52 | 0.36 | 0.34 | 0.40 | NA |
| C3-P3–C4-P4 | 0.50 | 0.45 | 0.34 | 0.22 | 0.37 | 0.34 |
| P3-O1–P4-O2 | 0.46 | 0.51 | 0.42 | 0.47 | 0.47 | 0.42 |
| Interhemispheric Lateral | ||||||
| F7-F3–F8-F4 | 0.22 | 0.36 | 0.39 | 0.22 | 0.32 | 0.31 |
| T7-C3–T8-C4 | 0.38 | 0.50 | 0.52 | 0.46 | 0.39 | NA |
| P7-P3–P8-P4 | 0.51 | 0.58 | 0.56 | 0.47 | 0.58 | NA |
| Intrahemispheric Lateral | ||||||
| F7-F3–T7-C3 | 0.32 | 0.45 | 0.45 | 0.33 | 0.38 | 0.37 |
| F8-F4–T8-C4 | 0.30 | 0.47 | 0.31 | 0.31 | 0.41 | 0.34 |
| F7-F3–P7-P3 | 0.50 | 0.47 | 0.47 | NA | NA | NA |
| F8-F4–P8-P4 | 0.35 | 0.60 | 0.43 | NA | NA | NA |
| T7-C3–P7-P3 | 0.38 | 0.39 | 0.47 | 0.38 | 0.34 | 0.33 |
| T8-C4–P8-P4 | 0.36 | 0.55 | 0.59 | 0.40 | 0.45 | 0.40 |
Examination of the coherence values finds that coherences are highest in low and high alpha, and, where distances are comparable, lower with longer distances. These results are presented in Table 2.
Table 2.
Summary of Mean Coherences by Band.
| Channel Pairs | Theta | Low Alpha | High Alpha | Low Beta | Mid Beta | High Beta |
|---|---|---|---|---|---|---|
| Interhemispheric Sagittal | ||||||
| FP1-F3–FP2-F4 | 0.28 | 0.29 | 0.28 | 0.19 | 0.15 | 0.13 |
| F7-T7–F8-T8 | 0.11 | 0.18 | 0.22 | 0.08 | 0.07 | 0.05 |
| F3-C3–F4-C4 | 0.28 | 0.34 | 0.35 | 0.20 | 0.17 | 0.16 |
| C3-P3–C4-P4 | 0.28 | 0.38 | 0.48 | 0.26 | 0.21 | 0.16 |
| T7-P7–T8-P8 | 0.17 | 0.27 | 0.37 | 0.15 | 0.11 | 0.08 |
| P3-O1–P4-O2 | 0.26 | 0.32 | 0.38 | 0.24 | 0.22 | 0.19 |
| Interhemispheric Lateral | ||||||
| F7-F3–F8-F4 | 0.21 | 0.26 | 0.26 | 0.15 | 0.13 | 0.11 |
| T7-C3–T8-C4 | 0.24 | 0.28 | 0.32 | 0.13 | 0.12 | 0.09 |
| P7-P3–P8-P4 | 0.17 | 0.24 | 0.34 | 0.16 | 0.13 | 0.10 |
| Intrahemispheric Lateral | ||||||
| F7-F3–T7-C3 | 0.32 | 0.36 | 0.38 | 0.25 | 0.23 | 0.19 |
| F8-F4–T8-C4 | 0.31 | 0.36 | 0.37 | 0.24 | 0.22 | 0.19 |
| F7-F3–P7-P3 | 0.14 | 0.17 | 0.21 | 0.10 | 0.08 | 0.06 |
| F8-F4–P8-P4 | 0.13 | 0.17 | 0.20 | 0.10 | 0.08 | 0.06 |
| T7-C3–P7-P3 | 0.28 | 0.37 | 0.44 | 0.29 | 0.26 | 0.20 |
| T8-C4–P8-P4 | 0.28 | 0.36 | 0.43 | 0.28 | 0.25 | 0.19 |
Examination of the heritability results shows that the highest heritabilities are found in the two alpha bands. Heritabilities involving only the frontal electrodes are the lowest. The sagittally oriented interhemispheric and intrahemispheric cases permit comparisons between shorter and longer distance coherence pairs. We note that in the sagittally oriented interhemispheric case the long distance heritability value is somewhat higher than short distance value in fronto-central high alpha, while in parieto-central theta the short distance heritability value is higher than long distance one. In the intrahemispheric case long distance heritabilities are comparable to and sometimes greater than the short distance ones.
In order to further understand the heritability results, bivariate genetic correlations were calculated for 16 pairs of related coherence pairs in each frequency band. Genetic correlations were high between intrahemispheric coherence pairs, whether between symmetrically placed pairs in opposite hemispheres or between pairs within each hemisphere. The genetic correlations were fairly uniform across frequency bands for each comparison. The pattern of genetic correlation for interhemispheric laterally oriented pairs was similar to that of the intrahemispheric coherence pairs. However, the pattern was different for the sagittally oriented interhemispheric pairs. Meaningful comparisons can be made either between midline and temporal pairs (pairs 1 and 2 of table 4) or between frontal-central and central-parietal pairs (pairs 3 and 4 of table 4). In the relations involving the more temporal channels, either paired with the corresponding more midline channels, or together, the genetic correlation in the theta band was notably less than that in the low alpha band (pairs 1–3 in table 4), and less than in any other theta genetic correlations.
Table 4.
Genetic Correlations between selected Interhemispheric coherence pairs with following keys.
| Pairs | Theta | Low Alpha | High Alpha | Low Beta | Mid Beta | High Beta |
|---|---|---|---|---|---|---|
| Sagittal Interhemispheric | ||||||
| 1 | 0.67 | 0.92 | 0.86 | NA | NA | NA |
| 2 | 0.65 | 0.78 | 0.82 | 0.49 | 0.68 | NA |
| 3 | 0.75 | 0.91 | 0.81 | NA | NA | NA |
| 4 | 0.88 | 0.89 | 0.79 | 0.52 | 0.64 | 0.73 |
| Lateral Interhemispheric | ||||||
| 5 | 0.86 | 0.93 | 0.91 | 1.00 | 0.94 | NA |
| 6 | 0.85 | 0.80 | 0.71 | 0.77 | 0.77 | NA |
| 7 | 0.94 | 0.98 | 0.86 | 0.86 | 0.89 | NA |
| Key to Coherence Pair Correlations | ||||||
| Pairs | Coherence Pair 1 | Coherence Pair 2 | ||||
| Sagittal Interhemispheric | ||||||
| 1 | F7-T7–F8-T8 | F3-C3–F4-C4 | ||||
| 2 | T7-P7–T8-P8 | C3-P3–C4-P4 | ||||
| 3 | F7-T7–F8-T8 | T7-P7–T8-P8 | ||||
| 4 | F3-C3–F4-C4 | C3-P3–C4-P4 | ||||
| Lateral Interhemispheric | ||||||
| 5 | F7-F3–F8-F4 | T7-C3–T8-C4 | ||||
| 6 | F7-F3–F8-F4 | P7-P3–P8-P4 | ||||
| 7 | T7-C3–T8-C4 | P7-P3–P8-P4 | ||||
4 Discussion
The results reported here are consistent with those reported in van Beijsterveldt et al. (1998) with regard to relative heritabilities by frequency band and not inconsistent with regard to relative heritabilities by distance between sites. Our largest heritabilities were not as large as the largest reported by van Beijsterveldt et al. (1998), perhaps as a result of the study of a population far more heterogenous in age. Additionally, the use of sib-pairs as opposed to MZ/DZ twins may also be a contributing factor. A number of facts suggest that the heritable aspect of intrahemispheric coherence in each frequency band is globally determined. First, distance does not seem to be a determinant of intrahemispheric coherence heritability. Second, there are very high genetic correlations between symmetrically situated intrahemispheric coherence pairs in opposite hemispheres. Third, the genetic correlations between intrahemispheric pairs situated in the same hemisphere are generally high. Results for the heritability of interhemispheric coherence have not previously been reported. Not surprisingly, the interhemispheric heritability values have the same range as the intrahemispheric values. Particularly noteworthy is that the sagittally oriented interhemispheric coherence pairs suggest a different pattern of genetic control in the theta band as compared to the alpha bands by use of of bivariate genetic correlations, as shown in Table 4. The high genetic correlations for most alpha comparisons indicate that the heritable components of alpha coherence are predominantly a product of one or more genetic sources operating globally. The heritable components of theta coherence seem to be more manifold, particularly shown by the relatively low genetic correlations between F7-T7–F8-T8 and F3-C3–F4-C4, and between C3-P3–C4-P4 and T7-P7–T8-P8. The low theta correlation between F7-T7–F8-T8 and F3-C3–F4-C4 compared to the high correlation for low alpha is especially striking. The genetic correlation between F7-T7–F8-T8 and T7-P7–T8-P8 is relatively low compared to that between F3-C3–F4-C4 and C3-P3–C4-P4 in theta, compared to their near equality in low alpha and high alpha. These features indicate a pattern of midline vs. peripheral differentiation combined with frontal vs. parietal differentiation in the peripheral region present in theta but not found in alpha.
We must note some differences between our study and those of van Beijsterveldt et al. (1998), and van Baal et al. (1998). Since, as we discussed in the introduction, uncertainties in coherence values propagate into any further analyses in which those values are employed, we have refrained from estimating heritabilities when the mean coherence is less than .1 even with 128 epochs. The use of coherence pairs for which the mean coherence is less than .1 requires that the minimum number of epochs allowed in the coherence calculation be at least 60 (Nunez et al., 1997, p 503); see also Wang and Tang (2002); Wang et al. (2004); Nunez et al. (2001). In the cases where the number of epochs falls below this value and the coherence is less than .1, it is statistically indistinguishable from 0, and thus all values less than .1 are statistically indistinguishable from each other. In the paper by van Beijsterveldt et al. (1998), confining ourselves to the same frequency bands we examined, 22 of the 42 heritability results are based on such low coherence values, primarily in the long distance pairs (van Beijsterveldt et al., 1998, Table I, p. 447). The problem with using low values is not distributional, so the use of the transform y = log(x/(1 − x)) to “normalize” the data in that paper does not ensure that the very low coherence values can be treated in the same manner as the larger values. The effect is apparent in examining the split-half reliability data for the theta and beta bands for coherence pairs with low mean values. In the paper by van Baal et al. (1998) the coherence values are presented only pictorially, so it is difficult to tell what the values for FPn-Pn and Fn-On are. However they seem to be close enough to .1 to regard heritability results for these coherence pairs, as well as FPn-On, as possibly based on coherence values falling below the limit discussed above. This point is reinforced by the low split-half reliability for these pairs. It is also claimed by van Baal et al. (1998) that the coherence results reported are free of volume conduction effects because of non-zero phase differences. However, statistical testing for this is necessary since random sampling from distributions with mean zero phase and even moderate levels of coherence will produce data which has a mean non-zero phase value.
In the case of coherences derived from bipolar data, we can be fairly sure that there is little volume conduction effect, except perhaps for close intrahemispheric pairs. However, the overall similarity in the pattern of heritabilities between the results reported here and those of van Baal et al. (1998) and van Beijsterveldt et al. (1998) seems to indicate that the heritability of long distance coherences is not particularly affected by volume conduction effects in their studies. Given the high genetic correlations between short and long distance coherence pairs in the alpha bands, we similarly conclude that short distance alpha coherence heritability is not particularly affected by volume conduction effects.
Our study confirms that EEG coherence is a heritable trait in humans. It gives stronger support to the hypothesis that the neurophysiological factors involved in the heritability of coherence are related to neural connectivity than previous studies, because of the elimination of the possible effect of volume conduction on long distance coherence values. It is likely that studies using denser electrode arrays, multiple data preprocessing strategies, and more sophisticated analyses of genetic correlations would provide further insight into the neurophysiological factors which contribute to the heritability of EEG coherence.
Table 3.
Genetic Correlations between selected Intrahemispheric coherence pairs with following keys.
| Pairs | Theta | Low Alpha | High Alpha | Low Beta | Mid Beta | High Beta |
|---|---|---|---|---|---|---|
| Lateral Intrahemispheric – between hemispheres | ||||||
| 1 | 1.00 | 0.94 | 0.92 | 1.00 | 1.00 | 1.00 |
| 2 | 0.99 | 1.00 | 0.95 | NA | NA | NA |
| 3 | 0.92 | 0.98 | 0.93 | 0.97 | 0.90 | 0.89 |
| Lateral Intrahemispheric – within left hemisphere | ||||||
| 4 | 1.00 | 1.00 | 0.97 | NA | NA | NA |
| 5 | 1.00 | 0.95 | 0.94 | 1.00 | 0.92 | 0.94 |
| 6 | 0.91 | 0.88 | 0.87 | NA | NA | NA |
| Lateral Intrahemispheric – within right hemisphere | ||||||
| 7 | 0.99 | 0.94 | 0.95 | NA | NA | NA |
| 8 | 0.79 | 0.78 | 0.79 | 0.85 | 0.83 | 0.74 |
| 9 | 0.94 | 0.89 | 0.92 | NA | NA | NA |
| Key to Coherence Pair Correlations | ||||||
| Pairs | Coherence Pair 1 | Coherence Pair 2 | ||||
| Lateral Intrahemispheric – between hemispheres | ||||||
| 1 | F7-F3–T7-C3 | F8-F4–T8-C4 | ||||
| 2 | F7-F3–P7-P3 | F8-F4–P8-P4 | ||||
| 3 | T7-C3–P7-P3 | T8-C4–P8-P4 | ||||
| Lateral Intrahemispheric – within left hemisphere | ||||||
| 4 | F7-F3–T7-C3 | F7-F3–P7-P3 | ||||
| 5 | F7-F3–T7-C3 | T7-C3–P7-P3 | ||||
| 6 | F7-F3–P7-P3 | T7-C3–P7-P3 | ||||
| Lateral Intrahemispheric – within right hemisphere | ||||||
| 7 | F8-F4–T8-C4 | F8-F4–P8-P4 | ||||
| 8 | F8-F4–T8-C4 | T8-C4–P8-P4 | ||||
| 9 | F8-F4–P8-P4 | T8-C4–P8-P4 | ||||
5 Acknowledgements
We would like to thank our colleagues at Henri Begleiter Neurodynamics Laboratory, particularly Arthur Stimus and Chamion Thomas, for their help in preparing this paper. The comments of two anonymous reviewers and of the action editor helped improve the paper immeasurably. We would like to dedicate this paper to the memory of Henri Begleiter, who inspired its creation.
Footnotes
This work was supported by NIAAA Investigator-Initiated Interactive Research Project Grant (IRPG): AA 12560 at SUNY Downstate Medical Center, AA 12555 at Indiana University School of Medicine, grant AA 12557 at Washington University School of Medicine, and AA 12553 at Howard University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David B. Chorlian, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
Yongqiang Tang, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
Madhavi Rangaswamy, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
Sean O’Connor, Indiana University School of Medicine, Indianapolis, IN, USA.
John Rohrbaugh, Washington University School of Medicine, St. Louis, MO, USA.
Robert Taylor, Howard University, Washington D.C., USA.
Bernice Porjesz, Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY.
References
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14(6):953–958. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Rushby JA. Age and gender effects in EEG coherence: I. Developmental trends in normal children. Clin Neurophysiol. 2004;115(10):2252–2258. doi: 10.1016/j.clinph.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M. Adjusting EEG coherence for inter-electrode distance effects: an exploration in normal children. Int J Psychophysiol. 2005;55(3):313–321. doi: 10.1016/j.ijpsycho.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bendat J, Piersol A. Random Data: Analysis and Measurement Procedures. New York: Wiley-Interscience; 1971. [Google Scholar]
- Bruce A, Gao H. S+ wavelets user’s manual. Seattle, WA: Mathsoft, Inc.; 1994. [Google Scholar]
- Courchesne E. Chronology of postnatal human development: event related potential, positron emission tomography, myelinogenesis, and synaptogenesis studies. In: Rohrbaugh JW, editor. Event Related Potentials of the Brain. New York: Oxford; 1990. [Google Scholar]
- Essl M, Rappelsberger P. EEG coherence and reference signals: experimental results and mathematical explanations. Med Biol Eng Comput. 1998;36(4):399–406. doi: 10.1007/BF02523206. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Harlow, Essex, U.K: Addison Wesley Longman; 1996. [Google Scholar]
- Gasser T, Sroka L, Mocks J. The transfer of EOG activity into the EEG for eyes open and closed. Electroencephalogr Clin Neurophysiol. 1985;61:181–193. doi: 10.1016/0013-4694(85)91058-2. [DOI] [PubMed] [Google Scholar]
- Gasser T, Sroka L, Mocks J. The correction of EOG artifacts by frequency dependentand frequency independent methods. Psychophysiology. 1986;23:704–712. 69581–69584. doi: 10.1111/j.1469-8986.1986.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Grieve PG, Emerson RG, Fifer WP, Isler JR, Stark RI. Spatial correlation of the infant and adult electroencephalogram. Clin Neurophysiol. 2003;114(9):1594–1608. doi: 10.1016/s1388-2457(03)00122-6. [DOI] [PubMed] [Google Scholar]
- Hamming R. Digital Filters. Englewood Cliffs, NJ: Prentice-Hall; 1983. [Google Scholar]
- Holland BA, Haas DK, Normal D, Brant-Zawadski M, Newton TH. The MRI of normal brain maturation. Am J Neurorad. 1986;7:201–208. [PMC free article] [PubMed] [Google Scholar]
- Lange KL, Little RJA, Taylor JMG. Robust Statistical Modeling Using the t distribution. JASA. 1989;84:881–896. [Google Scholar]
- Nunez PL. Quantitative states of neocortex. In: Nunez PL, editor. Neocortical dynamics and Human EEG rhythms. New York, Oxford: Oxford University Press; 1995. pp. 3–67. [Google Scholar]
- Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, Cadusch PJ. EEG coherency.I: Statistics,reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Clin Neurophysiol. 1997;103:499–515. doi: 10.1016/s0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB, Shi Z, Carpenter MR, Srinivasan R, Tucker DM, Doran SM, Cadusch PJ, Wijesinghe RS. EEG coherency II: experimental comparisons of multiple measures. Clin Neurophysiol. 1999;110(3):469–486. doi: 10.1016/s1388-2457(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13(3):125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Mulder EJ, Smit DJ, Boomsma DI, Stam CJ. Genetic cmponents of functional connectivity in the brain: the heritabilityof synchronization likelihood. Hum Brain Mapp. 2005;26(3):191–198. doi: 10.1002/hbm.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans Biomed Eng. 1998;45(7):814–826. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Srinivasan R. Spatial structure of the human alpha rhythm: global correlation in adults and local correlation in children. Clin Neurophysiol. 1999;110(8):1351–1362. doi: 10.1016/s1388-2457(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Strang G, Nguyen T. Wavelets and filter banks. Wellesley, MA: Wellesley-Cambridge Press; 1996. [Google Scholar]
- Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortical associations and EEG coherence: a two-compartmental model. Electroencephalogr. Clin. Neurophysiol. 1986;64(2):123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- van Baal GC, De Geus EJ, Boomsma DI. Genetic architecture of EEG power spectra in early life. Electroencephalogr Clin Neurophysiol. 1996;98(6):502–514. doi: 10.1016/0013-4694(96)95601-1. [DOI] [PubMed] [Google Scholar]
- van Baal GC, de Geus EJ, Boomsma DI. Genetic influences on EEG coherence in 5-year-old twins. Behav Genet. 1998;28(1):9–19. doi: 10.1023/a:1021400613723. [DOI] [PubMed] [Google Scholar]
- van Baal GC, Boomsma DI, de Geus EJ. Longitudinal genetic analysis of EEG coherence in young twins. Behav Genet. 2001;31(6):637–651. doi: 10.1023/a:1013357714500. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Genetic and environmental influences on EEG coherence. Behav Genet. 1998;28(6):443–453. doi: 10.1023/a:1021637328512. [DOI] [PubMed] [Google Scholar]
- Wang SY, Tang MX. Exact Confidence Interval for Magnitude-Squared Coherence Estimates. IEEE SIGNAL PROCESSING LETTERS. 2004;11(3):326–329. [Google Scholar]
- Wang SY, Lix X, Yianni J, Christopher Miall R, Aziz TZ, Stein JF. Optimising coherence estimation to assess the functional correlation of tremor-related activity between the subthalamic nucleus and the forearm muscles. J Neurosci Methods. 2004;136(2):197–205. doi: 10.1016/j.jneumeth.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Winterer G, Enoch MA, White KV, Saylan M, Coppola R, Goldman D. EEG phenotype in alcoholism: increased coherence in the depressive subtype. Acta Psychiatr Scand. 2003;108(1):51–60. doi: 10.1034/j.1600-0447.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation in the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Philadelphia: F.A. Davis; 1967. [Google Scholar]


