Abstract
Photodynamic therapy (PDT) is a promising new modality that utilizes a combination of a photosensitizing chemical and visible light for the management of a variety of solid malignancies. The mechanism of PDT-mediated cell killing is not well defined. We investigated the involvement of cell cycle regulatory events during silicon phthalocyanine (Pc4)-PDT-mediated apoptosis in human epidermoid carcinoma cells A431. PDT resulted in apoptosis, inhibition of cell growth, and G0-G1 phase arrest of the cell cycle, in a time-dependent fashion. Western blot analysis revealed that PDT results in an induction of the cyclin kinase inhibitor WAF1/CIP1/p21, and a down-regulation of cyclin D1 and cyclin E, and their catalytic subunits cyclin-dependent kinase (cdk) 2 and cdk6. The treatment also resulted in a decrease in kinase activities associated with all the cdks and cyclins examined. PDT also resulted in (i) an increase in the binding of cyclin D1 and cdk6 toward WAF1/CIP1/p21, and (ii) a decrease in the binding of cyclin D1 toward cdk2 and cdk6. The binding of cyclin E and cdk2 toward WAF1/CIP1/p21, and of cyclin E toward cdk2 did not change by the treatment. These data suggest that PDT-mediated induction of WAF1/CIP1/p21 results in an imposition of artificial checkpoint at G1 → S transition thereby resulting in an arrest of cells in G0-G1 phase of the cell cycle through inhibition in the cdk2, cdk6, cyclin D1, and cyclin E. We suggest that this arrest is an irreversible process and the cells, unable to repair the damages, ultimately undergo apoptosis.
Photodynamic therapy (PDT) is a promising new therapeutic procedure for the management of a variety of solid malignancies and is also showing promise as a modality for many nonmalignant diseases (1–4). PDT is a bimodal therapy having two components (chemical-photosensitization and light-irradiation) that individually are nontoxic, but tumoricidal in combination (1–4). Typically, the treatment relies on a selective uptake of porphyrin-based photosensitizing chemical in tumor relative to the surrounding normal tissue followed by light irradiation in visible or near infrared region that is typically derived through a laser. This combination causes a photoactivation leading to an oxidative damage to variety of cellular targets and a subsequent cell death resulting in tumor ablation. The model photosensitizer we have been working with is a silicon-phthalocyanine compound HOSiPcSi (CH3)2- (CH2)3N (CH3)2, or Pc4. The mechanism(s) of the PDT-mediated cytotoxic effect is not fully understood. PDT-mediated oxidative stress has been shown to increase the expression of early response genes (c-fos, c-jun, c-myc, and egr-1), as well as the cellular stress protein glucose-regulated protein and heme oxygenase (5–7). The involvement of heat shock protein HSP-70 (8) and NFκB (9) has also been documented in PDT-response. PDT causes apoptosis under both in vitro and in vivo situations (10–12). The mechanism of PDT-mediated apoptosis has received an increasing attention in the recent past. The involvement of phospholipases A2 and C as well as an increase in intracellular Ca2+ has been reported in PDT-mediated apoptosis (13). Recently, the involvement of ceramide generation has been shown during PDT-mediated apoptosis of mouse lymphoma (L5178Y) cells (14). Previous studies from this laboratory have shown the involvement of apoptosis as an early event in ablation of radiation-induced fibrosarcoma cell (RIF-1)-implanted tumors in C3H mice (12) and chemically induced squamous papillomas in mouse skin during PDT (15). A proper understanding of the mechanism involved in the PDT-induced cancer ablation may result in improving the efficacy of this treatment modality.
A growing body of evidence indicates that cell cycle perturbations may be related to apoptosis because certain cell cycle regulatory molecules such as the tumor suppressor protein p53 and the cyclin kinase inhibitor (cki), namely WAF1/CIP1/p21, are shown to be associated with apoptosis and cell cycle arrest (16–20). Studies have demonstrated the implication of WAF1/CIP1/p21-induction through a p53-dependent as well as p53-independent mechanism during apoptosis and cell cycle deregulation at G1 → S phase transition checkpoint (16–22). WAF1/CIP1/p21 is known to control the cell cycle by regulating the cyclin-dependent kinase (cdk)-cyclin complexes, in response to various physiological processes such as the cellular response to DNA damage, senescence, response to growth inhibitory signals, and tumor suppression. In this study we provide evidence for the involvement of cell cycle deregulation during PDT-mediated apoptosis.
MATERIALS AND METHODS
Cells.
The human epidermoid carcinoma cells A431 obtained from American Type Culture Collection (Manassas, VA) were used in this study. The cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin and kept in an atmosphere of 95% air/5% CO2 in a 37°C humidified incubator. In all the experiments, 70–80% confluent cells were used.
PDT.
The phthalocyanine photosensitizer Pc4 was provided by Ying syi Li and Malcolm E. Kenney (Department of Chemistry, Case Western Reserve University). The cells were treated with Pc4 (0.5 μM in DMEM complete media) overnight in a 100 × 20 mm style Falcon disposable cell culture dish. Next morning, the cells were washed with Hanks’ balanced salt solution (with Ca2+ and Mg2+), irradiated (in Hanks’ balanced salt solution with Ca2+ and Mg2+) with 15 kJ/m2 of light. The cells, after irradiation, were incubated (in DMEM complete media) for selected time point in a humidified incubator at 37°C. Appropriate controls, as specified at appropriate places, also were included. After the specified times, the medium was aspirated, the cells were washed with cold PBS (10 mM, pH 7.4) and processed as desired.
DNA Isolation and Gel Electrophoresis, and Quantitation of Apoptosis by Flow Cytometry.
After the treatment of cells with PDT the cellular DNA was isolated and resolved over 1.5% agarose gel to identify the formation of DNA ladder using standard procedure. Briefly, after PDT, the cells were washed twice with PBS (pH 7.2), suspended in 1.0 ml cytoplasm extraction buffer (10 mM Tris, pH 7.5, 150 mM NaCl/5 mM MgCl2/0.5% Triton X-100), left over ice for 15 min, and pelleted down by centrifugation (14,000 × g) at 4°C. The pellet was incubated with 1.0 ml of DNA lysis buffer (10 mM Tris, pH 7.5/400 mM NaCl/1 mM EDTA/1% Triton X-100) for 20 min on ice and then centrifuged at 14,000 × g at 4°C. The supernatant was incubated overnight with RNase (0.2 mg/ml) at room temperature and then with proteinase K (0.1 mg/ml) for 2 hr at 37°C. DNA was then extracted using phenol: chloroform (1:1) and precipitated with 95% ethanol for 2 hr at −80°C. The DNA precipitate was centrifuged at 14,000 × g at 4°C for 15 min and the pellet was air dried and dissolved in 20 μl of TE buffer (10 mM Tris⋅HCl, pH 8.0/1 mM EDTA). Total amount of DNA obtained was resolved over 1.5% agarose gel, containing 0.3 μg/ml ethidium bromide in Tris⋅borate⋅EDTA (1× TBE) buffer. The bands were visualized under UV transilluminator followed by polaroid photography.
In an additional experiment, after PDT, the extent of apoptosis was quantified by using APO-DIRECT flow cytometry kit (Phoenix Flow Systems, San Diego) as per the manufacturer’s protocol.
Cell Growth and Viability.
The cells were subjected to PDT and harvested at 3, 6, 12, and 24 hr post-PDT. The total number of cells and the viable cells were counted using trypan blue exclusion assay.
DNA Cell Cycle Analysis.
The cells were subjected to PDT and harvested at different time points. The cells were washed twice with cold PBS (10 mM, pH 7.2) and resuspended in 50 μl of cold PBS and 450 μl of cold methanol for 1 hr at 4°C. The cells were centrifuged at 1100 rpm for 5 min, pellet washed twice with cold PBS, suspended in 500 μl of PBS, and incubated with RNase (20 μg/ml final concentration) at 37°C for 30 min. The cells were chilled over ice for 10 min and stained with propidium iodide (50 μg/ml final concentration) for 1 hr and analyzed by flow cytometry.
Immunoprecipitation and Western Blot Analysis.
The cells were treated with as described above and harvested at different time points (3, 6, and 12 hr after PDT). In addition to 0 hr control, we also included 12 hr untreated control in further experiments because it was the longest time point post-PDT in our experimental protocol. After these specific time periods, the medium was aspirated, the cells were washed with cold PBS (10 mM, pH = 7.4) and ice cold RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.3/1% Triton X-100/1% sodium deoxycholate) was added to the plates that were then placed over ice for 30 min. The cells were scraped, the lysate was collected in a microfuge tube and passed through a 21G needle to break up the cell aggregates. The lysate was cleared by centrifugation at 14,000 × g for 15 min at 4°C and the supernatant (total cell lysate) was either used immediately or stored at −70°C. The protein concentration was determined by DC Bio-Rad assay using the manufacturer’s protocol. For Western blot analysis, 50–100 μg of protein was resolved over SDS/12% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot was blocked in blocking buffer (5% nonfat dry milk/1% Tween 20; in 20 mM TBS, pH 7.6) for 1 hr at room temperature, incubated with appropriate monoclonal or polyclonal primary antibody (human reactive anti-WAF1, cdk2, cdk6, cyclin D1, and cyclin E, Santa Cruz Biotechnology) in blocking buffer for 1 hr to overnight at 4°C followed by incubation with anti-mouse or anti-rabbit secondary antibody horseradish peroxidase conjugate (Amersham) and detected by chemiluminescence and autoradiography using XAR-5 film (Eastman Kodak).
For immunoprecipitation, the cell lysates containing 50–200 μg of protein were taken in 1.0 ml RIPA buffer and were precleared by incubating with normal mouse or rabbit IgG (1 μg) and protein A-Sepharose 4B fast flow (20 μl) (Sigma) for 1 hr at 4°C. The supernatant was collected and incubated with 1.0 μg of appropriate primary antibody and 20 μl of protein A-Sepharose overnight at 4°C. The beads were collected by centrifugation at 3,000 rpm for 10 min at 4°C, washed with RIPA buffer three times after centrifugation steps. The immunocomplexes were collected, heated at 100°C over boiling water in 2× denaturing sample buffer for 5 min and resolved over a SDS/12% polyacrylamide gel, followed by Western blot analysis and chemiluminescent detection.
Kinase Activity Assay.
The cells were treated and lysed as described above except that RIPA buffer was replaced with kinase lysis buffer containing 50 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, 20 mM NaF, 1.0 mM EDTA, 1.0 mM EGTA, 1% Triton X-100, 0.5% Nonidet P-40, and freshly added phenylmethylsulfonyl fluoride (0.01%), aprotinin (1 mg/ml), and sodium orthovanadate (1 mM). The cyclin-cdk complexes were immunoprecipitated as above using appropriate primary antibody raised to the C-terminus of the protein. The immunocomplexes were washed three times with the kinase lysis buffer and once with kinase buffer containing 50 mM Tris (pH 7.4), MgCl2 (10 mM), and DTT (1 mM). The beads were, incubated with 40 μl “hot” kinase solution containing 5.0 μg of histone H1 (for cdk2 and cyclin E associated kinase activity) or pRb-glutathione S-transferase (for cdk6 and cyclin D1 associated kinase activity) and 80 μM ATP, and 5 μCi of [γ-32P]ATP (6,000 Ci/mmol; 1 Ci = 37 GBq; Amersham) in kinase buffer, for 30 min at 37°C. The sample buffer (5×, 8 μl) was added to the tubes that were then heated at 100°C for 5 min over boiling water. The samples were resolved over 12% polyacrylamide gel. The gels were stained with Coomassie blue for the visualization of bands and then dried in a Bio-Rad gel drier (model 583) followed by detection by autoradiography with XAR-5 film. The kinase activity was quantified by counting the radioactive β-disintegrations of individually resolved bands on the dried gel.
RESULTS
PDT Results in the Induction of Apoptosis in A431 Cells.
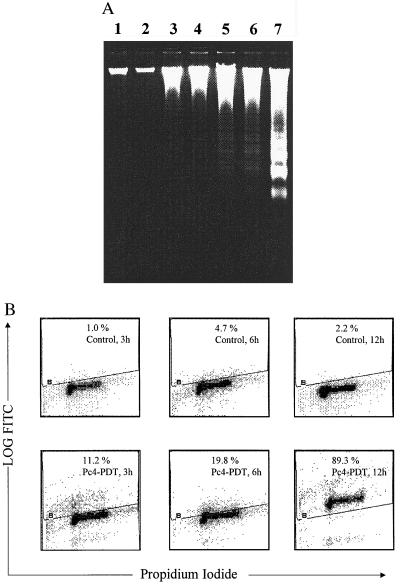
We characterized the induction of apoptosis in A431 cells after PDT using DNA fragmentation assay. As shown in Fig. 1A, the formation of DNA ladder was observed as early as 3 hr post-PDT and found to be more profound at 6 and 12 hr post-PDT. The treatment of the cells with light alone or Pc4 alone did not cause any fragmentation of the DNA. Next, we quantified the extent of apoptosis by flow cytometric analysis of the cells labeled with F-dUTP and propidium iodide. The PDT of A431 cells resulted in 12.8%, 21.7%, and 89.3% apoptotic cells at 3, 6, and 12 hr posttreatment, respectively, as compared with the corresponding untreated controls showing 1.0%, 3.7%, and 2.2% apoptotic cells respectively (Fig. 1B). While the induction of apoptosis was not significant at 1 hr post-PDT, the later time point of 24 hr post-PDT showed almost 99% apoptotic cells (data not shown) that was accompanied with a drastic decline in the cell population (due to exhaustive cellular degradation) that could be labeled for flow cytometry.
Figure 1.
Induction of apoptosis by PDT. (A) DNA ladder formation by PDT in A431 cells. The cells were treated with Pc4 only, light only or Pc4+light. After designated times, the cells were harvested, cellular DNA isolated and subjected to agarose gel electrophoresis followed by visualization of bands as described in Materials and Methods. The data shown here are from a representative experiment repeated three times with similar results. Lane 1, untreated control; lane 2, Pc4 only; lane 3, light only; lane 4, Pc4+light (1 hr post-PDT); lane 5, Pc4+light (3 hr post-PDT); lane 6, Pc4+light (6 hr post-PDT); lane 7, Pc4+light (12 hr post-PDT). (B) Quantification of apoptosis by flow cytometry. The untreated control cells or PDT treated cells were harvested after designated times, labeled with deoxyuridine triphosphate using terminal deoxynucleotide transferase and propidium iodide, and analyzed by flow cytometry. The results are displayed using dual parameter method where the x and y axes represent DNA and deoxyuridine triphosphate fluorescence respectively. The cells displaying deoxyuridine triphosphate fluorescence above that of the control population, as indicated by the line in each histogram are considered as apoptotic cells and their percent population is shown at right top in each box. Similar data were obtained in two repeat experiments.
PDT Results in a Growth Inhibition and a G0-G1 Cell Cycle Arrest in A431 Cells.
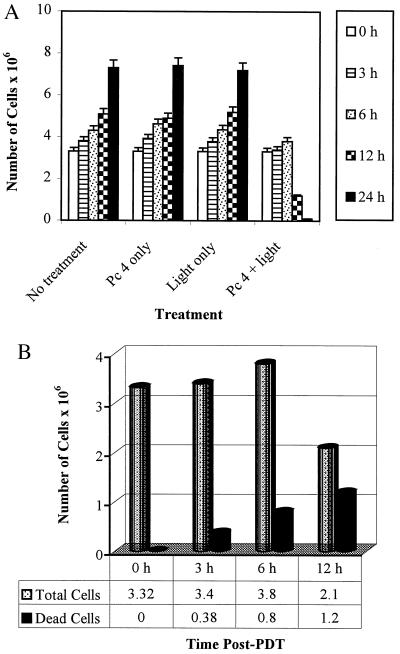
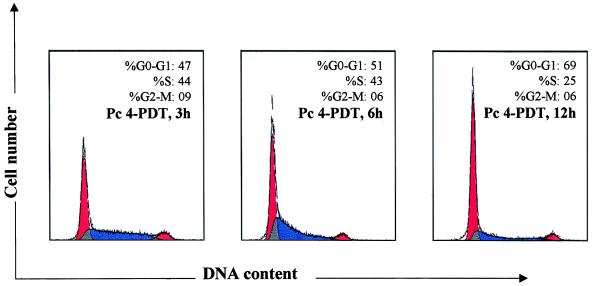
The cells subjected to PDT resulted in a growth arrest as compared with their untreated controls at all the time points observed (Fig. 2A). PDT treatment of cells also resulted in a significant time-dependent loss of cell viability (Fig. 2B). We argued about the possibility that the mechanism of PDT action may also involve an arrest of cells at specific check point(s) in the cell cycle. We, therefore, assessed the effect of PDT on cell cycle perturbations in growing A431 cells. Data in Fig. 3 show an appreciable arrest of cells in G0-G1 phase of the cell cycle after PDT. The treatment caused an accumulation of 47%, 51%, and 69% cells at 3, 6, and 12 hr post-PDT, respectively. The G0-G1 cell population of controls at these times did not change and ranged between 39% and 43% (data not shown) probably because we employed growing (unsynchronized) cells in these experiments. The increase in G0-G1 cell population was accompanied with a decrease of cell number in S-phase. At earlier time points there was no significant change in distribution of cells in the cell cycle whereas at 24 hr post-PDT the population of cells in G0-G1 phase did not show any significant change when compared with 12 hr post-PDT (data not shown).
Figure 2.
Effect of PDT on cell growth and viability. (A) Growth of A431 cells after PDT. The cells were subjected to PDT and harvested at designated times post-PDT and the total number of cells was counted. The data shown are means ± SEM of three independent experiments, each run in triplicate. (B) Viability of A431 cells after PDT. The cells were treated as above and the number of viable cells was counted using trypan blue exclusion assay. The cell viability is represented as number of viable cells × 106. The data shown are representative of three independent experiments, each run in triplicate. The variation between the experiments is <5%.
Figure 3.
DNA flow cytometric analysis. The A431cells were treated with Pc4+light and harvested for designated times as shown in the figure and described under Materials and Methods, and analyzed by flow cytometry. The appropriate controls were also incorporated. The percentage of cells in G0-G1, S, and G2-M phase were calculated using cellfit computer software and are represented within the histograms. The data shown here is from a representative experiment repeated four times with similar results. Because the G0-G1 cell population of controls did not show any significant change and ranged between 39% and 43%, the data are not supplied.
The Cell Cycle Arrest Due to PDT in A431 Cells Is Mediated via an Induction in WAF1/CIP1/p21 and Consequent Inhibition in Cyclin D1, Cyclin E, cdk2, and cdk6.
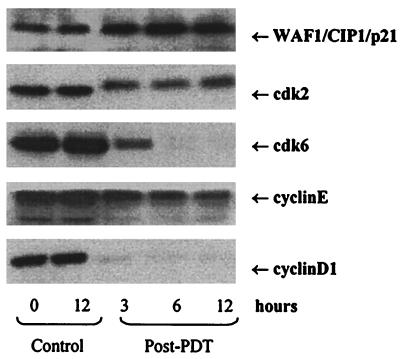
We next assessed the effect of PDT on the induction of WAF1/CIP1/p21, which is known to regulate the entry of cells at G1 → S transition checkpoint, and induce apoptosis. Western blot analysis revealed that PDT results in a significant induction of WAF1/CIP1/p21 at 3, 6, and 12 hr after the treatment, when compared with the basal levels (Fig. 4).
Figure 4.
Effect of PDT on protein expression of WAF1/CIP1/p21, cdk2, cdk6, cyclin E, and cyclin D1. The cells were subjected to PDT and harvested at 3, 6, and 12 hr post-PDT. Total cell lysates were prepared and 50–100 μg protein was subjected to SDS/PAGE followed by Western blot analysis and chemiluminescent detection. The details are described under Materials and Methods.
Employing Western blot analysis, we also assessed the protein expression of the cyclins and cdks which are known to be operative in G1-phase to investigate if their function is altered. PDT resulted in a time-dependent decrease in cyclin D1, cyclin E, cdk2, and cdk6 (Fig. 4). The decrease in cyclin D1 and cdk2 protein expression was more pronounced than that of cyclin E and cdk6 respectively. The decrease in the protein expression of cdk6 was much more pronounced at 6 and 12 hr post-PDT than at 3 hr posttreatment. PDT also resulted in decreased mobility of cdk2 bands.
PDT of A431 Cells Results in Decrease in the Kinase Activities Associated with cdks and Cyclins.
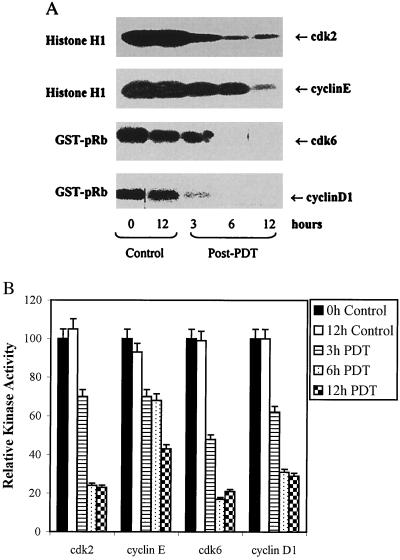
Because the kinase activity of cdks is the driving force the progression of cell cycle as it activates the cyclins that are essential component of cyclin-cdk complexes, we assessed the effect of PDT on kinase activities associated with cdk2, cdk6, cyclin D1, and cyclin E. The radioactive kinase activity assay (Fig. 5A) and its quantification (Fig. 5B) showed that PDT, though to a different extent, almost universally resulted in a time-dependent decrease in kinase activities associated with all the cdks and cyclins examined.
Figure 5.
Effect of PDT on kinase activities. (A) Kinase activity associated with cdk2 and cdk6, and cyclin E and cyclin D1. The cells were subjected to PDT and harvested at designated times post-PDT. Total cell lysates were prepared using kinase lysis buffer, 200 μg protein were immunoprecipitated and kinase activities were measured using appropriate substrates and [γ-32P]ATP as described under Materials and Methods. (B) Quantification of kinase activity associated with cdk2 and cdk6, and cyclin E and cyclin D1. The kinase activities were quantified by counting the radioactive β-integrations of individually resolved bands and is expressed in terms of percent relative intensity of the bands as compared with the untreated controls.
PDT of A431 Cells Alters Interbinding Between WAF1/CIP1/p21, Cyclins and cdks.
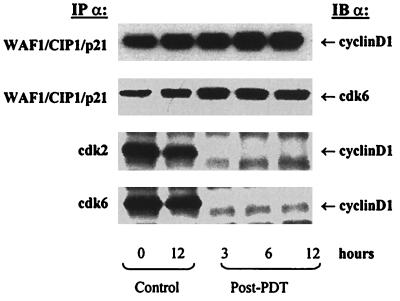
For a normal progression of cells through the cell cycle, a balance between each component of cki-cyclin-cdk complex plays an important role. We therefore investigated the effect of PDT on the binding between WAF1/CIP1/p21-cyclin, WAF1/CIP1/p21-cdk, and cyclin-cdk. For this, one of the two proteins was immunoprecipitated using appropriate antibody and the effect on the binding was assessed by probing the immunoblot using antibody directed against the other protein. Data in Fig. 6 show that PDT resulted in an increased binding of cyclin D1 and cdk6 toward WAF1/CIP1/p21 whereas the binding of cyclin E and cdk2 toward WAF1/CIP1/p21 did not change (data not shown). PDT also resulted in a decrease in the binding of cyclin D1 toward cdk2 and cdk6 (Fig. 6) whereas the binding of cyclin E toward cdk2 remained unaltered (data not shown).
Figure 6.
Effect of PDT on inter-binding between WAF1/CIP1/p21, cyclins, and cdks. The cells were subjected to PDT and harvested at designated times post-PDT. Total cell lysates were prepared, 50–100 μg protein were immunoprecipitated and subjected to SDS/PAGE and the binding with the other protein was measured by immunoblot analysis using antibodies directed against the other protein. The details are described in Materials and Methods.
DISCUSSION
In the recent past, PDT has emerged as a promising therapeutic procedure for ablating a variety of solid tumors in selected patients with advanced esophageal cancer, lung cancer, nonmelanoma superficial skin cancer, and Kaposi’s sarcoma. PDT is also showing promise for the management of certain nonmalignant diseases. In vivo and in vitro studies have shown the involvement of apoptosis in PDT mediated cell-kill in cell culture systems as well as in animal models (10–12). Studies have also suggested the involvement of singlet oxygen and possibly other reactive oxygen species as a prerequisite for PDT-effect (23). The involvement of phospholipase A2 and C (13), and ceramide (14) is shown in PDT-mediated apoptosis. Recently, the stimulation of stress-activated protein kinase and p38 high osmolarity glycerol protein kinase in murine keratinocytes after PDT with benzoporphyrin derivative has been shown (24). Another recent report has documented the involvement of caspases in PDT-mediated cell killing (25). The understanding of the cascade of events playing a role in PDT-mediated apoptosis is far from complete. Defining these events will be helpful in designing better PDT protocols, which could have wider applications as a cancer therapeutic modality. This study may be an important step in this direction.
The tumor suppressor gene p53 is regarded as a key element in maintaining a balance between cell growth and cell death in the living system (26–28). P53, in response to DNA damage, triggers a variety of cell cycle regulatory events to limit the proliferation of damaged cells. This “gate keeper” gene, in a number of human tumors, is inactivated by mutation resulting in unlimited cellular proliferation (29, 30). It is, therefore, important to study of the mechanism(s) by which PDT results in tumor growth inhibition and induction of apoptosis, in the absence of active (wild-type) p53 in the transformed cells. In the present study we employed A431 cells that are shown to have nonfunctional (mutated) p53 carrying G→A mutation at codon 273 resulting in an arginine to histidine substitution (mp53) (31). Our data showed the induction of apoptosis by PDT as early as 3 hr posttreatment, showing a progressive increase at 6 hr and 12 hr post-PDT. This apoptotic response of A431 cells is slower than certain other cell types such as L5178Y cells, which are shown to undergo rapid apoptosis after PDT (14). Certain other cell types such as RIF-1 cells even show resistant to apoptosis by PDT (12). The viral infection state has also shown to affect the kinetics of PDT-induced apoptosis (32). Therefore the kinetics of PDT-mediated apoptosis seems to be cell type specific. The mutation in p53 could be another reason for slower apoptotic response of A431 cells. We argued that this induction in apoptosis might be mediated through cell cycle perturbations resulting in cell growth arrest. The cell growth and viability assay have shown that PDT results in an arrest of cell growth and an increase in the loss of viability in a time-dependent fashion that could be related to apoptosis. To further prove the point we conducted DNA cell cycle analysis that showed a progressive arrest of the cells in G0-G1 phase of the cell cycle. It is important to emphasize here that the induction of cell cycle arrest as well as apoptosis follows a similar pattern of time-dependent response.
The passage through the cell cycle, in eukaryotes is orchestrated by the function of a family of protein kinase complexes (33). Each complex is composed minimally of a catalytic subunit, the cdk, and its essential activating partner, the cyclin (33). These complexes are activated at various checkpoints after specific intervals during the cell cycle, but can also be induced and regulated by the exogenous factors. The cdks are subjected to inhibition by the binding of a class of proteins known as cki such as the CIP/KIP family of proteins that includes WAF1/CIP1/p21 as its most important member (16–20). Certain diseases such as cancer have been reported to carry defects at various checkpoints regulated by cki-cyclin-cdk machinery, which can serve as targets for intervention against the growth of cancer. Our interest was to elucidate the modulation(s) in these master cell cycle regulatory events during PDT-mediated cell cycle deregulation and apoptosis.
Studies have shown that certain exogenous stimuli may result in a p53-dependent as well as p53-independent induction of WAF1/CIP1/p21, which may result in a cell cycle arrest and apoptosis. Our data demonstrated an induction of WAF1/CIP1/p21 at 3, 6, and 12 hr after PDT. In absence of active p53, this induction of WAF1/CIP1/p21 appears to be independent of wild-type p53 in A431 cells. The role of mutant p53 during this process, however, remains to be examined. Because WAF1/CIP1/p21 is regarded as a universal inhibitor of cdks, we assessed the effect of PDT on the expression of cdk2 and cdk6. PDT resulted in significant inhibition of these two critical molecules operative in G0-G1 phase of the cell cycle. The results also demonstrated a decrease in the mobility of cdk2 band due to the treatment. This could be attributed to decrease in the phosphorylation of cdk2 at Thr-160 that is an important event for progression of the cell cycle (ref. 34 and references therein). WAF1/CIP1/p21 is also shown to inhibit the kinase activity of cdk-cyclin complexes that may modulate the phosphorylation events, which are very important for cell cycle transitions. We therefore examined the effect of PDT on kinase activities associated with cdk2, and cdk6, as well as their regulatory subunits cyclin E, and cyclin D1. PDT resulted in a time-dependent inhibition in the kinase activities associated with these G1-phase cell cycle regulatory molecules. Our data also demonstrate a decrease in the levels of the, cyclin D1 and cyclin E as a result of PDT. These results are in agreement with the fact that the cdks and cyclins operate in association with each other by forming a complex that may bind to and inhibited by WAF1/CIP1/p21 for the regulation of cell cycle.
Because the normal progression of cell cycle is dependent on a balance between each component of cki-cyclin-cdk complex, we were also interested to investigate the effect of PDT on the binding of (i) WAF1/CIP1/p21 with cdk2, cdk6, cyclin D1, and cyclin E, and (ii) cdk2 and cdk6 with their regulatory subunits cyclin D1 and cyclin E. To delineate the effect of PDT on the binding of WAF1/CIP1/p21 with the cdks and cyclins, we immunoprecipitated WAF1/CIP1/p21 and probed the blot with antibodies against the cdks and cyclins. The data revealed that PDT resulted in an increased binding of cyclin D1 and cdk6 toward WAF1/CIP1/p21 whereas the binding of cyclin E and cdk2 toward WAF1/CIP1/p21 did not change. This indicates that PDT-induced WAF1/CIP1/p21 binds to and inhibits (i) cyclin D1-cdk2 complex through a binding domain present in cyclin D1, (ii) cyclin D1-cdk6 complex through the binding domain(s) residing at cdk6 and/or cyclin D1. The mode of action of WAF1/CIP1/p21 on cyclin E-cdk2 complex is our system however, is not clear from our data. However, this may be an important observation that PDT inhibits the protein expression as well as the kinase activities associated with cyclin E and cdk2 without any alteration in their binding with WAF1/CIP1/p21. To understand the effect of PDT on the binding of cdks with the cyclins, we immunoprecipitated cdks and probed the blot with antibodies against the cyclins. PDT also resulted in a decreased binding of cyclin D1 to cdk2 and cdk6 but did not affect the binding between cyclin E and cdk2. This effect is presumably because PDT results in an increased binding of cyclin D1 to WAF1/CIP1/p21 with a resultant decreased availability of this cyclin in cyclin D1-cdk2 and cyclin D1-cdk6 complexes. On the other hand, because PDT does not affect the binding of cdk2 or cyclin E with WAF1/CIP1/p21, the binding between cdk2 and cyclin E remains unaltered. In this experiment also, PDT did not cause any change in the binding between cyclin E and cdk2 that, further strengthens our presumption that PDT inhibits the protein expression as well as the kinase activities associated with cyclin E and cdk2, by some other mechanism presently not known to us.
The importance of WAF1/CIP1/p21 as the key regulator of G1-checkpoints, in response to exogenous stress is also supported by the recent observation that gamma irradiation induced arrest of the cell cycle is compromised in WAF1/CIP1/p21 deficient mouse embryo fibroblasts (35, 36). The present studies, therefore, implicate that PDT-induced WAF1/CIP1/p21 is the major inhibitor of G1-phase cyclins (cyclin D1 and cyclin E) and the cdks (cdk2 and cdk6).
Based on this study, we believe that PDT-mediated induction of WAF1/CIP1/p21 results in an imposition of artificial checkpoint at G1 → S transition thereby resulting in an arrest of cells in G0-G1 phase of the cell cycle through inhibition in the cdk2, cdk6, cyclin D1, and cyclin E. This arrest in cell cycle is an irreversible process and the cells, unable to repair these damages, ultimately undergo apoptosis. Our data show that at 12 hr post-PDT only 69% cells are arrested in G0-G1 phase whereas 89% cells are apoptotic at this time. This observation indicates that in addition to the cell cycle-mediated apoptosis, some other mechanism of apoptosis may also be operative. The involvement of two independent mechanisms for apoptosis and cell cycle arrest cannot be ruled out. This study, for the first time establishes the involvement of cell cycle deregulation by cki-cyclin-cdk network during PDT-mediated cell kill. The exact determinants that are critical for apoptosis still remains unclear and further studies involving the cell lines having functional and nonfunctional p53 may identify targets for developing better strategies for rational therapy.
Acknowledgments
This work was supported by U.S. Public Health Service Grants RO1 CA 51802, PO1 CA 48735, P30 CA 43703, and P30 AR 39750.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Pc, phthalocyanine; PDT, photodynamic therapy; cki, cyclin kinase inhibitor; cdk, cyclin-dependent kinase.
References
- 1.Gomer C J. Photochem Photobiol. 1991;54:1093–1107. doi: 10.1111/j.1751-1097.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher A M, Murphree A L, Gomer C J. Lasers Surg Med. 1995;17:2–31. doi: 10.1002/lsm.1900170103. [DOI] [PubMed] [Google Scholar]
- 3.Pass H I. J Natl Cancer Inst. 1993;85:145–157. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- 4.Tom R. J Natl Cancer Inst. 1997;89:112–114. [Google Scholar]
- 5.Luna M C, Wong S, Gomer C J. Cancer Res. 1994;54:1374–1380. [PubMed] [Google Scholar]
- 6.Gomer C J, Luna M C, Ferrario A, Rucker N. Photochem Photobiol. 1991;53:275–279. doi: 10.1111/j.1751-1097.1991.tb03934.x. [DOI] [PubMed] [Google Scholar]
- 7.Gomer C J, Ferrario A, Rucker N, Wong S, Lee A. Cancer Res. 1991;51:6574–6579. [PubMed] [Google Scholar]
- 8.Gomer C J, Ryter S W, Ferrario A, Rucker N, Wong S, Fisher A M R. Cancer Res. 1996;56:2355–2360. [PubMed] [Google Scholar]
- 9.Ryter S W, Gomer C J. Photochem Photobiol. 1993;58:753–756. doi: 10.1111/j.1751-1097.1993.tb04964.x. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal M L, Clay M E, Harvey E G, Evans H H, Antunez A R, Oleinick N L. Cancer Res. 1991;51:5993–5996. [PubMed] [Google Scholar]
- 11.He X-Y, Sikes S, Thomsen L, Chung L W K, Jacques S L. Photochem Photobiol. 1994;59:468–473. doi: 10.1111/j.1751-1097.1994.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 12.Zaidi S I A, Oleinick N L, Zaim M T, Mukhtar H. Photochem Photobiol. 1993;58:771–776. doi: 10.1111/j.1751-1097.1993.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal M L, Larkin H E, Zaidi S I A, Evans, Mukhtar H, Oleinick N L. Cancer Res. 1993;53:5897–5902. [PubMed] [Google Scholar]
- 14.Separovic D, He J, Oleinick N L. Cancer Res. 1997;57:1717–1721. [PubMed] [Google Scholar]
- 15.Agarwal R, Korman N J, Mohan R R, Feyes D K, Jawed S, Zaim M T, Mukhtar H. Photochem Photobiol. 1996;63:547–552. doi: 10.1111/j.1751-1097.1996.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 16.Ho Y S, Wang Y J, Lin J K. Mol Carcinog. 1996;16:20–31. doi: 10.1002/(SICI)1098-2744(199605)16:1<20::AID-MC4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Gartenhaus R B, Wang P, Hoffmann P. Proc Natl Acad Sci USA. 1996;93:265–268. doi: 10.1073/pnas.93.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu C A, Rishi A K, Su-Li X, Gerald T M, Dawson M I, Schiffer C, Reichert U, Shroot B, Poirer G C, Fontana J A. Blood. 1997;89:4470–4479. [PubMed] [Google Scholar]
- 19.Kondo S, Barna B P, Kondo Y, Tanaka Y, Casey G, Liu J, Morimura T, Kaakaji R, Peterson J W, Werbel B, Barnett G H. Oncogene. 1996;13:1279–1285. [PubMed] [Google Scholar]
- 20.Huang T S, Kuo M L, Shew J Y, Chou Y W, Yang W K. Oncogene. 1996;13:625–632. [PubMed] [Google Scholar]
- 21.Xiaong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. Nature (London) 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 22.Russo T, Zambrano N, Esposito F, Ammendola R, Cimino F, Fiscella M, Jackman J, O’Connor P M, Anderson C W, Appella E. J Biol Chem. 1995;270:29386–29391. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- 23.Girotti A W. In: Photodynamic Therapy of Neopastic Disease. Kessel D, editor. Vol. 1. Boca Raton, FL: CRC; 1990. pp. 229–246. [Google Scholar]
- 24.Tao J-s, Sanghera J S, Pelech S L, Wong G, Levy J G. J Biol Chem. 1996;271:27107–27115. doi: 10.1074/jbc.271.43.27107. [DOI] [PubMed] [Google Scholar]
- 25.Granville D J, Levy J G, Hunt D W C. Cell Death Differ. 1997;4:623–626. doi: 10.1038/sj.cdd.4400286. [DOI] [PubMed] [Google Scholar]
- 26.Vogelstein B, Kinzler K W. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 27.Harris C C. J Natl Cancer Inst. 1996;88:1442–1455. doi: 10.1093/jnci/88.20.1442. [DOI] [PubMed] [Google Scholar]
- 28.Velculescu V E, El-Deiry W S. Clin Chem. 1996;42:858–868. [PubMed] [Google Scholar]
- 29.Lee J M, Bernstein A. Cancer Metastasis Rev. 1995;14:149–161. doi: 10.1007/BF00665797. [DOI] [PubMed] [Google Scholar]
- 30.Neubauer A, Thiede C, Huhn D, Wittig B. Leukemia. 1996;3:S2–S4. [PubMed] [Google Scholar]
- 31.Kwok T T, Mok C H, Menton-Brenanan L. Cancer Res. 1994;54:2834–2836. [PubMed] [Google Scholar]
- 32.Ben-Hur E, Oetjen J, Horowitz B. Photochem Photobiol. 1997;65:456–460. doi: 10.1111/j.1751-1097.1997.tb08589.x. [DOI] [PubMed] [Google Scholar]
- 33.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 34.Petrocelli T, Poon R, Drucker D J, Slingerland J M, Rosen C F. Oncogene. 1996;12:1387–1396. [PubMed] [Google Scholar]
- 35.Brugarolas J C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 36.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]