Abstract
Studies have suggested that teens with alcohol use disorder (AUD) can demonstrate memory deficits, but the underlying neuroanatomical substrates are unclear. The hippocampus is crucial to intact memory functioning, and it actively develops during adolescence. The current study attempted to replicate and extend previous findings suggesting that adolescents with AUD show smaller hippocampal volumes than healthy adolescents. Manual tracings of bilateral hippocampi were performed on structural magnetic resonance images of 14 adolescents (ages 15 to 17 years) with AUD and 17 healthy comparison teens. Intracranial, white, and gray matter volumes, as well as memory abilities, were also measured. Results revealed that adolescents with AUD had significantly smaller left hippocampal volumes than healthy teens, even after removal of teens with comorbid conduct disorder from the analyses. In contrast, the groups did not differ in right hippocampal, intracranial, gray or white matter volumes, or memory performance. Hippocampal volumes were not related to alcohol-consumption rates. These findings indicate that adolescents with AUD, but free from other psychiatric comorbidities, have reduced left hippocampal volume. Because hippocampal volume did not relate to alcohol use characteristics, it is possible that premorbid volumetric differences could account for some of the observed group differences in hippocampal volume.
Keywords: Adolescence, Hippocampus, Alcoholism, MRI, Brain
1. Introduction
Heavy alcohol use is common in adolescence, with 31% of 12th graders reporting getting drunk in the past month (Johnston et al., 2004), and 6−10% of teens meeting diagnostic criteria for an alcohol use disorder (AUD) (Clark et al., 2002; Rohde et al., 1996). AUDs are diagnostically encompassed within other substance-related disorders, and they include both alcohol abuse, which is characterized by a “maladaptive pattern of alcohol use manifested by recurrent and significant adverse consequences related to the repeated use of alcohol”, and alcohol dependence, which is defined as “a cluster of cognitive, behavioral, and physiological symptoms indicating that the individual continues to use alcohol despite significant alcohol-related problems” (DSM-IV, 1994). Despite the high prevalence of AUD among teens, few studies have examined the effects of heavy alcohol consumption on the adolescent brain. While a wealth of literature demonstrates the deleterious effects of alcohol on the adult brain (for review, see Kril and Halliday, 1999), the adolescent brain is still actively developing (Durston et al., 2001; Giedd et al., 1999; Gogtay et al., 2004), making it difficult to draw conclusions about adolescents based on findings from adults. It is essential to understand the neuromaturational and cognitive implications of chronic alcohol use during adolescence, as neural and functional abnormalities related to maladaptive drinking differ from those of adults and could limit educational, occupational, and social opportunities.
One brain structure that has consistently demonstrated sensitivity to alcohol-related neurotoxicity is the hippocampus—a structure crucial to intact learning and memory formation (Eichenbaum, 1999; Squire, 1992). Several studies on alcoholic older adults have documented reduced hippocampal volumes (Jernigan et al., 1991; Sullivan et al., 1995) as well as verbal and spatial memory impairments (e.g., Grant, 1987; Munro et al., 2000). While the research on human adolescents is limited, rodent studies suggest that alcohol-induced hippocampal pathology and functional impairments are greater in adolescent than adult rats (for review, see White and Swartzwelder, 2004), further indicating that adolescence may be a particularly vulnerable period for alcohol-related hippocampal and associated neurocognitive dysfunction.
A few studies have examined alcohol-related neurocognitive impairments among human adolescents, and suggest that chronic alcohol use among teens is associated with lower executive functioning, visuo-spatial skills, attention, and verbal and nonverbal memory scores (Brown et al., 2000; Moss et al., 1994; Tapert et al., 2002). However, very few studies have examined the neural correlates of these deficits, and most investigations of adolescents with AUD have not controlled for psychiatric comorbidities, including other substance use disorders, so the unique effects of heavy drinking remain unclear.
Only one study to date has examined the structural neuropathological consequences of heavy alcohol use on the developing brain (De Bellis et al., 2000), and it revealed that even with no differences in cerebral, cortical gray and white matter, corpus callosum, and amygdala volumes, adolescents and young adults with adolescent-onset AUD had significantly smaller bilateral hippocampal volumes than case-matched controls. However, as with other previous investigations of neurocognition among AUD youths, findings may have been influenced by comorbid psychiatric disturbances, such as post-traumatic stress disorder (PTSD) and depression, which have been independently associated with hippocampal volume reduction in adults (Bremner et al., 2000; Nutt and Malizia, 2004). Poly-substance use may also be related to results, as some participants with AUD also met criteria for other substance use disorders. While that sample was largely representative of adolescents with AUD, the current study was designed to address issues of comorbidity by examining hippocampal volumes in teens with AUD but without mood, anxiety, or other substance use disorders. It was hypothesized that AUD teens would have smaller hippocampi than demographically similar controls.
2. Methods
2.1. Participants
Adolescents, ages 15 to 17, were recruited from local high schools, and included 14 teens who met DSM-IV criteria for alcohol abuse (n = 5) or dependence (n = 9) and 17 demographically similar non-abusing controls. Participants and their parents were telephone-screened for eligibility, as detailed elsewhere (Tapert et al., 2003). This study was conducted in accordance with and approved by the University of California San Diego Institutional Review Board, and written consent/assent was obtained from teens and their legal guardians. Exclusionary criteria were as follows: a lifetime history of a DSM-IV substance use disorder other than AUD or psychiatric disorder (determined by a positive diagnosis from the parent or youth version of the Diagnostic Interview Schedule for Children-4.0) (Shaffer et al., 2000), serious medical problems or neurological illness (e.g., seizure disorder, migraine); head trauma with loss of consciousness > 2 min; learning disability; current use of medications that could affect the central nervous system; smoking > four cigarettes per day; significant maternal drug use or drinking (≥ 4 drinks per occasion or ≥ 7 drinks per week) during pregnancy; parental history of bipolar I or psychotic disorders (determined by the Family History Assessment Module screener; Rice et al., 1995); left-handedness; pregnancy (verified with urine test); non-fluent English skills; sensory impairments; and MRI contraindications. In addition, no teen had an estimated IQ<80. Of the 729 teens who responded to recruitment efforts, many were ineligible due to psychiatric symptomatology or treatment (n = 80), other drug involvement (n = 42), braces (n = 41), history of a neurological disorder or head trauma (n = 30), or a diagnosis of a learning disability (n = 12). Beyond those who were ineligible, many teens were not included in the study because they did not meet clear inclusion criteria for either group (e.g., moderate alcohol use), lost interest or were unable to be contacted beyond the initial screening, or were a potential control but did not match demographic characteristics of the AUD group. Because of high comorbidity with substance use disorders (Brown et al., 1996; Myers et al., 1998), teens meeting criteria for both AUD and DSM-IV mild or moderate conduct disorder (n = 5) were not excluded from study.
Groups were demographically similar except that families of AUD teens had significantly higher household incomes (P = 0.025), although all teens came from middle to upper class families (see Table 1). AUD teens also endorsed greater levels of self-reported anxiety on the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1970) and depression on the Beck Depression Inventory (Beck, 1978), despite all scores falling well within the subclinical range (see Table 1).
Table 1.
Participant demographic and substance use characteristics
| AUD (n = 14), mean (S.D.) [range] or ratio/% | Controls (n = 17), mean (S.D.) [range] or ratio/%) | P | |
|---|---|---|---|
| Age | 16.75 (0.68) [15.17−17.66] | 16.46 (0.88) [15.33−17.92] | NS |
| Gender ratio (female/male) | 5:9 | 7:10 | NS |
| Ethnicity ratio (Caucasian/non-Caucasian) | 14:0 | 14:3 | 0.098 |
| Body weight (pounds) | 147.86 (24.32) [104−190] | 154.00 (40.10) [110−260] | NS |
| Grades completed | 10.07 (0.83) [8−11] | 9.71 (0.85) [8−11] | NS |
| Parent annual salary (thousands) | 150.77 (68.34) [55−280] | 99.69 (31.15) [46−150] | 0.025 |
| % Family history negative for AUD | 35.71 | 41.18 | NS |
| % Conduct disorder positive | 35.71 | 0.00 | 0.007 |
| Spielberger State-Anxiety T-score | 43.08 (9.07) [33.11−63.91] | 25.47 (5.09) [30.03−47.48] | 0.021 |
| BDI total scorea | 5.31 (5.04) [0−19] | 1.94 (2.28) [0−8] | 0.021 |
| CBCL externalizing T-scoreb | 42.65 (4.35) [38.13−51.47] | 43.29 (6.25) [38.13−58.13] | NS |
| Age of first regular drinkingc | 14.90 (1.10) [14−17] | N/A | N/A |
| Years of regular drinkingc | 2.03 (0.91) [0.58−3.50] | 0.00 (0.00) [0−0] | NS |
| Lifetime alcohol use episodesd | 137.38 (144.45) [45−505] | 3.06 (5.25) [0−15] | 0.004 |
| Typical peak BAC, past 3 months | 0.109 (0.087) [0.022−0.353] | 0.008 (0.020) [0−0.078] | 0.001 |
| Drinks/month, past 3 months | 43.00 (31.89) [3−108] | 1.53 (4.49) [0−18] | < 0.0001 |
| Days since last drinke | 16.71 (15.03) [5−60] | 66.50 (67.66) [5−180] | 0.077 |
| Lifetime alcohol withdrawal or hangover symptoms | 2.14 (1.92) [0−6] | 0.07 (0.31) [0−1] | 0.125 |
| Lifetime DSM-IV abuse and dependence criteria met | 4.43 (1.51) [2−7] | 0.24 (0.56) [0−2] | 0.000 |
| % who smoked cigarettes in past month | 42.86 | 5.88 | 0.012 |
| Lifetime marijuana use episodes | 11.00 (12.52) [0−40] | 0.47 (1.28) [0−5] | 0.008 |
| Lifetime other drug use episodes | 0.79 (2.67) [0−10] | 0.00 (0) [0−0] | NS |
NS=non-significant group mean difference.
Beck Depression Inventory.
Child Behavior Checklist.
Self-reported drinking once a week (excludes 4 AUD participants who denied weekly drinking).
Number of lifetime alcohol use episodes was assessed at the time of screening (up to several months before the scan procedure).
Only includes 4 controls with drinking experience.
Lifetime and past 3-month substance involvement, withdrawal symptomatology, and DSM-IV abuse and dependence criteria were assessed using the Customary Drinking and Drug Use Record (Brown et al., 1998), which has good internal consistency, test–retest, and inter-rater reliability with adolescents (Brown et al., 1998; Stewart and Brown, 1995). Both controls and teens with AUD had limited usage of drugs other than alcohol (see Table 1), had not used marijuana for at least 12 days before scanning, and had not used other drugs for 30 days before scanning. Participants with AUD were primarily weekend binge drinkers, reflected by an average 16.71 days since last use (most recent use was 5 days before study) and estimated typical peak blood-alcohol concentration (BAC) of 0.109. Two AUD teens reported no drinking in the month before the scan. Participants were asked to abstain from alcohol and other drugs for at least 48 h before scanning and provided Breathalyzer (Intoximeter, Inc., St. Louis, MO) and urine samples for toxicology screening. All breath and urine samples were negative, confirming self-reported recent abstinence.
2.2. Neuropsychological assessment
All teens underwent a neuropsychological assessment by a trained psychometrist, typically conducted the same day as neuroimaging, and included the Vocabulary subtest from the Wechsler Intelligence Scale for Children-Third Edition (Wechsler, 1993) for teens under age 17 or from the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981) for 17-year-olds; the Rey–Osterrieth Complex Figure Test copy and long delay (Rey–O) (Osterrieth, 1944); the California Verbal Learning Test-Children's version (CVLT-C) (Delis et al., 1994); and the Reading subtest from the Wide Range Achievement Test-Third Edition (WRAT-3) (Wilkinson, 1993).
2.3. Magnetic resonance imaging (MRI) acquisition
Participants were imaged in a 1.5 Tesla General Electric scanner. The protocol consisted of a high-resolution structural image collected in the sagittal plane using an inversion recovery prepared T1-weighted 3D spiral fast spin echo (Wong et al., 2000) sequence (TR = 2000 ms, TE = 16 ms, FOV = 240 mm, resolution = 0.9375 × 0.9375 × 1.328 mm, 128 continuous slices, acquisition time = 8:36). No sedation was used, all participants complied well with procedures, and no abnormal anatomies were detected by a neuroradiologist.
2.4. Image processing
Due to significant individual variability in overall brain size (Giedd et al., 1996a), gray matter, white matter, and hippocampal volume, measurements were examined as a ratio to overall intracranial volume and, separately, with intracranial volume statistically controlled in analyses of covariance (ANCOVA). Intracranial volumes were determined using a semiautomated hybrid skull-stripping method (Segonne et al., 2004) in addition to manual editing. Tissue volume estimates for white and gray matter were acquired using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain's (FMRIB) automated segmentation tool (FAST), which is based on a hidden Markov random field model and an associated expectation–maximization algorithm (Zhang et al., 2001). All manual editing and hippocampal tracings were performed using the Analysis of Functional NeuroImages package (Cox, 1996) by independent raters without knowledge of participant group. Good inter- and intra-rater reliabilities were established before data collection: for skull-stripping, inter- and intra-rater intraclass correlation coefficients (ICC) = 0.99; for right hippocampal tracings, inter-rater ICC = 0.96 and intra-rater ICC = 0.95; for left hippocampal tracings, inter- and intra-rater ICCs = 0.96.
Hippocampal tracings were manually performed on contiguous 1.3-mm slices in the coronal plane, perpendicular to the anterior commissure/posterior commissure plane, and included the dentate gyrus, subiculum, and cornu ammonis. Right and left hippocampal volumes were calculated by multiplying voxel counts by associated voxel dimensions. The criteria used for defining boundaries of the hippocampus are detailed elsewhere (Nagel et al., 2004). Briefly, the anterior boundary of the hippocampus was identified as the coronal slice that cut through the fullest portion of the mamillary bodies. The superior and lateral borders of the hippocampus were demarcated by the temporal horn and alveus. The ventral boundary was delineated by the white matter of the parahippocampal gyrus. Medially, hippocampal boundaries were defined by the ambient cistern. Posteriorly, the hippocampus was bounded by the columns of the fornix (see Fig. 1 for pictorial examples of the boundaries).
Fig. 1.
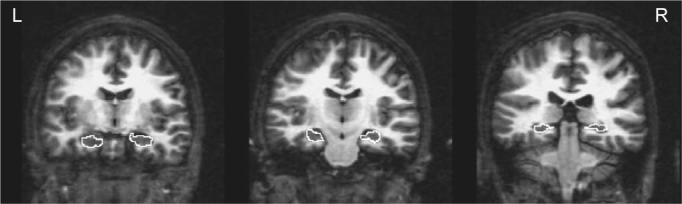
Examples of anterior, middle, and posterior hippocampal boundary delineation on a control participant's coronal T1 image.
2.5. Statistical analysis
Normality of data distributions was examined using Shapiro and Wilk's W statistic. The only non-normally distributed variable (white matter to intracranial volume ratio) was due to one significant male control outlier (> 3 S.D. below the mean) whose data were subsequently removed from white matter analyses. Between-group differences on demographic variables were examined using t-tests (for continuous variables) and chi-square analyses (for categorical variables). Pearson correlation coefficients revealed no associations between demographic variables and the dependent variables of right and left hippocampal volumes. Spearman rank-order correlations were used to examine the relationship between conduct disorder severity and right and left hippocampal volumes. Since no covariates were needed in the analyses, hippocampal volumes were compared between adolescents with and without AUD by t-tests. To examine the possibility of a group (AUD and control) by side (right and left hippocampal volume) interaction, we used a repeated measures analysis of variance (ANOVA). Equality of variance was assessed with Levene's test, and if indicated, the significance value associated with unequal variance was used. A follow-up t-test compared the two groups after excluding teens with comorbid conduct disorder. Group differences in hippocampal volumes were also examined with analysis of covariance (ANCOVA) controlling for intracranial volume.
3. Results
3.1. Brain volumes
Adolescents with AUD had significantly smaller left hippocampal volume ratios (expressed as a ratio to intracranial volume) than healthy matched control teens (t = 2.90, df = 29, P < 0.01; Cohen's d = 1.08) (see Fig. 2), while no statistically significant differences were present between groups for right hippocampal volume ratios (Fig. 3) (t = 0.51, df = 29, P = 0.61), intracranial volume (t = −0.67, df = 29, P = 0.51), or overall gray (t = 0.06, df = 29, P = 0.95) and white matter (t = −1.20, df = 28, P = 0.24) volume ratios (see Table 2). There was a significant group by hippocampal side interaction (F = 8.34, df = 29, P < 0.01), suggesting a reverse right/left hippocampal volume asymmetry between the AUD and control groups. After excluding the five AUD teens with comorbid conduct disorder, left hippocampal volume ratios remained significantly smaller in the AUD group (t = 1.71, df = 24, P < 0.05; Cohen's d = 0.70). Group left hippocampal volume differences were also significant when examined using ANCOVA and covarying for intracranial volume (F = 6.81, df = 28, P = 0.01).
Fig. 2.
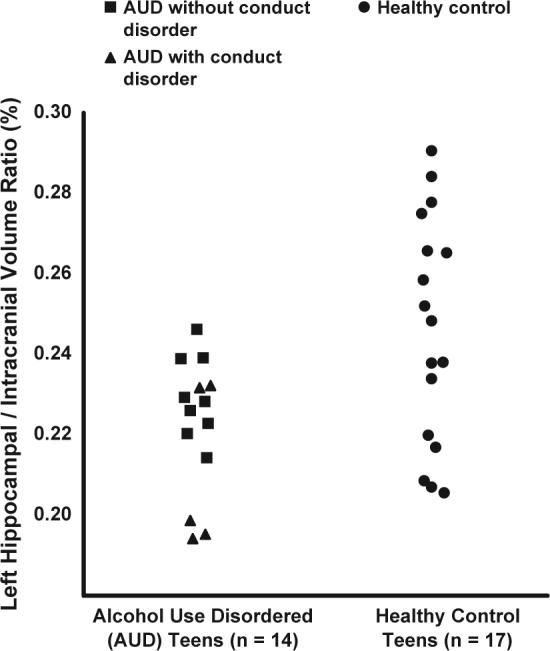
Left hippocampal volumes expressed as a ratio to overall intracranial volumes in AUD and healthy control teens. Ratios of left hippocampal volume to overall intracranial volume (%) were significantly smaller in teens with alcohol use disorder than in healthy control teens (t = 2.90, df = 29, P < 0.01).
Fig. 3.
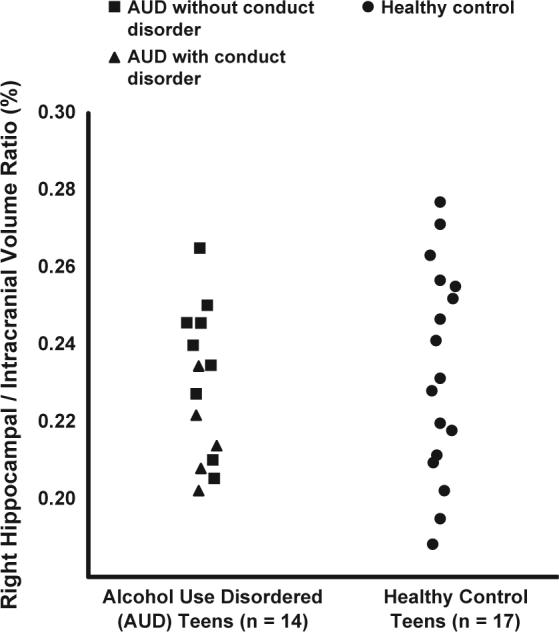
Right hippocampal volumes expressed as a ratio to overall intracranial volumes in AUD and healthy control teens. Ratios of right hippocampal volume to overall intracranial volume (%) did not differ significantly between teens with alcohol use disorder and healthy control teens.
Table 2.
Brain volumes of AUD and healthy control teens (in cm3)
| AUD (n = 14), mean (S.D.) | Controls (n = 17), mean (S.D.) | P | |
|---|---|---|---|
| Intracranial volume | 1619.64 (96.04) | 1592.37 (123.95) | NS |
| White mattera | 494.28 (50.95) | 468.64 (54.51) | NS |
| Gray mattera | 744.99 (66.61) | 729.73 (60.51) | NS |
| Right hippocampusa | 3.71 (0.33) | 3.71 (0.47) | NS |
| Left hippocampusa | 3.60 (0.32) | 3.92 (0.52) | 0.007 |
NS=non-significant group mean difference.
Values statistically examined as ratio to intracranial volume.
3.2. Demographic, behavioral, and alcohol characteristics
Right and left hippocampal volumes were not significantly associated with any demographic or behavioral characteristics, including family history of substance use disorders. Further, among teens with AUD, there were no significant relationships between hippocampal volumes and alcohol-involvement characteristics, including age of onset of regular drinking, years of regular drinking, drinks consumed per month, alcohol withdrawal symptoms, estimated typical peak BAC, or lifetime number of abuse/dependence criteria. Among teens with AUD (n = 14), there was no significant relationship between conduct severity (0 for absence, 1 for mild, 2 for moderate) and left hippocampal volume; however, there was a trend toward a significant negative relationship between conduct severity and right hippocampal volume (r = −0.46, P = 0.10).
3.3. Cognitive performance
No significant differences were found between groups on any neuropsychological measure. In addition, there were no significant correlations between neuropsychological performance and right or left hippocampal, intracranial, or gray or white matter volumes.
4. Discussion
This study replicated and extended the results of a previous study examining brain morphology associated with AUD in adolescents. Here, we examined teens who were free from other substance use disorders and psychiatric disorder, with the exception of conduct disorder, and observed significantly smaller left hippocampal volumes among adolescents with AUD than non-abusing matched control teens. Even after exclusion of teens with comorbid AUD and conduct disorder from the analyses, teens with AUD alone continued to show smaller left hippocampal volumes than controls. Unlike the first study to examine structural neuropathology in teens with AUD (De Bellis et al., 2000), the current study did not demonstrate smaller volumes of the right hippocampus among adolescents with AUD. Consistent between the two studies were statistically similar overall cerebral, gray matter, and white matter volumes between youth with and without AUD.
One possible explanation for reduced hippocampal volume in adolescents with AUD may relate to N-methyl-d-aspartate (NMDA) neurotoxicity in the hippocampus. Animal models have demonstrated that ethanol-induced suppression of NMDA receptor-mediated synaptic potentials and long-term potentiation is greater in hippocampal slices taken from juvenile rats than adult rats (Pyapali et al., 1999; Swartzwelder et al., 1995a,b). In addition, rats given a brief ethanol binge during periadolescence showed similar hippocampal electrophysiological abnormalities as rats exposed during adulthood, despite shorter periods of ethanol exposure among the juvenile rats (Slawecki et al., 2001), thus providing support for the hypothesis that human adolescents are especially vulnerable to alcohol-related neurotoxicity in the hippocampus. However, particularly in light of other findings in the current study, there is reason to suspect alternative explanations for the aberrant hippocampal volumes in the AUD group.
Some findings from the current study suggest that perhaps the group differences in hippocampal volume may relate to premorbid developmental factors as opposed to being due entirely to alcohol-related neurotoxicity. In contrast to findings by De Bellis et al. (2000), the current analyses demonstrated no significant correlation between alcohol use characteristics (i.e., age of first regular drinking, years of regular drinking) and hippocampal volume. As these characteristics are inherent in the diagnosis of AUD, one would expect such a correlation to exist if the differences in hippocampal volume were truly attributable to the AUD itself.
Another notable feature of the current study was the failure to replicate past findings of volumetric differences in the right hippocampus between teens with and without AUD. It is important to note that while the previous study in this area evaluated youths with multiple types of psychiatric and substance use comorbidities (De Bellis et al., 2000), the current investigation attempted to remove such sources of variance from the AUD sample. While limited work has been done in adolescent populations, previous research has demonstrated bilaterally reduced hippocampal volumes in adolescents with depression compared with healthy control peers (MacMaster and Kusumakar, 2004), reduced right temporal lobe volume in adolescents with conduct disorder (as well as other psychiatric comorbidities) compared with controls (Kruesi et al., 2004), and reduced right hippocampal volume in adults with PTSD compared with controls (Wignall et al., 2004). Further, while the data did not suggest AUD and control group differences in right hippocampal volume, there was a trend toward a significant negative correlation between conduct disorder severity and right hippocampal volume. These findings may partially explain why the previous study that included subjects with comorbid depression, conduct disorder, and PTSD in their AUD sample (De Bellis et al., 2000) demonstrated reduced right hippocampal volume (in addition to left), while the present study examining teens without psychiatric comorbidity did not.
Surprisingly, there were no significant differences between AUD and control teens on any test of memory performance. This finding is inconsistent with a study suggesting that adolescents with AUD demonstrate verbal and spatial memory impairments compared with healthy controls (Brown et al., 2000); however, the findings from that study were based on a larger sample of teens recruited from treatment facilities with greater severity of AUD symptomatology. Moreover, despite abnormal left hippocampal volumes among AUD teens, neither right nor left hippocampal volumes significantly related to memory functioning in this sample. A recent meta-analysis revealed that, among typically developing adolescents, there is a negative relationship between hippocampal volume and memory performance (Van Petten, 2004). However, the author suggests that if we look to the aging and neuropsychological literature, smaller hippocampal volumes are associated with impaired memory performance when there is reason to suspect hippocampal tissue loss as opposed to premorbid developmental differences in hippocampal size. These findings again raise the question of whether the difference in hippocampal volume between AUD and control teens observed in the current study reflects alcohol neurotoxicity or pre-existing developmental or comorbid differences between adolescents with and without AUD.
While this study had the benefit of clean and well-matched groups, there are several limitations to consider. First, statistical analyses were constrained by a small sample size, and we had insufficient power to examine the influence of gender. As males and females differ in neurodevelopment (Giedd et al., 1999) and vulnerability to alcohol effects on the brain (Hommer et al., 2001; Mann et al., 1992; Schweinsburg et al., 2003), future studies should examine gender differences. By excluding youths with comorbid psychiatric and substance use disorders, this study captures a subset of adolescents with AUD who have above average socioeconomic backgrounds and verbal intelligence, which may not be representative of adolescents with AUD in the overall population. An anomalous finding with regards to left versus right hippocampal asymmetry should be noted. While past research suggests that a right greater than left hippocampal volume asymmetry is more common in normally developing populations (Giedd et al., 1996b), this study found right greater than left volumes only in our AUD sample. Although the atypical symmetry pattern in control teens may be a function of small sample size, it may have contributed to the group difference in left hippocampal volumes or lack of group difference in right hippocampus, and thus should be considered as a potential limitation of the study.
These findings raise several questions to be addressed by further research. First, although not supported in the current study, previous work has suggested a relationship between drinking severity and hippocampal volumes in youths (De Bellis et al., 2000). Thus, longitudinal studies could elucidate the structural changes associated with continued heavy drinking in these youths, as well as the potential for neural recovery among those who sustain abstinence. Further, as observed in the current study, others have also demonstrated no relationship between hippocampal volumes and familial risk for AUD among adolescents (Hill, 2004), challenging future studies to examine other premorbid factors that may help to explain smaller hippocampal volumes in adolescents with AUD. To this end, longitudinal investigations of children and adolescents at risk for developing AUD should attempt to identify the behavioral and neurocognitive characteristics that may mediate the relationship between hippocampal volume and AUD. By understanding the neural vulnerabilities and consequences of AUD, as well as the functional implications, prevention programs may be able to better target youths at risk for AUD, and intervention efforts may more efficiently treat adolescents with drinking problems.
Acknowledgements
This work was supported by grants R21 AA12519 and R01 AA13419 from the National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD (Dr. Tapert) and the UCSD Fellowship in Biological Psychiatry and Neuroscience (Dr. Nagel). The authors thank M.J. Meloy, Ph.D., Randy Notestine, Christine Fennema-Notestine, Ph.D., Korak Sarkar, and Erick Cheung for their valuable contributions to this research.
References
- Beck AT. Beck Depression Inventory (BDI) Psychological Corp.; San Antonio, TX: 1978. [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. American Journal of Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Brown SA, Gleghorn AA, Schuckit MA, Myers MG, Mott MA. Conduct disorder among adolescent alcohol and drug abusers. Journal of Studies on Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism, Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Clark DB, Bukstein O, Cornelius J. Alcohol use disorders in adolescents: epidemiology, diagnosis, psychosocial interventions, and pharmacological treatment. Paediatric Drugs. 2002;4:493–502. doi: 10.2165/00128072-200204080-00002. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test-Children's Version. Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- DSM-IV . Diagnostic and Statistical Manual of Mental Disorders, Fourth edition. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural Brain Research. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4−18. Cerebral Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4−18 years. Journal of Comparative Neurology. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I. Alcohol and the brain: neuropsychological correlates. Journal of Consulting and Clinical Psychology. 1987;55:310–324. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- Hill SY. Trajectories of alcohol use and electrophysiological and morphological indices of brain development: distinguishing causes from consequences. Annals of the New York Academy of Sciences. 2004;1021:245–259. doi: 10.1196/annals.1308.029. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Jernigan T, Schafer K, Butters N, Cermak L. Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology. 1991;4:174–186. [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. The Monitoring the Future National Survey Results on Adolescent Drug Use: Overview of Key Findings, 2003. National Institute on Drug Abuse; Bethesda, MD: 2004. [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Progress in Neurobiology. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Research: Neuroimaging. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Medicine. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Batra A, Gunthner A, Schroth G. Do women develop alcoholic brain damage more readily than men? Alcoholism, Clinical and Experimental Research. 1992;16:1052–1056. doi: 10.1111/j.1530-0277.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcoholism, Clinical and Experimental Research. 1994;18:159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcoholism, Clinical and Experimental Research. 2000;24:1510–1516. [PubMed] [Google Scholar]
- Myers MG, Stewart DG, Brown SA. Progression from conduct disorder to antisocial personality disorder following treatment for adolescent substance abuse. American Journal of Psychiatry. 1998:155. doi: 10.1176/ajp.155.4.479. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Palmer SL, Reddick WE, Glass JO, Helton KJ, Wu S, Xiong X, Kun LE, Gajjar A, Mulhern RK. Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. American Journal of Neuroradiology. 2004;25:1575–1582. [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. Journal of Clinical Psychiatry. 2004;65:11–17. [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Archives of Psychology. 1944;30:206–356. [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism, Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Psychiatric comorbidity with problematic alcohol use in high school students. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:101–109. doi: 10.1097/00004583-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I. Effects of alcoholism and gender on brain metabolism. American Journal of Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Peri-adolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Developmental Brain Research. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism, Clinical and Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcoholism, Clinical and Experimental Research. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcoholism, Clinical and Experimental Research. 1995;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-III. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Annals of the New York Academy of Sciences. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Wignall EL, Dickson JM, Vaughan P, Farrow TF, Wilkinson ID, Hunter MD, Woodruff PW. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biological Psychiatry. 2004;56:832–836. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test-3 Administration Manual. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank RL. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society of Magnetic Resonance in Medicine. 2000;8:683. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]


