Abstract
Recent studies have described neuromaturation and cognitive development across the lifespan, yet few neuroimaging studies have investigated task-related alterations in brain activity during adolescence. We used functional magnetic resonance imaging (fMRI) to examine brain response to a spatial working memory (SWM) task in 49 typically developing adolescents (25 females and 24 males; ages 12−17). No gender or age differences were found for task performance during SWM. However, age was positively associated with SWM brain response in left prefrontal and bilateral inferior posterior parietal regions. Age was negatively associated with SWM activation in bilateral superior parietal cortex. Gender was significantly associated with SWM response; females demonstrated diminished anterior cingulate activation and males demonstrated greater response in frontopolar cortex than females. Our findings indicate that the frontal and parietal neural networks involved in spatial working memory change over the adolescent age range and are further influenced by gender. These changes may represent evolving mnemonic strategies subserved by ongoing adolescent brain development.
Keywords: Adolescent, Functional MRI, Cognition, Neuropsychology, Gender, Development
INTRODUCTION
Modern neuroimaging techniques have provided a wealth of information about human brain development. Whereas it was once believed that the human brain was largely developed by the onset of puberty, it has now been established that the brain continues to develop throughout adolescence and well into adulthood (Durston et al., 2001; Giedd, 2004; Sowell et al., 2003). A recent longitudinal investigation demonstrated that higher order association cortices, such as superior temporal, posterior parietal, and prefrontal cortex, develop later than primary sensorimotor cortices, with the dorsolateral prefrontal cortex developing last (Gogtay et al., 2004). This later occurring development is predominantly a function of the progressive and regressive processes of myelination and synaptic pruning that result in increasing white matter volumes and cortical thinning (Huttenlocher, 1990; Paus et al., 1999) and a more efficient central nervous system.
During adolescence and this time of active neural maturation, many cognitive processes are also developing. One such process is working memory. Working memory refers to the ability to actively store and manipulate information online over brief periods of time (Baddeley, 1986). This ability is fundamental to intact performance in a variety of other cognitive domains, including language comprehension, abstract reasoning, and learning and memory (Baddeley, 1992; Gathercole, 1999). Verbal and spatial working memory abilities improve throughout childhood and adolescence (Gathercole et al., 2004; Luna et al., 2004), with accuracy and reaction times increasing and decreasing respectively during spatial n-back (Kwon et al., 2002; Vuontela et al., 2003) and spatial delayed response tasks (Zald & Iacono, 1998). It is likely that these behavioral improvements in working memory are the result of the described neuromaturational processes that are occurring during the child and adolescent years.
With the advent of functional magnetic resonance imaging (fMRI), the neural substrates of working memory functioning have begun to be identified. Adult studies of working memory have consistently revealed prefrontal and posterior parietal cortical activation in response to intact performance during working memory tasks (for review, see Wager & Smith, 2003). In contrast to the large number of fMRI studies in adult populations, very few studies have examined fMRI response to working memory tasks in typically developing adolescents, and most have focused on the development of spatial (as opposed to verbal) working memory. The few studies examining fMRI response during verbal and spatial working memory in children and adolescents suggest that, overall, children and adolescents demonstrate similar frontal and parietal patterns of response as adults (Casey et al., 1995; Thomas et al., 1999), but show greater (Klingberg et al., 2002; Kwon et al., 2002) and more widespread (Kwon et al., 2002) activation in these regions with increasing age. To our knowledge, only two of these studies have examined fMRI response to working memory across a sample of typically developing adolescents. One study of spatial working memory (SWM) among 34 7- to 22-year-olds suggested age-related increases in both the intensity and spatial extent of SWM activation in bilateral dorsolateral prefrontal cortex, left ventrolateral prefrontal cortex, left premotor cortex, and bilateral superior and inferior posterior parietal cortices (Kwon et al., 2002). However, although age was the best predictor of activation in these brain regions, there were significant improvements in SWM performance across the study age range that may have contributed to age-related activation patterns. Another study examined SWM in 13 9- to 18-year-olds and demonstrated increased neural response in bilateral superior frontal and intraparietal cortex and left middle occipital gyrus, and decreased intensity of response with age in right inferior frontal cortex (Klingberg et al., 2002), but no significant relationship between age and the spatial extent of brain response was demonstrated. Thus, although we have some understanding of the developmental changes in the neural systems involved in adolescent working memory, these studies are preliminary and are based on small sample sizes across relatively broad age ranges.
Likely due to limited statistical power, to date, no studies have examined gender differences in fMRI response to cognitive tasks across normal adolescent development. Despite this, previous neuroanatomical and cognitive research suggests that developmental gender differences may be present in SWM activation. Specifically, there are established gender differences in the rate of neural development, with females developing earlier than males in frontal and parietal brain regions (Giedd et al., 1999), which have been consistently implicated in working memory (Wager & Smith, 2003). In addition, gender differences in working memory ability have been identified, specifically for SWM skills. Although adult studies have demonstrated a general spatial information processing advantage for males over females that emerges with increasing age (Voyer et al., 1995), this is primarily a result of differences in active spatial processing (e.g., spatial rotation or manipulation) (Vecchi & Girelli, 1998), which is often not required in traditional n-back or delayed matching working memory tasks. Studies of SWM abilities suggest gender differences for accuracy and reaction time in children (Vuontela et al., 2003) and adults (Barn-field, 1999; Duff & Hampson, 2001; Loring-Meier & Halpern, 1999). Overall, these studies indicate that adult females demonstrate more accurate SWM performance than adult males (Barnfield, 1999; Duff & Hampson, 2001), but males tend to show faster reaction times (Loring-Meier & Halpern, 1999). One investigation suggested a similar profile of gender differences for SWM performance in children that diminishes towards adolescence (Vuontela et al., 2003), and another SWM study in adolescents found no performance differences between the genders (Barnfield, 1999). Although the pattern of findings is somewhat difficult to interpret based on the different tasks and samples used across studies, it does suggest that gender discrepancies in SWM performance may vary based on visuospatial processing demands and stage of development.
Given that the majority of developmental working memory research using fMRI has focused on SWM, we chose to further contribute to this literature by utilizing a relatively large sample of normally developing teens to carefully investigate the neural substrates involved in SWM across adolescent development and between the genders using fMRI. Based on the findings from the limited previous research in the area, we predicted that working memory brain activation would increase in frontal and parietal regions as a function of age. In addition, based on known differences in rates of neuromaturation and a potential female advantage in SWM accuracy, we hypothesized that females would demonstrate a more mature pattern of fMRI response than males.
METHODS
Research Participants
Adolescent participants were recruited from local junior high and high schools as part of an ongoing adolescent brain imaging project (Tapert et al., 2003, 2004). This study was approved by the University of California San Diego Institutional Review Board, and written consent and assent were obtained from teens and their guardians. Adolescents were administered a 90-minute telephone screening interview to ascertain eligibility, and a guardian (usually a parent), separately provided corroborative reports. Exclusion criteria for the study were: use of psychotropic medications; head injury with loss of consciousness >2 minutes; neurological or medical illness; learning disabilities; DSM-IV (American Psychiatric Association, 1994) psychiatric disorder including attention deficit hyperactivity disorder and substance use disorders; significant maternal drinking during pregnancy (≥4 drinks/day or ≥7 drinks/week); parental history of bipolar I, psychotic disorders or substance use disorders; left handedness; and MRI contraindications. Eligible participants were 49 youth ages 12 to 17, including 24 males and 25 females. Males and females were similar on demographics such as age, ethnicity, and socioeconomic status (Table 1).
Table 1.
Participant characteristics
| Mean (SD) or % |
||
|---|---|---|
| Males (n = 24) | Females (n = 25) | |
| Years of age (range: 12 to 17) | 15.06 (1.72) | 14.49 (1.73) |
| Caucasian | 75% | 80% |
| Annual household income (thousands) | 87.13 (37.31) | 91.67 (51.72) |
| Grade point average | 3.46 (0.51) | 3.66 (0.44) |
| Number of grades completed | 8.39 (1.64) | 7.80 (1.53) |
| Vocabulary (T-score)a | 57.71 (7.33) | 57.80 (9.12) |
| Digit Span (T-score)a | 49.80 (8.28) | 51.08 (9.69) |
| Block Design (T-score)a | 56.92 (8.65) | 55.25 (9.19) |
Subtest from the Wechsler Intelligence Scale for Children—Third Edition (Wechsler, 1993) and the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) for those under age 17, or from the Wechsler Adult Intelligence Scale—Third Edition (Wechsler, 1997) for 17-year-olds.
Measures
Spatial working memory task
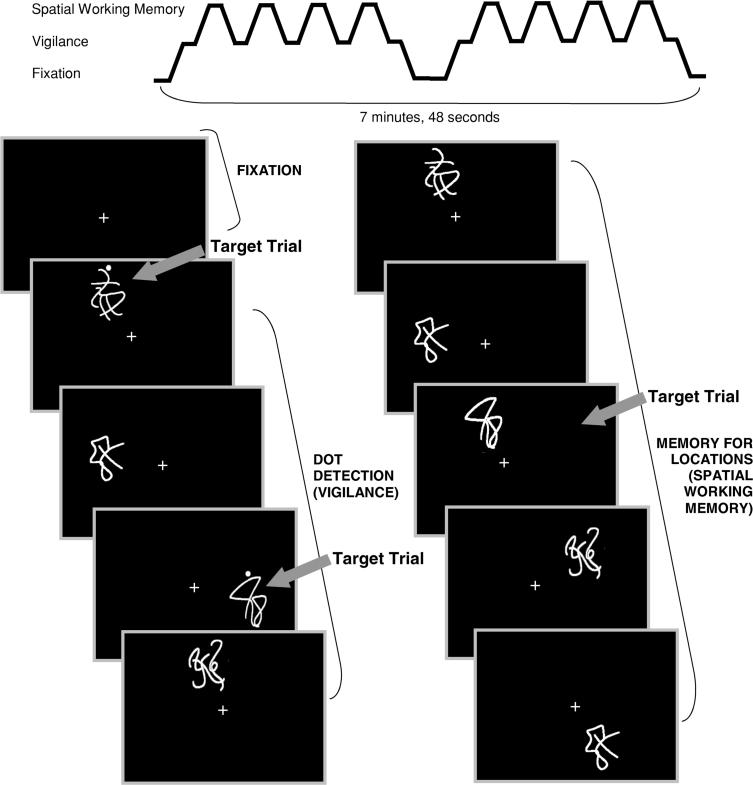
The spatial working memory (SWM) task (Kindermann et al., 2004; Tapert et al., 2001) consisted of 18 21-second blocks that alternated between experimental and baseline conditions, and three blocks of rest (two 21-second blocks and one 42-second block). The task also included six seconds of blank screen at the beginning (not analyzed), allowing the scanner to reach steady state. Total task time was 7 minutes and 48 seconds (see Figure 1). Each block started with a one-second word cue at the center of the screen to inform the participant of the upcoming block type. Stimuli were presented for 1000 ms, and each interstimulus interval was 1000 ms. During rest blocks, the word “LOOK” appeared at the center of the screen, then a centered fixation cross appeared for 20 seconds. The experimental (spatial working memory) condition was a memory for locations task in which abstract line drawings (Kimura figures) were projected one at a time in one of eight locations in a circular array. Locations were chosen to minimize verbal labeling (e.g., not in the four cardinal compass points). The word “WHERE” appeared for one second at the beginning of the block, and participants were asked to press a button when a figure appeared in a location in which a design had already appeared during that block. Unbeknownst to participants, target trials were always repeat locations of items displayed two trials prior (2-back). In each block, an average of 3 of the 10 stimuli presented were target items. During the vigilance baseline condition, the word “DOTS” appeared at the beginning of the block to alert participants to the block type. Then the same abstract line drawings used in the SWM blocks were presented one at a time in the same eight locations, but a dot appeared above figures on 30% of trials. Participants were asked to press a button when they saw a design with a dot. The purpose of the baseline condition was to control for the motor, sensory, and attention processes involved in the experimental condition.
Fig. 1.
Spatial working memory task design.
Neurocognitive ability
To examine potential gender differences in neurocognitive performance, all teens were administered the Vocabulary, Digit Span, and Block Design subtests from the Wechsler Intelligence Scale for Children–Third Edition (Wechsler, 1993) and the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) for teens under age 17, and from the Wechsler Adult Intelligence Scale–Third Edition (Wechsler, 1997) for 17-year-olds.
Pubertal maturation
Given the variability in the onset and timing of pubertal development, chronological age can be an inaccurate indicator of biological maturation. For this reason, all teens completed the Pubertal Development Scale (PDS)—a five-item self-report measure of pubertal status with demonstrated reliability and validity (Petersen et al., 1988). The PDS correlates significantly (r = .61 to .80) with physician ratings and Sexual Maturation Scale self-ratings of pubertal maturation (Brooks-Gunn et al., 1987).
Procedures
To minimize head motion in the scanner, a soft cloth was placed on the participant's forehead then taped to the head tray, and foam pads were inserted around the head. Task stimuli were projected onto a screen at the foot of the MRI bed, and participants viewed the images from a mirror attached to the head coil. An MRI-safe button box collected responses during the task.
Anatomical and functional imaging data were acquired on a 1.5 Tesla General Electric Signa LX system. Structural imaging consisted of a sagittally acquired inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (repetition time = 2000 ms, echo time = 16 ms, field of view = 240 mm, resolution = 0.9375 × 0.9375 × 1.328 mm, 128 continuous slices, acquisition time = 8:36) (Wong et al., 2000). During task presentation, functional imaging was collected in the axial plane using T2*-weighted spiral gradient recall echo imaging (repetition time = 3000 ms, echo time = 40 ms, flip angle = 90°, field of view = 240 mm, 20 slices covering the whole brain, slice thickness = 7 mm, in-plane resolution = 1.875 × 1.875 mm, 156 repetitions, acquisition time = 7:48).
Data Analyses
SWM task accuracy and reaction time were examined in relationship to age using regression analyses. Gender differences in task performance were analyzed using within-subjects analysis of variance (ANOVA) with task condition (SWM vs. vigilance performance) as the within subjects factor and gender as the between subjects factor. Because age did not significantly relate to task performance, it was not used as a covariate in the ANOVA. Significant interactions were followed up with t tests to examine simple effects. Neuropsychological test performance was analyzed using regression analyses examining age, gender, and their interaction.
Imaging data were processed and analyzed using Analysis of Functional NeuroImages (AFNI) (Cox, 1996). First, we applied a motion-correction algorithm to align each volume in the time series with a base volume, yielding three rotational and three displacement parameters across the time series for each participant. Two independent raters inspected time series data to remove any repetitions on which the algorithm did not adequately adjust for motion; all participants retained at least 80% of repetitions. Using a deconvolution process (Ward, 2002), the time series data were correlated with a vector representing the design of the task (see Figure 1) that modeled 1- and 2-repetition delays in hemodynamic response (Bandettini et al., 1993), and covaried for estimated degree of motion and linear trends. This process yielded fit coefficients representing the blood oxygen level dependent (BOLD) response contrast between SWM and vigilance, SWM and fixation, and vigilance and fixation in each voxel for every subject. Imaging datasets were transformed into standard Talairach coordinates for structure localization and comparisons among subjects (Lancaster et al., 2000; Talairach & Tournoux, 1988), and functional data were resampled into 3.5-mm3 isotropic voxels. We applied a spatial smoothing Gaussian filter (full-width half maximum = 3.5 mm) to functional data to account for anatomic variability.
Group-level analyses conducted regressions in each voxel of the brain to predict the fit coefficient representing the contrast between SWM and vigilance from gender, age, and their interaction. To control for Type I error when determining clusters that showed significant effects, we used a combination of t-statistic magnitude and cluster volume thresholding (Forman et al., 1995; Ward, 1997) by only interpreting clusters exceeding 943 microliters, equal to 22 contiguous significant (α < .05) 3.5-mm3 voxels, yielding a clusterwise α < .05. To understand the nature of these group level clusters, we performed exploratory follow-up analyses (uncorrected) examining contrasts between SWM and fixation and vigilance and fixation in each significant cluster. We utilized the Talairach Daemon (Lancaster et al., 2000; Ward, 1997) and AFNI (Cox, 1996) to confirm gyral labels for significant clusters.
To examine the role of pubertal maturation on SWM BOLD response, mean fit coefficients for each participant were computed for each significant activation cluster, and hierarchical regression analyses determined whether PDS scores explained significant variance in brain response above and beyond that explained by chronological age.
Planned follow-up analyses examined whether age, gender, and their interaction were related to spatial extent of neural response in brain regions showing significant age-related activation during SWM relative to vigilance. Because we were particularly interested in age-related changes in the spatial extent of activation during SWM (and not in regions showing less SWM response than vigilance response), we only examined clusters in which there was greater activation to SWM relative to vigilance, and not regions that were deactivated by the task. Therefore, we created posterior parietal and left prefrontal regions of interest (ROI), and counted the number of voxels exhibiting significantly greater activation to SWM relative to vigilance for each participant. In order to best represent functionally important frontal and parietal regions within this sample of adolescents, our ROIs were determined by identifying significant clusters activated by the task, rather than anatomically defined based on specific gyri or Brodmann's Areas (BA).
Because regression analyses indicated a change in location of parietal activation across adolescence (see Results), an ROI based on the average activation map for the whole group would not accurately represent regions used for the task in both young and old teens (Passarotti et al., 2003). Therefore, we divided teens on age with median (14.93 years) split, and determined significant clusters activated by the task in young and old teens separately using single sample t tests (cluster volume ≥ 943 microliters, p < .05). This yielded separate posterior parietal clusters for young and old teens. We created a posterior parietal ROI for examining spatial extent of activation by including all voxels that occupied the posterior parietal clusters for young teens and old teens, and all voxels within clusters showing a significant positive or negative relationship to age. A similar procedure determined an appropriate left prefrontal ROI. Because young teens did not demonstrate significant clusters of left prefrontal activation, significant clusters activated by the task in older teens were added to the average left prefrontal cluster showing a significant relationship with age. To assess volume of activation within posterior parietal and left prefrontal ROIs, we calculated the number of voxels showing significantly greater (p < .025) activation during SWM relative to vigilance for each participant within each ROI. We then performed regression analyses to predict volume of activation from age, gender, and their interaction.
RESULTS
Behavioral Performance
SWM task performance data were available for 47 participants (button box failed during two examinations). Teens performed at 95.61 ± 2.54% accuracy on the vigilance condition and 88.87 ± 8.35% accuracy on SWM [F(1,45) = 30.44, p < .001]. There was no significant gender difference nor was there a gender × task condition interaction for task accuracy. Participants responded slower to vigilance (636.63 ± 73.12 ms) than to SWM (605.00 ± 70.01 ms; F(1,45) = 10.54, p < .005). Males' overall reaction time (601.11 ± 59.72 ms) was faster than females' (641.38 ± 59.71 ms; F(1,45) = 5.34, p < .025). A gender × condition interaction was found for reaction time (p < .05); while both males and females performed faster on SWM than vigilance, the difference was greater for females (607.14 ± 67.21 ms on vigilance and 595.08 ± 70.02 ms on SWM for males; 667.41 ± 67.21 ms on vigilance and 615.36 ± 70.02 ms on SWM for females), and males responded faster than females during vigilance [t(45) = 3.07, p < .005]. Age was negatively associated with vigilance reaction time (r = 2.292, p < .05). There were no significant gender differences on the Wechsler Vocabulary, Digit Span, or Block Design subtest scores (see Table 1).
Movement
To determine whether movement during fMRI scanning might affect results, we examined relationships between age and bulk motion in two ways. Both total number of removed repetitions and average movement in each direction throughout the task (i.e., roll, pitch, yaw, superior, left, posterior) were examined in relation to age and gender using correlational analyses. The number of repetitions removed for excessive motion during the task declined with age (r = −.44, p < .01). However, in brain regions demonstrating a relationship between SWM response and age, number of removed repetitions did not significantly relate to brain response (all p's > .025), and the relationship between age and brain response in each cluster remained significant after controlling for number of removed repetitions. Mean rotational and translational motion were not significantly related to age. The average rotational movement throughout the task was 0.07, 0.22, and 0.09 degrees for roll, pitch, and yaw, respectively; the average translational movement was 0.16, 0.06, and 0.09 mm for superior, left, and posterior, respectively. There were no significant gender differences for number of repetitions removed for movement or on any directional movement parameter, with the exception of males demonstrating significantly greater rotational motion than females in the pitch direction [t(1,47) = −2.08, p < .05].
fMRI Response
Main effect for age
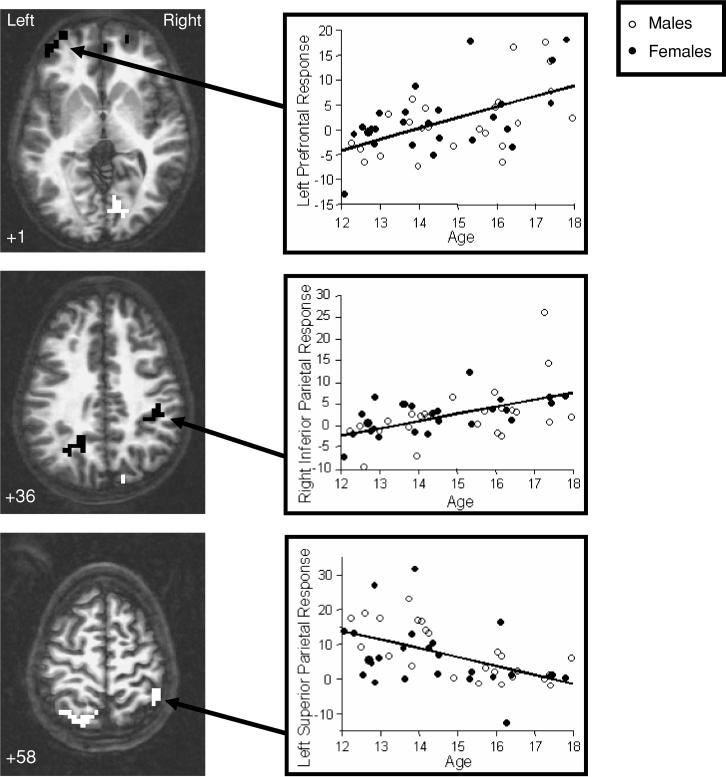
Age positively predicted SWM brain response in bilateral medial portions of superior frontal gyrus (BA 10); left superior and middle frontal gyri (BA 9, 10); inferior aspects of the left precuneus and angular gyrus (BA 31); and a cluster encompassing the right inferior parietal lobule, postcentral gyrus, and insula (BA 40, 2, 13; p's < .05; see Table 2 and Figure 2). A negative relationship between age and SWM response was observed in: the left superior frontal gyrus (BA 11), left precuneus and superior parietal lobule (BA 7), superior portions of the right inferior parietal lobule (BA 40), and the right lingual gyrus (BA 18). Exploratory follow-up analyses revealed that in the medial superior frontal cluster, teens evidenced less response during SWM than during vigilance, with younger youths showing greater vigilance response than older teens. Further, in the right lingual gyrus, youths demonstrated less response during SWM than during rest (SWM deactivation), with older teens showing a greater decrease in SWM response relative to fixation (i.e., more deactivation) than younger teens (uncorrected p's < .05). In the left superior frontal gyrus (BA 11), most participants showed no significant response to SWM relative to vigilance.
Table 2.
Regions showing significant SWM brain response changes in adolescence
| Brodmann's Area | Volume (μl) | Talairach coordinates |
Effect size Cohen's d | |||
|---|---|---|---|---|---|---|
| Anatomic region | x | y | z | |||
| Main Effect for Age | ||||||
| Positive Relationship | ||||||
| Bilateral medial superior frontal gyrus | 10 | 2401 | 2r | 52a | 7s | 1.40 |
| Left superior & middle frontal gyri | 9, 10 | 1972 | 30l | 62a | 3s | 1.45 |
| Left inferior precuneus | 31 | 1415 | 26l | 61p | 35s | 0.83 |
| Right inferior parietal lobule, postcentral gyrus, insula | 40, 2, 13 | 1501 | 44r | 29p | 35s | 0.85 |
| Negative Relationship | ||||||
| Left superior frontal gyrus | 11 | 1158 | 12l | 62a | 8i | −0.77 |
| Left precuneus & superior parietal lobule | 7 | 1200 | 5l | 57p | 63s | −1.33 |
| Right inferior parietal lobule | 40 | 943 | 44r | 43p | 52s | −1.17 |
| Right lingual gyrus | 18 | 2358 | 5r | 78p | 3s | −1.10 |
| Main Effect for Gender | ||||||
| Males > Females | ||||||
| Right anterior cingulate | 24, 32 | 1072 | 2r | 31a | 4i | 15.50 |
| Right superior frontal gyrus | 10, 11 | 986 | 9r | 66a | 11i | −11.01 |
| Age × Gender Interaction | ||||||
| Right superior frontal gyrus | 10, 11 | 1029 | 9r | 66a | 11i | 0.69 |
Talairach coordinates and Cohen's d refer to maximum signal intensity group difference within the cluster; R, right; L, left; A, anterior; P, posterior; S superior; I, inferior.
Fig. 2.
Brain regions showing significant relationships between age and fMRI response to spatial working memory relative to vigilance across adolescence. Black clusters indicate areas showing a positive relationship between age and fMRI response, and white regions represent clusters showing a negative relationship between age and fMRI response (p < .05, cluster volume > 943 microliters). Numbers below images refer to axial slice positions.
Main effect for gender
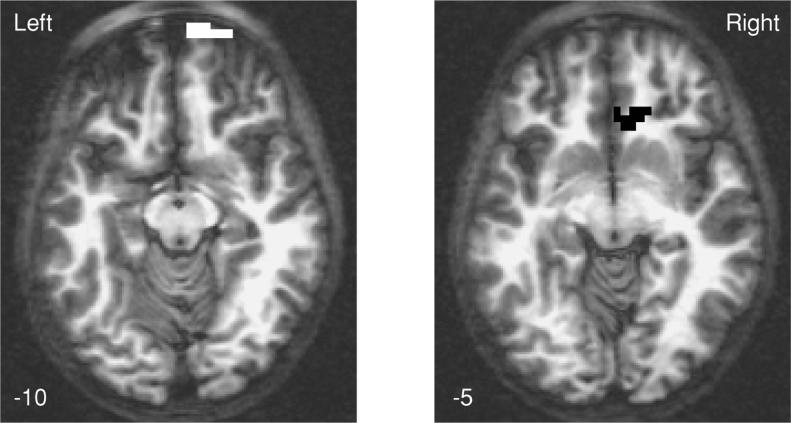
Males showed more SWM brain response than females in the right frontopolar superior frontal gyrus (BA 10, 11) and right anterior cingulate (BA 24, 32; p's < .05; see Table 2 and Figure 3). Follow-up analyses revealed that females showed reduced response to SWM relative to vigilance in the anterior cingulate.
Fig. 3.
Brain regions showing significant relationships between gender and fMRI response to spatial working memory relative to vigilance in adolescents. Black cluster indicates area where girls showed greater vigilance fMRI response than boys; white region represents cluster where boys showed more SWM fMRI response than girls (p < .05, cluster volume > 943 microliters). Numbers below images refer to axial slice positions.
Age × Gender Interaction
A significant age × gender interaction was observed in the right frontopolar superior frontal gyrus (BA 10, 11), in the same location as the gender difference described above (see Table 2). In this cluster, males showed a negative relationship between age and SWM response, but females showed a positive relationship.
fMRI and task performance
To understand whether age and gender related differences in BOLD response could be accounted for by task performance (SWM or vigilance accuracy or reaction time), we examined mediational models using a series of regressions (Baron & Kenny, 1986; Judd & Kenny, 1981). As vigilance reaction time was the only task performance index related to age or gender, regression analyses examined whether it mediated the relationship between age or gender and BOLD response in any of the clusters listed in Table 2. Vigilance reaction time was not significantly related to brain response in any region that was related to age or gender, and therefore did not mediate the relationship between age or gender and BOLD response.
Pubertal development
Age and PDS scores were highly correlated (r = .77, p < .001). However, even after entering age into the model, PDS score significantly negatively related to BOLD response in the superior right parietal cluster (BA 40, 7) [F(2,46) = 11.57, p < .001; β = −.40, p < .05; R2Δ = .07]. PDS scores did not explain variance above and beyond the age relationship in any other activation cluster listed in Table 2.
Spatial extent of frontal and parietal brain response
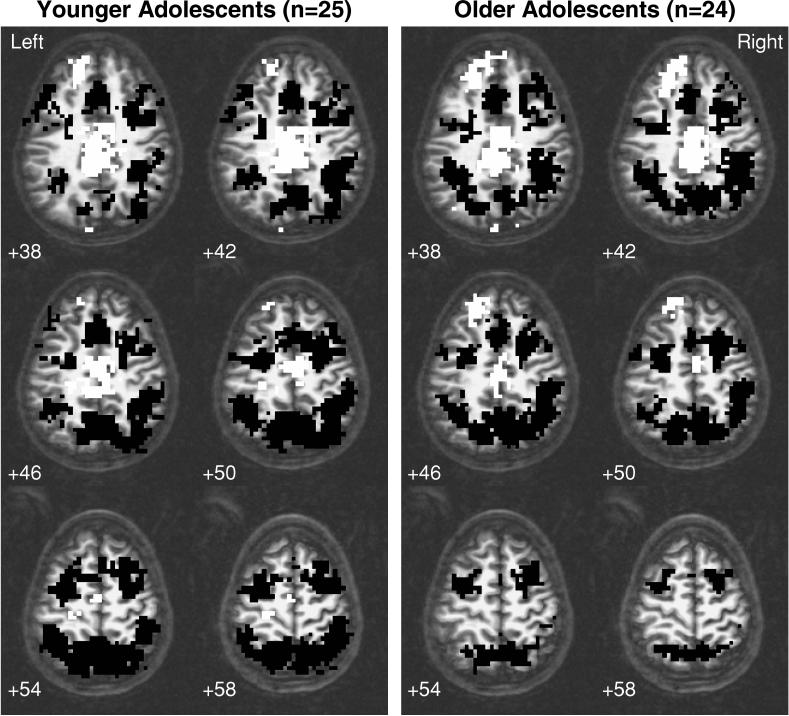
The posterior parietal ROI encompassing areas demonstrating significant activation to SWM relative to vigilance in young and old youths was 96,876 microliters, and spanned bilateral portions of the precuneus and superior and inferior parietal lobules. Within that cluster, ROI analyses demonstrated that activation for young adolescents was mostly in superior regions of the parietal cortex, whereas response for old teens was mostly in inferior parietal areas (see Figure 4). Although age and the age × gender interaction did not predict volume of parietal activation within the combined parietal ROI, males demonstrated larger volumes of activations than females [12,348 ± 7,643 microliters for females, 16,828 ± 7,217 microliters for males, F(1,43) = 5.62, p < .025]. Furthermore, males showed a significant negative relationship between age and volume of activation (r = −.41, p < .05), whereas females showed no relationship between age and volume of response. Within the left prefrontal ROI (94,607 microliters in size), age significantly predicted the volume of activation, with larger volumes of activation demonstrated by older teens [F(1,43) = 4.95, p = .03]. There was no significant effect of gender or the age × gender interaction on the volume of left prefrontal activation.
Fig. 4.
Brain regions showing significant fMRI response to spatial working memory relative to vigilance among older adolescents (>14.93 years) and younger adolescents (<14.93 years). Black clusters indicate areas where teens showed greater fMRI response to spatial working memory than vigilance, and white regions represent clusters where teens showed less fMRI response to spatial working memory than vigilance (p < .05, cluster volume > 943 microliters). Numbers below images refer to axial slice positions. Note that while both groups of adolescents demonstrate posterior parietal activation, localization of response is more superior among younger youths.
DISCUSSION
This cross-sectional study examined the effects of age and gender on brain response during a SWM task among 12- to 17-year-olds. In general, we observed comparable task performance across the age range and between genders, and all teens showed typical response patterns for SWM, with activation in bilateral prefrontal and posterior parietal cortices. This pattern parallels adult activation during spatial working memory tasks (for a review, see Wager & Smith, 2003) and supports occipitoparietal, or “dorsal stream,” processing of spatial locations (e.g., Ungerleider & Haxby, 1994), suggesting that, in general, teens use similar working memory and spatial processing strategies as adults. However, specific localization and intensity of response varied across the adolescent age range, and males and females showed slightly different activations. These differential patterns emerged despite similar task performance across the age range and between genders, suggesting that developmental changes in SWM brain response are driven by factors other than task performance.
Behavioral Performance
In contrast to the literature suggesting that SWM abilities on n-back tasks improve across the adolescent age range (Kwon et al., 2002), we did not observe age-related improvements in performance on our SWM task. Although this was likely a result of the low difficulty level of the task used (only 8 spatial locations and 2-back working memory load), which approached ceiling effects, it is a benefit to the neuroimaging component of this study as it prevented confounding performance effects on the neural activation patterns observed across this age range.
Although task performance was not related to age, teens performed more accurately on vigilance than SWM, yet reaction times were faster on SWM. Our previous studies using this task demonstrated similar findings, with slightly faster performance on SWM than vigilance (Tapert et al., 2001, 2004). While the reason for this difference is unclear, it could be that the small visual discrimination necessary for dot detection during vigilance blocks is more time consuming than the broader location detection required during SWM blocks. Future studies should attempt to eliminate this difference in reaction times between experimental and control conditions, perhaps by designing a task with easier visual discrimination (e.g., Kwon et al., 2002).
fMRI Response and Age
As hypothesized, age positively predicted SWM activation within the prefrontal cortex. Specifically, age was positively associated with both the intensity and extent of brain response in the left middle and superior frontal gyri (BA 9, 10). This cluster spanned frontopolar cortex but also encompassed parts of dorsolateral prefrontal cortex. Frontopolar prefrontal cortex activation has been associated with subgoal processing (Braver & Bongiolatti, 2002) and evaluation of internally generated information (Christoff & Gabrieli, 2000; Christoff et al., 2003). Thus, older teens may invoke more self-generated strategies, including rule induction or more efficient retrieval processes. This cluster also included portions of dorsolateral prefrontal cortex, which has been consistently implicated in working memory tasks. Adult studies have suggested that prefrontal activation is often left-lateralized during verbal working memory tasks (Wager & Smith, 2003); thus the greater left prefrontal response among older teens may suggest that older teens employ more verbal rehearsal strategies during the task than younger adolescents. Although the task was designed to minimize verbal encoding, older teens may have imagined the eight possible stimulus locations as positions on a clock face, thereby facilitating verbal labeling and resulting in greater left prefrontal activity.
Also consistent with our hypotheses, we observed a positive relationship between age and SWM response in posterior parietal regions. However, while we detected a positive relationship between age and SWM activation in bilateral inferior parietal regions, including inferior aspects of the right precuneus and left inferior parietal lobule, our data also revealed a negative relationship between age and brain response in bilateral superior parietal cortex, comprising superior portions of the right inferior parietal lobule, left precuneus, and left superior parietal lobule. Exploratory ROI analyses confirmed these findings, demonstrating that while both young and old teens evidenced overlapping posterior parietal activation, younger youths showed activation mostly in superior regions, but older teens showed activation mostly in inferior regions. Together, these results indicate a shift from superior to inferior parietal areas utilized during SWM across adolescence. Previous fMRI studies of SWM have suggested that parietal activation intensity increases across adolescence (Klingberg et al., 2002; Kwon et al., 2002), yet small sample sizes and different task designs may have prevented the observation of additional negative relationships identified in the current study. Although functional parcellation of parietal involvement in subcomponents of spatial working memory is largely unknown, some researchers of adult populations have suggested that superior parietal regions may be important for spatial rehearsal during working memory (Wager et al., 2004), whereas inferior parietal regions may be implicated in short-term storage during working memory (Smith & Jonides, 1998). Therefore, the superior to inferior shift in parietal activation across adolescence could represent a change in spatial working memory strategies. Younger adolescents may rely more on spatial rehearsal, which could become more automated throughout adolescence, requiring less superior parietal activation. Along those lines, older adolescents may be better able to engage inferior parietal regions involved with spatial storage, and rely less on spatial rehearsal. Moreover, if older adolescents are employing greater verbal rehearsal strategies, as discussed earlier, then spatial rehearsal may be less efficient, and therefore utilized to a lesser degree.
In addition, stage of pubertal development was negatively associated with response in the superior right inferior parietal lobule cluster, above and beyond the effects of chronological age. Previous literature has demonstrated the impact of sex hormones on the development of cerebral lateralization (e.g., Diamond, 1991), and pubertal timing has been related to functional asymmetry (Nikolova et al., 1994). Similarly, in this study, right parietal maturation appears linked to pubertal stage whereas left parietal development is not, suggesting asymmetrical cortical development that may be hormonally influenced. This finding points to the importance of individual variation in biological maturation that may not be accounted for by chronological age, and suggests that indices of pubertal development may further characterize neural maturation and help explain changes in SWM brain response patterns and cognitive strategies across adolescence.
As well as showing changing fMRI response patterns to SWM tasks across adolescence (Klingberg et al., 2002; Kwon et al., 2002), previous adolescent research has also demonstrated age-related increases in the spatial extent of frontal and parietal SWM activation (Kwon et al., 2002). We found a greater number of significantly activated voxels in left prefrontal cortex with increasing adolescent age, suggesting that in some regions, both the magnitude of response and the volume of significant activation increase across adolescence. However, the results of our spatial extent analysis in posterior parietal cortex showed no significant relationship between age and volume of significant SWM response. Taken together with our results demonstrating age-related regional changes in the intensity of activation, these findings suggest that in late developing frontal brain regions, intense and more widespread activation emerges, whereas in slightly earlier developing posterior parietal networks, there is a focal shift in localization of activity. When examined in light of the adult working memory literature, adolescent age-related changes in frontal and parietal networks involved in SWM support the evolution of more efficient cognitive strategies.
In the lingual gyrus, we observed deactivation (reduced activation during SWM compared to rest) that increased with age. Occipital deactivations are thought to relate to perceptual priming as information is reprocessed (e.g., Cabeza & Nyberg, 2000). Increasing occipital deactivation across adolescence could represent enhanced priming, and therefore greater recognition and reprocessing of repeated spatial locations among older youths.
Teens in this study also demonstrated less response in the medial superior frontal cortex during SWM than during the vigilance condition, yet this discrepancy dissipated across adolescence, such that this area was no longer “under active” in older teens. Medial frontal cortex is highly active at rest, during which it is involved in attentional monitoring of various internal and external stimuli (McKiernan et al., 2003). Medial frontal cortex under-activation during a cognitive task may represent reallocation of limited attentional resources to areas directly involved in task performance (Lawrence et al., 2003; McKiernan et al., 2003). Thus, SWM task demands may be more difficult for younger youths, who require greater attentional allocation to maintain performance, and therefore greater under-activation of medial frontal cortex.
fMRI Response and Gender
This is the first known fMRI study to attempt to examine the role of gender in relation to the neural substrates involved in SWM across adolescent development. Although our findings do not entirely support the hypothesis that females would evidence more mature SWM response patterns than males, several interesting gender-specific findings suggest that males and females utilize slightly different brain regions to perform well on a SWM task. Specifically, females demonstrated more right anterior cingulate response during the vigilance condition than did males. The anterior cingulate has been implicated for its role in attentional control and conflict monitoring (Luks et al., 2002; MacDonald et al., 2000) and has been shown to be involved during adult SWM performance (Wager & Smith, 2003). Diminished anterior cingulate activity during a cognitive task may be related to reorganization of attentional resources as task demands arise (Lawrence et al., 2003; McKiernan et al., 2003). Such cingulate deactivation was observed only in females in this study, suggesting that adolescent females may require greater reallocation of attentional resources than males during SWM. Males consistently perform better than females on visuospatial tasks (for a review, see Voyer et al., 1995). Further, recent fMRI studies have demonstrated gender-specific activation patterns during mental rotation, theorizing that females use more detail-oriented analytic strategies, whereas males use more “gestalt” perceptual strategies (Jordan et al., 2002; Thomsen et al., 2000; Weiss et al., 2003). Thus, anterior cingulate deactivation among females in this study could represent greater attentional demand to maintain performance. In addition to gender differences in cingulate activation, males in this study evidenced greater activation in right frontopolar cortex. Further, an interaction between age and gender was observed in the frontopolar cluster, with activation in this region decreasing with age in males and increasing with age in females. The right frontopolar cortex has been associated with SWM in adults (Manoach et al., 2004), and has also received attention for its more general role in subgoal processing and integration during working memory tasks, as well as more efficient retrieval during episodic memory (Braver & Bongiolatti, 2002; Christoff et al., 2003). Such activation among males may indicate a more economical strategy to achieve task demands. This could preclude the need for increased attentional control, and therefore anterior cingulate deactivation, as demonstrated in females. Furthermore, the age-related decrease in frontopolar activity among males may reflect more efficient processing as development progresses, whereas the age-related increase among females could indicate increased ability to reallocate attention from extraneous regions to task-relevant areas, including the frontopolar cortex. Although it remains unclear as to when in the course of development the male advantage on spatial tests emerges, meta-analysis has indicated that this gender difference appears some time in early adolescence and increases with age (Voyer et al., 1995). Similarly, the observed gender differences in brain response in this study could represent the emergence of sexually dimorphic activation patterns and cognitive strategies as neural maturation progresses.
fMRI Response and Task Performance
It is critical to interpret fMRI results in the context of task performance. We therefore examined whether performance indices mediated the relationship between age or gender and SWM BOLD response. Although vigilance reaction time was negatively associated with age, it did not predict brain response in any region where age and BOLD response were related. This suggests that age-related differences in brain response represent changes in neural utilization and strategy, rather than behavioral alterations. Likewise, boys had faster vigilance reaction times than girls, yet vigilance reaction time was not related to brain response in either region demonstrating a gender difference in SWM BOLD response. This provides evidence that gender differences in brain response are related to gender differences in neurocognitive features other than those manifested behaviorally.
Limitations and Future Directions
This study is the first of its kind to utilize a well-stratified adolescent age sample with enough power to detect gender differences; however, it remains limited by its cross-sectional design. Longitudinal investigations are the only accurate way to depict evolving neural networks involved in SWM during adolescent development. The study was also limited by a sample size that may not have been sufficient to detect more subtle variations between the genders. In addition, participants in the current study came from relatively high-income families, which may not accurately represent the general population. Motion during scanning is a concern for all functional neuroimaging studies, and constraints of existing motion management programs are a limitation. Furthermore, while this SWM task was used because it has been previously reported on by our group in adolescents (Caldwell et al., 2005; Schweinsburg et al., 2005; Tapert et al., 2004) and young adults (Tapert et al., 2001), the high accuracy on both conditions in this study suggests a potential ceiling effect among healthy adolescents. A more difficult task, with greater working memory load and/or more spatial locations, could elicit age-related performance differences and elucidate different patterns of functional development. Moreover, the fact that teenagers performed somewhat faster during the spatial working memory condition than during the vigilance baseline condition warrants consideration. Although this discrepancy has been observed in our previous studies with this task (Tapert et al., 2001, 2004), it is not clear whether reaction time differences may have contributed to fMRI findings. Therefore, future tasks should be designed in attempt to equate reaction times for experimental and control conditions. Finally, future investigations might attempt to more objectively characterize pubertal development using biological assays, as opposed to retrospective self-report measures that can be influenced both by participant recollection of pubertal events and willingness to disclose information.
Conclusions
In sum, the present findings suggest that significant age-related changes occur across adolescence in the neural networks involved in SWM, and highlight the importance of using developmentally appropriate models for understanding SWM in youths. Specifically, the emergence of left prefrontal activation and superior to inferior shift in parietal response across development suggest that older teens rely less on rote spatial rehearsal and employ more verbally-mediated procedures. In addition, this is the first known study to demonstrate gender differences in neural response to SWM across adolescent development, indicating gender-related strategic differentiation in adolescence. Greater frontopolar response in males may suggest more efficient subgoal processing and episodic encoding, whereas diminished anterior cingulate activation among females could indicate attentional reallocation to maintain performance. In addition to employing longitudinal methods of investigation, future developmental fMRI studies should be aimed at further parceling the component processes of working memory, as well as describing gender-specific developmental trajectories in neural strategy. Better characterization of the neuroanatomical substrates of working memory development may improve understanding of adolescent populations impacted by working memory dysfunction.
ACKNOWLEDGMENTS
Portions of this study were presented at the annual meeting of the International Neuropsychological Society, February 5−8, 2003, Honolulu, Hawaii. This research was supported by the National Institute on Alcohol Abuse and Alcoholism grants R21 AA12519 and R01 AA13419 and National Institute on Drug abuse grant DA15228 to S. F. Tapert, and the UCSD Fellowship in Biological Psychiatry and Neuroscience to B. J. Nagel. The authors express appreciation to Valerie Barlett, Lisa Caldwell, Lauren Killeen, Dr. Greg Brown, Dr. Sandra A. Brown, Dr. Sandra Kindermann, Dr. M. J. Meloy, and Dr. Martin Paulus for their assistance with this project.
REFERENCES
- American Psychiatric Association . DSM-IV: Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford University Press; Oxford: 1986. [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Barnfield AM. Development of sex differences in spatial memory. Perceptual and Motor Skills. 1999;89:339–350. doi: 10.2466/pms.1999.89.1.339. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Development. 1987;58:829–841. [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol and Alcoholism. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JD. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behavioral Neuroscience. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Diamond MC. Hormonal effects on the development of cerebral lateralization. Psychoneuroendocrinology. 1991;16:121–129. doi: 10.1016/0306-4530(91)90074-4. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A sex difference on a novel spatial working memory task in humans. Brain and Cognition. 2001;47:470–493. doi: 10.1006/brcg.2001.1326. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: What have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Cognitive approaches to the development of short-term memory. Trends in Cognitive Sciences. 1999;3:410–419. doi: 10.1016/s1364-6613(99)01388-1. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, U.S.A. 2004;17:17. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wustenberg T, Heinze HJ, Peters M, Jancke L. Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia. 2002;40:2397–2408. doi: 10.1016/s0028-3932(02)00076-3. [DOI] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Process analysis: Estimating mediation in evaluation research. Evaluation Review. 1981;5:602–619. [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophrenia Research. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences, U.S.A. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. Journal of Cognitive Neuroscience. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Loring-Meier S, Halpern DF. Sex differences in visuo-spatial working memory: Components of cognitive processing. Psychonomic Bulletin and Review. 1999;6:464–471. doi: 10.3758/bf03210836. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL. Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage. 2002;17:792–802. [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manoach DS, White NS, Lindgren KA, Heckers S, Coleman MJ, Dubal S, Holzman PS. Hemispheric specialization of the lateral prefrontal cortex for strategic processing during spatial and shape working memory. Neuroimage. 2004;21:894–903. doi: 10.1016/j.neuroimage.2003.10.025. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Nikolova P, Stoyanov Z, Negrev N. Functional brain asymmetry, handedness and menarcheal age. International Journal of Psychophysiology. 1994;18:213–215. doi: 10.1016/0167-8760(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: An fMRI study. Developmental Science. 2003;6:100–117. [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-dimensional proportional system: An approach to cerebral imaging. Thieme; New York: 1988. Coplanar stereotaxic atlas of the human brain. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown GG, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thomsen T, Hugdahl K, Ersland L, Barndon R, Lundervold A, Smievoll AI, Roscher BE, Sundberg H. Functional magnetic resonance imaging (fMRI) study of sex differences in a mental rotation task. Medical Science Monitor. 2000;6:1186–1196. [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Vecchi T, Girelli L. Gender differences in visuo-spatial processing: The importance of distinguishing between passive storage and active manipulation. Acta Psychologica (Amst) 1998;99:1–16. doi: 10.1016/s0001-6918(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Vuontela V, Steenari MR, Carlson S, Koivisto J, Fjallberg M, Aronen ET. Audiospatial and visuospatial working memory in 6−13 year old school children. Learning and Memory. 2003;10:74–81. doi: 10.1101/lm.53503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 1997. [Google Scholar]
- Ward BD. Deconvolution analysis of FMRI time series data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 2002. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children–III. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale–III. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Weiss E, Siedentopf CM, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, Felber S, Fleischhacker WW, Delazer M. Sex differences in brain activation pattern during a visuospatial cognitive task: A functional magnetic resonance imaging study in healthy volunteers. Neuroscience Letters. 2003;344:169–172. doi: 10.1016/s0304-3940(03)00406-3. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank LR. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society for Magnetic Resonance in Medicine. 2000;8:683. [Google Scholar]
- Zald DH, Iacono WG. The development of spatial working memory abilities. Developmental Neuropsychology. 1998;14:563–578. [Google Scholar]