Abstract
Aims
To determine how alcohol use differentially affects brain functioning in male and female adolescents.
Methods
Adolescents with alcohol use disorders (AUDs; 7 female, 11 male) and control adolescents without AUDs (9 female, 12 male), aged 14−17 years, performed spatial working memory and vigilance tasks during functional magnetic resonance imaging.
Results
Gender, AUD and their interaction were significantly associated with brain activation patterns to the tasks. There were interactions in the superior frontal, superior temporal, cingulate and fusiform regions, in which female and male adolescents with AUDs showed a different brain response from each other and control subjects. Overall, female adolescents with AUDs showed a greater departure from normal activation patterns than male adolescents with AUD.
Conclusions
Adolescent alcohol involvement may affect male and female brains differently, and adolescent females may be somewhat more vulnerable to adverse alcohol effects. With continued drinking, these adolescents may be at an increased risk for behavioural deficits.
INTRODUCTION
The use of alcohol among adolescents represents a serious problem. By the 12th grade, 58% of adolescents have gotten drunk (Johnston et al., 2003) and 6% satisfy the diagnostic criteria for an alcohol use disorder (AUD) (SAMHSA, 2003). Adolescence is the only stage when drinking rates for females equal those for males (SAMHSA, 2003).
During this time of adolescent alcohol use, significant neurodevelopment occurs. While the size of the brain stabilizes by age 5 (Durston et al., 2001), the processes underlying cognitive development, such as myelination and synaptic pruning, occur throughout adolescence (Giedd et al., 1999; Durston et al., 2001), with males and females undergoing different rates of neuromaturation (Giedd et al., 1999). It is unclear how alcohol consumption interacts with gender to influence the developing brain.
Among adults, alcohol-dependent women appear somewhat more susceptible to brain and corpus callosum shrinkage (Mann et al., 1992; Hommer et al., 1996, 2001) as well as neuronal injury (Schweinsburg et al., 2003) than alcohol-dependent men. Although alcohol-dependent men and women have similar degrees of neuropsychological impairments, women generally have shorter drinking histories, suggesting a greater vulnerability to alcohol-related deficits (Nixon, 1994; Sullivan et al., 2002).
Animal studies suggest that the adolescent brain may be particularly vulnerable to the neurotoxic effects of alcohol (Smith, 2003). While few studies have examined the effects of heavy alcohol use on the developing human adolescent brain, neuropsychological studies demonstrate that adolescents with AUDs perform poorly on tests for verbal and non-verbal memory, attention, executive and visuospatial skills than their light-drinking peers (Tapert and Brown, 1999; Brown et al., 2000; Tapert et al., 2002). These impairments are similar to those seen in adults, but of lesser magnitudes and consistent with smaller durations of AUDs. However, the functional pathology underlying the reduced performance in adolescents is unclear. One study examining the hippocampi in adolescents with AUDs suggested smaller volumes of hippocampi compared with age-matched non-drinkers (De Bellis et al., 2000), although the influence of gender was not examined.
Functional magnetic resonance imaging (fMRI) studies have identified abnormalities in the brain activation patterns among adults with AUDs. Compared with controls, alcohol-dependent women (aged 18−25 years) demonstrated a diminished blood oxygen level dependent (BOLD) response in the frontal and parietal regions during a spatial working memory (SWM) task, as well as a poorer task performance (Tapert et al., 2001). Pfefferbaum et al. (2001) found a reduced BOLD response in adult male alcoholics in the prefrontal areas, but an increased response in the posterior and the inferior frontal cortex, indicating that the brain of older alcoholic males may functionally reorganize to achieve an adequate performance. Despite its utility in examining in vivo brain functioning, no fMRI study has examined the differential effects of AUD on brain activations in male and female adolescents.
The current study examined BOLD response differences in male and female adolescents with and without an AUD during an SWM task. Based on the adult literature and the limited research with adolescents, it was hypothesized that adolescent AUD would be associated with a poorer cognitive performance and less brain activation than the control subjects. Furthermore, based on studies suggesting a greater female vulnerability to alcohol neurotoxicity, it was predicted that females with AUDs would demonstrate a reduced BOLD response than male counterparts in areas subserving SWM, specifically the prefrontal and the posterior parietal cortices.
METHODS
Participants
Thirty-nine adolescents, aged 14−17 years, participated in the study: 18 (7 females, 11 males) were diagnosed with AUDs [i.e. met DSM-IV (American Psychiatric Association, 1994) criteria for alcohol abuse or dependence], and 21 (9 females, 12 males) were demographically similar adolescents without a history of substance use disorder (Table 1). Of the female adolescents diagnosed with AUDs, three satisfied the criteria for alcohol dependence and four for alcohol abuse. Five male adolescents with AUDs satisfied the criteria for alcohol dependence and six for alcohol abuse. The participants were compensated for their participation. The exclusion criteria were: history of head injury with loss of consciousness for >2 min, serious medical problems, neurological disorders, learning disabilities and axis I psychiatric disorders other than AUD or conduct disorder; current psychotropic medication use; lifetime marijuana use >100 times; lifetime other drug use >10 times; smoking >10 cigarettes/day; alcohol or drug use in the past 72 h; left-handedness; maternal drug use or drinking during gestation; family history of bipolar I or psychotic disorders; poor comprehension of English; sensory disorders; and MRI contraindications. Conduct disorder (n = 7) was not an exclusion criterion, due to high comorbidity with adolescent AUD (Brown et al., 1996; Myers et al., 1998).
Table 1.
Participant characteristics
| Female controls n = 9 mean (SD) | Male controls n = 12 mean (SD) | Female AUD n = 7 mean (SD) | Male AUD n = 11 mean (SD) | |
|---|---|---|---|---|
| Caucasian | 89% | 58% | 100% | 100% |
| Age (years) | 16.21 (1.11) | 16.61 (0.93) | 16.94 (0.76) | 16.60 (0.65) |
| Hollingshead socioeconomic status | 23.89 (10.07) | 22.82 (12.39) | 22.57 (16.54) | 27.27 (17.48) |
| WRAT3 Reading standard score | 107.89 (9.84) | 104.00 (6.48) | 107.71 (5.94) | 105.09 (8.68) |
| Pubertal Development Scale score | 3.87 (0.26) | 3.72 (0.30) | 3.86 (0.22) | 3.76 (0.23) |
| State Anxiety T-Score (gender normed)a | 38.09 (4.84) | 35.37 (4.24) | 49.58 (10.66) | 43.38 (9.38) |
| Alcohol use episodes, lifetimea | 7.56 (15.30) | 7.50 (12.27) | 105.14 (94.73) | 99.18 (120.26) |
| Drinks per month, past 3 monthsa | 1.33 (2.31) | 6.25 (7.27) | 53.14 (42.57) | 38.82 (24.87) |
| Average BAC when drinking, past 3 monthsa,b,* | 0.01 (0.01) | 0.03 (0.03) | 0.18 (0.09) | 0.08 (0.04) |
| Number of alcohol withdrawal symptoms, past 3 monthsa | 0.00 (0.00) | 0.13 (0.35) | 2.86 (1.57) | 2.55 (2.25) |
| Days since last alcohol usec | 120.00 (51.96) | 41.63 (48.89) | 10.71 (7.48) | 18.45 (16.46) |
| Years of regular alcohol use | — | — | 1.65 (0.85) | 2.05 (0.87) |
| Marijuana use episodes, lifetimea | 2.33 (6.63) | 2.67 (7.19) | 30.71 (33.96) | 24.45 (38.12) |
| Days per month marijuana use, past 3 monthsa | 0.00 (0.00) | 0.67 (1.56) | 4.80 (8.59) | 1.38 (1.79) |
| Days since last marijuana use | 539.00 (649.12) | 407.67 (520.46) | 262.40 (421.76) | 53.75 (44.60) |
| Drug use times per month, past 3 months | 0.00 (0.00) | 0.00 (0.00) | 4.40 (8.74) | 1.20 (1.64) |
| Days per month nicotine use, past monthc | 0.00 (0.00) | 0.58 (2.02) | 5.71 (5.35) | 2.09 (4.66) |
P < 0.05 for normal control vs AUD adolescents.
P < 0.05 for interaction between AUD diagnoses and gender.
P < 0.05 for gender, group and interaction.
Calculated based on self-report (Widmark, 1922; Fitzgerald, 1995).
Procedures
The procedures are detailed elsewhere (Tapert et al., 2003, 2004). Briefly, pamphlets were distributed at high schools and the respondents were administered a brief screening interview. If they were found to be eligible, written informed consent and assent, approved by the University of California San Diego Institutional Review Board, were obtained from the adolescents and their parents.
The participants and their parents were separately administered confidential structured clinical interviews (Brown et al., 1989) to ascertain demographic, medical, developmental history, and social and academic functioning. The Computerized Diagnostic Interview Schedule for Children version 4.0 (Shaffer et al., 2000) assessed for psychiatric diagnoses, the Family History Assessment Module (Rice et al., 1995) was used to diagnose family history of psychiatric disorders and the Pubertal Development Scale (Petersen et al., 1988) was used to stage pubertal development. The Customary Drinking and Drug Use Record (Brown et al., 1998) was used to evaluate the lifetime and the past 3-month alcohol, nicotine and other drug use, DSM-IV abuse and dependence criteria, withdrawal symptomatology, and other negative consequences. The Timeline Followback (Sobell and Sobell, 1992) was used to quantify the past 30-day substance use. Parents reported the familial socioeconomic status (Hollingshead, 1965) and completed the Child Behavior Checklist (Achenbach, 1991). On the day of the fMRI scan, the participants submitted samples for breathalyzer (Intoximeter, St Louis) and urine drug toxicology tests. State measures of mood (Beck, 1978), anxiety (Spielberger et al., 1970) and sleepiness (Glenville and Broughton, 1978) were obtained immediately before scanning. Reading level was assessed with the Wide Range Achievement Test-3 (Wilkinson, 1993) reading subtest. Right-handedness was confirmed with the Edinburgh Handedness Inventory (Oldfield, 1971).
fMRI acquisition
Imaging was conducted on Thursday evenings to facilitate maximal recovery from weekend drinking. The adolescents were trained on the task before scanning. Images were acquired on a 1.5 T GE Signa LX scanner. Scanning consisted of a high-resolution structural imaging (inversion recovery prepared T1-weighted sagittally acquired 3D spiral fast spin echo, TR = 2000, TI = 700, TE = 16, FOV = 240 mm, in-plane resolution = 0.9375 mm2, through-plane resolution = 1.328 mm for 128 continuous slices, 8:36) (Wong et al., 2000) and axially acquired BOLD-weighted spiral gradient recall echo imaging (TR = 3000, TE = 40, flip angle = 90°, FOV = 240 mm, 19−21 axial slices, slice thickness = 7 mm, reconstructed in-plane resolution = 1.875 mm2, 156 repetitions, 7:48).
Cognitive task
During fMRI data collection, an SWM task (Tapert et al., 2001; Kindermann et al., 2004) consisting of 18 blocks alternating between experimental and baseline conditions, was administered. In the experimental condition (SWM), abstract line drawings were sequentially presented in one of eight locations, chosen to minimize verbal labelling. Subjects pressed a button when a design appeared in a location already occupied within that block. Ten stimuli were presented per block, and 30% were repeats. In the baseline condition (vigilance), the same stimuli were presented in the same locations, but a dot appeared above the figure in 30% of the trials, and the subjects pressed a button when they saw a design with a dot. The baseline condition controlled for the brain response to the simple motor and visual attention processes involved in the experimental condition. In both conditions, the stimuli were presented for 1000 ms, and each interstimulus interval was 1000 ms (20 s per block). Three resting blocks occurred, during which a ‘+’ appeared at the centre of the screen.
fMRI analysis
Data were analysed with the Analysis of Functional NeuroImages (Cox, 1996). A 3D motion correction algorithm aligned each volume in the time series to a selected base volume (Cox and Jesmanowicz, 1999). A task reference vector that coded alternating conditions of the task was shifted forward in six 1-s increments to account for delays in haemodynamic responses (Bandettini et al., 1993). The time series data were correlated with this set of reference vectors, while covarying for linear trends and the motion adjustments, yielding fit coefficients for every subject in each voxel, representing the correspondence between the observed and hypothesized signal. Imaging results were transformed into standardized coordinates (Talairach and Tournoux, 1988) and resampled into cubic voxels (3.5 mm3), and a spatial smoothing Gaussian filter (full-width half maximum = 3.5 mm) was applied. Analyses of variance evaluated the influence of gender, AUD group and the interaction of the BOLD response to SWM relative to vigilance. Type I error was controlled by requiring that voxels surpass a specified alpha value (α = 0.05, two-tailed) and form clusters >943 μl (Forman et al., 1995; Ward, 1997). Significant interactions were examined by calculating the mean activations across the cluster for each participant, and simple effects t-tests were performed on each level for each factor (α = 0.025).
RESULTS
Behavioural results
Accuracy for SWM and vigilance tasks yielded no main effects or interactions. The AUD adolescents performed faster on the SWM tasks than the control subjects [F(1, 33) = 4.79, P < 0.05], and also showed a trend for faster performance on the vigilance tasks [F(1, 33) = 4.12, P = 0.05]. Reaction times did not show a gender effect or interaction (see Table 2).
Table 2.
Group comparisons of SWM task performance
| Female controls mean (SD) | Male controls mean (SD) | Female AUD mean (SD) | Male AUD mean (SD) | |
|---|---|---|---|---|
| Vigilance accuracy (% correct) | 95.13 (0.02) | 94.92 (0.03) | 94.00 (0.06) | 96.18 (0.01) |
| SWM accuracy (% correct) | 84.84 (0.11) | 88.33 (0.08) | 89.83 (0.06) | 90.75 (0.05) |
| Vigilance reaction time (ms) | 656.96 (66.44) | 595.69 (82.57) | 582.33 (73.20) | 577.83 (37.85) |
| SWM reaction time (ms)a | 620.98 (58.30) | 593.47 (67.20) | 553.41 (43.63) | 575.99 (49.00) |
SWM task data logging failed for two subjects.
P < 0.05 AUD faster than controls.
fMRI results: main effects of gender
The female adolescents showed significantly greater brain activation patterns in the left middle frontal gyrus, thalamus and right superior frontal gyrus than the male adolescents. Male adolescents had significantly more activation in the left precuneus, angular and subcallosal gyri (P < 0.05) than female adolescents.
fMRI results: main effects of AUD diagnosis
The AUD adolescents showed significantly greater brain activation patterns in the bilateral superior frontal gyri, left inferior frontal gyrus, right middle frontal gyrus, inferior parietal lobule, precuneus, fusiform and middle temporal gyrus than the control subjects. The AUD adolescents had significantly less activation in the inferior frontal gyrus, right middle frontal gyrus, left precentral gyrus, insula, bilateral precuneus and cerebellum (P < 0.05) than the control subjects.
fMRI results: gender × AUD diagnosis interactions
A gender × diagnosis interaction significantly affected SWM, relative to vigilance BOLD response, in 10 areas. Simple effects tests, described below, revealed the nature of these interactions in 7 of the 10 areas (listed in Table 3), but were not significant in the three middle frontal clusters (α =0.025). In one right superior temporal area (44R, 43P, 17S), AUD females showed more response than female controls (Cohen's d = 2.56) and AUD males (d = 2.42); however, AUD males showed less response than male controls (d = 1.48), and female controls showed less response than male controls (d = 1.678). In another right superior temporal area (44R, 57P, 17S), AUD females showed a greater response than female controls (d = 2.21) and males with AUD (d = 1.55), while female controls showed less response than male controls (d = 1.82). In the right superior frontal gyrus (see Figure 1), AUD females showed a diminished response than female controls (d = 1.67) and AUD males (d = 2.24), while AUD males had a greater response than male controls (d = 1.22). In the cingulate gyrus, AUD females showed less response than female controls (d = 1.63) and males with AUD (d = 1.40), while AUD males had a greater response than male controls (d = 1.46). In addition, in the cingulate gyrus, the response of female controls was greater than that of male controls (d = 1.91). In the left superior frontal gyrus (see Figure 1), AUD females showed less response than female controls (d = 2.73) and AUD males (d = 2.20), and female controls showed more response than male controls (d = 1.40). AUD females showed more response than female controls in the right (d = 2.10) and left (d = 2.019) fusiform gyri, as well as a greater response than AUD males (right d = 1.46, left d = 1.58), while female controls showed less response than male controls in these areas (right d = 1.35, left d = 1.45). As the average blood alcohol concentration (BAC), when drinking, differed between males and females with AUDs (see Table 1), the comparison between these two groups was re-run, controlling for a typical BAC in an ANCOVA. All results remained the same, except that differences in the fusiform gyri were no longer significant.
Table 3.
Regions showing significant gender × AUD diagnosis interactions and follow-up t-tests on BOLD response to SWM relative to vigilance
| Talairach coordinates |
t-statistic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anatomic region | Brodmann's area | Cluster volume (μl) | x | y | z | Female controls | Male controls | Female AUD | Male AUD |
| Right superior temporal gyrusa,b,c,d | — | 2358 | 44R | 43P | 17S | −1.112 | 1.114 | 4.527 | −0.688 |
| Right superior temporal gyrusa,b,d | 22 | 1886 | 44R | 57P | 17S | −2.686 | 1.49 | 1.232 | −0.768 |
| Right superior frontal gyrusa,b,c | 8 | 1029 | 23R | 34A | 45S | 1.44 | −1.206 | −1.762 | 2.747 |
| Left cingulate gyrusa,b,c,d | 24 | 1415 | 5L | 8P | 35S | 0.028 | −6.489 | −1.902 | −1.11 |
| Left superior frontal gyrusa,b,d | 8 | 943 | 33L | 20A | 52S | 0.364 | −1.989 | −5.438 | 0.588 |
| Right fusiform gyrusa,d | 37 | 1243 | 44R | 40P | 11I | −1.832 | 0.836 | 3.396 | −0.533 |
| Left fusiform gyrusa,d | 36/37 | 986 | 47L | 47P | 15I | −4.602 | 0.84 | 1.657 | −1.369 |
R, right; L, left; A, anterior; P, posterior; S, superior; I, interior.
Talairach coordinates refer to maximum intensity of interaction effect; t-statistics represent single sample t-tests for each group.
Female AUD significantly different than female controls (P < 0.025).
Female AUD significantly different than male AUD (P < 0.025) after controlling for average BAC.
Male AUD significantly different than male controls (P < 0.025).
Female controls significantly different than male controls (P < 0.025).
Fig. 1.
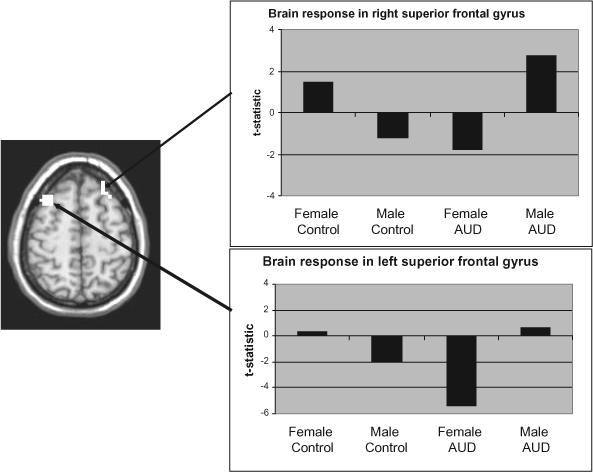
AUD group × gender interaction in superior frontal areas.
DISCUSSION
As hypothesized, gender and adolescent alcohol involvement were significantly related to BOLD response patterns during an SWM task. As expected, adolescents with AUDs showed less activation in the precentral, middle and inferior frontal, precunei, and cerebellar areas than the control subjects. However, AUD adolescents showed more activation in the middle and superior frontal, inferior parietal, and temporal regions. Although adolescents with AUDs showed an abnormal BOLD activation to the SWM task, there were no group differences on the task performance. The activation differences among adolescents with AUD may be due to neuronal reorganization, reflecting that, in order to perform adequately on the task, more brain response and neuonal recruitment from new areas compensated for areas of decreased activation (Tapert et al., 2004).
This is the first study of its kind to examine gender differences in brain response during adolescence. In this study, female adolescents demonstrated a greater activation in the right superior and left middle frontal regions than male adolescents, while male adolescents showed a greater activation in the left precuneus, angular and subcallosal gyri. These adolescent gender differences may be due to the gender-specific brain development (Giedd et al., 1999), as males and females have different patterns of neuromaturation, and fMRI studies have suggested that brain response to SWM changes across adolescent development in the frontal and parietal regions (Klingberg et al., 2002; Kwon et al., 2002).
Female adolescents with AUDs showed a greater departure from normal activation patterns than AUD males, potentially indicating a greater female vulnerability to alcohol neurotoxicity. AUD females showed a greater brain response than males with AUDs and, importantly, compared with female controls in the temporal areas, possibly to compensate for the reduced frontal and cingulate activation. In addition, differences in alcohol metabolism may contribute to female vulnerability. Women with chronic alcohol use have lower alcohol dehydrogenase levels than alcoholic men, which decreases alcohol metabolism (Lieber, 2000), resulting in higher BAC levels among females. Female rats show lower blood glucose levels compared with female control rats and male rats, which, when combined with a higher plasma alcohol level, could also contribute to alcohol toxicity (Sumida et al., 2004). Finally, hormonal and receptor differences in response to alcohol may also play a part in these gender differences. Since hormonal receptor levels are thought to be related to gender differences in brain functioning on spatial tasks (Williams and Meck, 1991), alcohol-induced changes in hormone distributions (Emanuele et al., 2001; Kim et al., 2003) could partially account for gender differences in alcohol-related neural abnormalities.
Despite the fact that the female and male adolescents with AUDs demonstrated an abnormal brain response during the SWM task, they performed similarly to the same-gender controls and even showed a trend for faster response (60 ms faster for AUD females vs controls, on average). Similarly, we have found abnormalities in the fMRI response but not on the neuropsychological tests among adolescents with AUDs relative to control subjects (Tapert et al., 2004). This pattern of BOLD response abnormalities albeit intact performance, among adolescents with 1−2 years of heavy drinking, might suggest subtle neuronal disruption that can be compensated for by reorganization of the circuitry used for the task. However, if heavy drinking continues for perhaps two or three more years, neuronal disruption may increase and compensation may no longer be possible, and performance might then diverge from that of non-drinkers (Tapert et al., 2001). Longitudinal studies utilizing functional neuroimaging and cognitive testing will be needed to verify this model of how alcohol-related cognitive changes might develop.
While this is the first study that attempts to examine the patterns of brain activation in male and female adolescents with AUDs, several limitations should be considered. First, although the samples were well matched, the small sample size limited the power to detect differences. Second, AUD females averaged a higher BAC, despite drinking as frequently as males, potentially accounting for the greater neural disruption. Nonetheless, after statistically controlling for a typical BAC, similar results emerged. Third, longitudinal studies are needed to clearly investigate neurodevelopmental trajectories among males and females with and without AUDs. Although the current study found that adolescents with AUDs had an intact performance, if current drinking patterns persist, they may risk further changes in brain chemistry that lead to behavioural impairments, as evidenced in fMRI studies using older subjects with longer AUD courses (Tapert et al., 2001). In addition, the potential for recovery from neurotoxic effects should be examined longitudinally. Adult alcoholics show recovery from neurotoxicity after continued abstinence (O'Neill et al., 2001), but heavy alcohol use during brain development may alter neurodevelopment, interfering with neural recovery. Finally, premorbid factors should be examined to dissociate alcohol neurotoxicity from any differences preceding the development of an AUD.
This preliminary study indicates that male and female adolescents with AUDs have different patterns of BOLD response when compared with demographically similar controls, although these early brain differences do not produce observable behavioural deficits. Furthermore, adolescent girls with AUDs appear more likely to show neural abnormalities than boys. With continued heavy drinking, these adolescents may be at a higher risk for developing behavioural impairments, and girls and boys may be differentially affected.
Acknowledgements
This study was supported by grants AA12519 and AA13419 (S.F.T.). The authors thank Drs Gregory Brown, Sandra Kindermann and Lawrence Frank.
REFERENCES
- Achenbach TM. Manual for the Child Behavior Checklist/4−18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, et al. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) Psychological Corp; San Antonio, TX: 1978. [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addictive Behaviors. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Brown SA, Gleghorn A, Schuckit MA, et al. Conduct disorder among adolescent alcohol and drug abusers. Journal of Studies on Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, et al. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, et al. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, et al. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, et al. Anatomical MRI of the developing human brain: what have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, et al. Effect of chronic ethanol exposure on female rat reproductive cyclicity and hormone secretion. Alcohol: Clinical and Experimental Research. 2001;25:1025–1029. [PubMed] [Google Scholar]
- Fitzgerald EF. Intoxication Test Evidence. Clark Boardman Callaghan; Deerfield, IL: 1995. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glenville M, Broughton R. Reliability of the Stanford Sleepiness Scale compared to short duration performance tests and the Wilkinson Auditory Vigilance Task. Advances in the Biosciences. 1978;21:235–244. [PubMed] [Google Scholar]
- Hollingshead AB. Two-Factor Index of Social Position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Hommer D, Momenan R, Rawlings R, et al. Decreased corpus callosum size among alcoholic women. Archives of Neurology. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, et al. Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG. Monitoring the Future National Survey Results on Adolescent Drug Use: Overview of Key Findings, 2002, Report NIH Publication No. 03−5374. National Institute on Drug Abuse; Bethesda, MD: 2003. [Google Scholar]
- Kim JH, Kim HJ, Noh HS, et al. Suppression by ethanol of male reproductive activity. Brain Research. 2003;989:91–98. doi: 10.1016/s0006-8993(03)03372-9. [DOI] [PubMed] [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, et al. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophrenia Research. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V, et al. Neural basis of protracted developmental changes in visuo-spatial working memory: Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Ethnic and gender differences in ethanol metabolism. Alcoholism: Clinical and Experimental Research. 2000;24:417–418. [PubMed] [Google Scholar]
- Mann K, Batra A, Gunthner A, et al. Do women develop alcoholic brain damage more readily than men? Alcoholism: Clinical and Experimental Research. 1992;16:1052–1056. doi: 10.1111/j.1530-0277.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Myers MG, Stewart DG, Brown SA. Progression from conduct disorder to antisocial personality disorder following treatment for adolescent substance abuse. American Journal of Psychiatry. 1998;155:479–485. doi: 10.1176/ajp.155.4.479. [DOI] [PubMed] [Google Scholar]
- Nixon SJ. Cognitive deficits in alcoholic women. Alcohol Health & Research World. 1994;18:228–232. [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcoholism: Clinical and Experimental Research. 2001;25:1673–1682.. [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, et al. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA . National Survey on Drug Use and Health, 2002. Office of Applied Studies; Bethesda, MD: 2003. [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, et al. Effects of alcoholism and gender on brain metabolism. American Journal of Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, et al. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicology and Teratology. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press; Totowa, NJ: 1992. pp. 41–72.. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, et al. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sumida KD, Qureshi T, Catanzaro MJ, et al. Chronic alcohol consumption yields sex differences in whole-body glucose production in rats. Alcohol and Alcoholism. 2004;39:418–426. doi: 10.1093/alcalc/agh082. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme; New York: 1988. Coplanar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. Journal of the International Neuropsychological Society. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, et al. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, et al. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, et al. BOLD response and spatial working memory in alcohol use disordered adolescents. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 1997. [Google Scholar]
- Widmark E. A micromethod for the evaluation of alcohol in blood. Biochemistry. 1922;131:473. [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test-3 Administration Manual. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, et al. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society for Magnetic Resonance in Medicine. 2000;8:683. [Google Scholar]


