Abstract
Neurodevelopmental changes induced by environmental stress exposure play a significant but poorly defined role in the etiology of schizophrenia. Exposure of pregnant female rats to a series of unpredictable stresses during the final week of pregnancy generates behavioral deficits and molecular changes in the offspring similar to those observed in schizophrenic individuals. We used this rat prenatal stress preparation to investigate social withdrawal behaviors that may have relevance to the negative symptoms of schizophrenia. The cumulative time adult male offspring of stress-exposed pregnant female rats actively interacted with a weight-matched, same-sex peer was decreased approximately 76% relative to non-stress exposed control rats. Prenatal stress exposure also diminished the quality of the social interaction behavior indicative of reduced social drive. Analysis of the oxytocinergic system in the prenatally stressed male rats revealed significantly less oxytocin mRNA in the paraventricular nucleus and increased oxytocin receptor binding in the central amygdala. Moreover, oxytocin, but not vasopressin, administration into the central amygdala reversed the social incompetence of the prenatally stressed rats without increasing behavior in non-stressed control animals. In addition, cross-fostering pups from prenatally stressed mothers to non-stressed mothers failed to improve the social deficit of the prenatally stressed male offspring. Two behavioral assays designed to measure anxiety did not differentiate the prenatally stressed rats from non-stressed controls. These data indicate that prenatal stress may be an etiologically appropriate animal model for some aspects of schizophrenic social withdrawal. Furthermore, unpredictable prenatal stress exposure selectively degrades social interaction behaviors without increasing anxiety measures.
Keywords: oxytocin, amygdala, rats, hypothalamus, social interaction, schizophrenia, prenatal stress
1. Introduction
Schizophrenia is a neuropsychiatric disease of unknown pathophysiology that is composed of positive, negative and cognitive symptom domains (Andreasen and Carpenter, 1993, Carpenter and Buchanan, 1994). Recent research indicates that aberrant brain development may play a prominent role in the origin of schizophrenia (for review see Weinberger and Lipska, 1995, Lewis and Levitt, 2002, Rapoport et al., 2005). A parsimonious mechanism that can alter fetal brain development is maternal exposure to stresses during the second trimester of pregnancy. Maternal bereavement, viral infection, malnutrition or natural disaster exposure during this gestational window increase the likelihood of the offspring developing schizophrenia (for review see Brixey et al., 1993, Walker and Diforio, 1997, Koenig et al., 2002, Sullivan, 2005).
Like the developing human brain, the emergent rat brain is vulnerable to environmental stress. Exposing pregnant female rats to stressful manipulations during the third week of pregnancy, the developmental equivalent to the second trimester of human pregnancy (Bayer et al., 1993), reprograms the hypothalamic-pituitary-adrenal (HPA) axis and enhances behavioral responses to psychostimulants (Fride et al., 1986, Takahashi et al., 1992, Henry et al., 1994, Koehl et al., 1999, Ward et al., 2000, Weinstock, 2001, Boksa and El-Khodor, 2003, Zuckerman et al., 2003, Bowman et al., 2004, Koenig et al., 2005). Repeated exposure of pregnant rats to the same stress fails to produce deficits in gating sensory information or cognition, which are integral elements of the schizophrenia phenotype (Lehmann et al., 2000, Bowman et al., 2004), while exposure of pregnant female rats to an unpredictable stress paradigm during the final week of pregnancy has been found to induce deficits in sensory gating and cognition [49, 50].
Unlike the positive symptoms of schizophrenia, there has been relatively little investigation into the negative symptoms of schizophrenia. Schizophrenic patients with negative symptoms suffer from social withdrawal, anhedonia, and blunted affect (Carpenter and Buchanan, 1994, Kirkpatrick et al., 2006). While the morbidity associated with these social symptoms is profound, currently available antipsychotic drugs fail to alleviate these symptoms (Breier et al., 1994, Bellack et al., 2004). The neurobiological substrates integral to the negative symptoms of schizophrenia remain to be identified.
Oxytocin, a peptide produced by the neurons of the hypothalamic paraventricular (PVN) and supraoptic nuclei (Buijs, 1978, Buijs, 1980), is essential for lactation and maternal behavior (Pedersen and Prange, 1979, Pedersen et al., 1982, Young et al., 1997). Oxytocin also plays an important role within the brain as a mediator of social behavior (Witt et al., 1992, Carter et al., 1995, Insel, 1997, Young et al., 1997, Young, 1999). Oxytocin binds to G-protein coupled receptors, which are localized in numerous brain regions, including the amygdala, hippocampus, septum and PVN (Gimpl and Fahrenholz, 2001). Moreover, oxytocin activates neurons in the central nucleus of the amygdala (CeA) where the peptide is anxiolytic and promotes affiliative behaviors (Neumann, 2002, Lee et al., 2005). Several studies have identified oxytocin as an important mediator of human social behavior (Fries et al., 2005, Kosfeld et al., 2005, Zak et al., 2005) and there are indications that oxytocin neurotransmission may be altered in schizophrenia (Mai et al., 1993, Bernstein et al., 1998).
Using the overarching hypothesis that mid-gestational stress is a risk factor for schizophrenia, we exposed pregnant female rats to an unpredictable variable stress paradigm, which produces rat offspring with molecular signatures, sensorimotor gating deficits and hyperdopaminergia similar to what has been observed in schizophrenic patients (Kinnunen et al., 2003, Koenig et al., 2005). In the present report, we examined the social phenotype of the adult male rats after exposure to an unpredictable prenatal stress paradigm. Our studies revealed a disruption of social behaviors in prenatally stressed male rats, which is accompanied by deficits in oxytocin mRNA production and changes in oxytocin receptor expression in the CeA. Microinjection of oxytocin into the central amygdala mitigates the asociality of the prenatally stressed rats. These results may provide insights into the origin and treatment of schizophrenia’s negative symptoms.
2. RESULTS
Litter Information following Unpredictable Prenatal Stress Exposure
Exposing pregnant female rats to unpredictable stresses during pregnancy can potentially have adverse consequences on the viability of the offspring. In the course of these studies, we exposed over 20 pregnant female rats to an unpredictable stress paradigm that is outlined in Table 1. Table 2 shows the characteristics of the litters exposed to this series of stresses compared to litters derived from dams that were exposed to daily animal room procedures that were used as the control rats for this series of studies. Exposure to the unpredictable prenatal stress paradigm did not cause significant changes in litter size, the number of male and female pups comprising each litter or the weight of the pups at weaning (Table 2). A preliminary analysis of maternal behavior revealed no obvious differences in pup care between the dams exposed to the unpredictable stress procedure and dams not exposed to these manipulations (data not shown).
Table 1.
Schedule of Stress Exposures for Pregnant Female Rats
| Time of Day | ||||
|---|---|---|---|---|
| Gestation Day | AM | Mid-day | PM | |
| 14 | Restraint - 60 min | Swim - 15 min | Restraint - 60 min | |
| 15 | Cold Exposure - 6 hr | Fast - overnight | ||
| 16 | Swim - 15 min | Restraint - 60 min | Swim - 15 min | |
| 17 | Open | Swim -15 min | Restraint - 60 min | |
| 18 | Social Stress - 12 hr | |||
| 19 | Restraint - 60 min | Swim - 15 min | Lights On Overnight | |
| 20 | Cold Exposure - 6 hr | |||
| 21 | Swim - 15 min | Restraint - 60 min | Swim -15 min | |
Table 2.
Comparison of litter characteristics between non-stress (NS) control litters and litters exposed to unpredictable prenatal stress.
| Characteristic | NS Control | Prenatal Stress | |
|---|---|---|---|
| Litter Size | 12.0 ± 0.58 (22) | 12.3 ± 0.51 (23) | |
| Number of Female Pups | 6.23 ± 0.34 | 6.39 ± 0.54 | |
| Number of Male Pups | 5.73 ± 0.50 | 5.96 ± 0.39 | |
| Weight at Weaning | |||
| Female Pups | 63.3 ± 0.64 gm | 62.1 ± 0.72 gm | |
| Male Pups | 67.1 ± 0.80 gm | 65.8 ± 1.14 gm |
Values given are mean ± SEM for 22 litters of non-stressed control rats and 23 litters of prenatally stressed rats. The male offspring of these litters were used as the subjects for these experiments. Pups weights were determined at weaning so there was no disruption of dam-pup interactions.
EXPERIMENT 1. Social Interaction Behavior in Rats following Prenatal Stress
Social interaction of adult rats exposed to prenatal stress (Figure 1a)
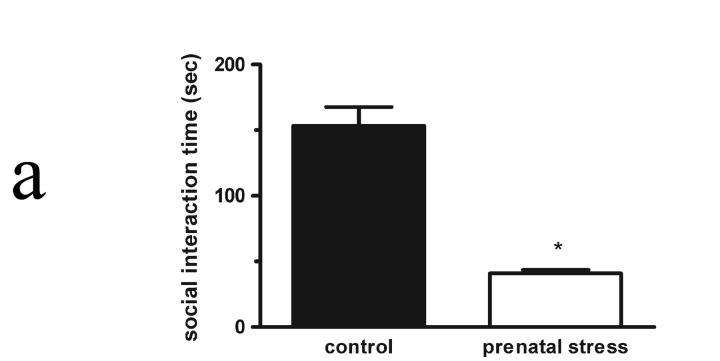
Figure 1a.
Social interaction behaviors in adult male rats (56 days of age) following exposure no stress during pregnancy or following exposure to an unpredictable prenatal stress regimen. Exposure in utero to an unpredictable stress paradigm caused a significant decrease in the cumulative mean time spent by adult males in social interaction (*p < 0.001, n=6) when compared to the mean time of male rats not exposed to stress (no stress control, n=6). Data shown are mean total social interaction times (sec) ± SEM for each group.
Adult male rats exposed to prenatal stress exhibited diminished social behavior (t = 7.70; df = 12; p < 0.001) when compared to unstressed control male peers. Prenatally stressed male rats exhibited total interaction times approximately 76% lower than unstressed males’ times (Figure 1a). No prolonged (> 5 seconds) freezing, indicative of an acute fear response, was observed in any subject, nor were there incidents of overtly aggressive actions (e.g., biting to injury) by any experimental or target animal.
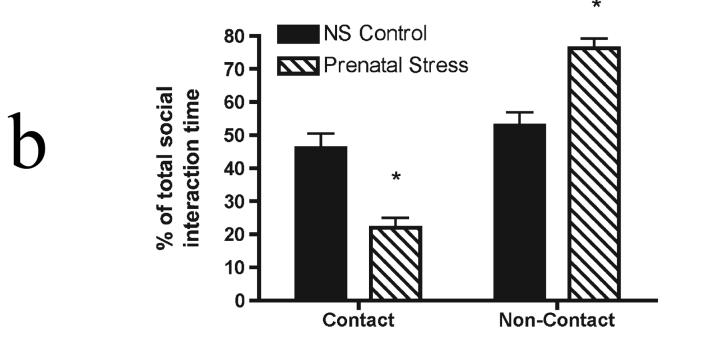
Figure 1b displays a further subdivision of the social interaction behaviors exhibited by the young adult male rats shown in Figure 1a into the percentage of the time the experimental rat spent in direct physical contact with the novel target rat or in close proximity to the target animal. This breakdown of the behavior revealed a significant qualitative difference in how rats engaged the novel male target. A repeated measures ANOVA performed on the behavioral data from prenatally stressed rats revealed significant main effects for behavior (F(1,10) = 38.3, p < 0.001) and an interaction effect (stress condition × behavior) (F(2,10) = 29.1, p < 0.0001) in the comparison to no stress control rats’ behaviors. Male rats exposed to the unpredictable prenatal stress spent a significantly larger fraction of their total social interaction (76 ± 3%) engaged in non-contact interaction with the target rat compared to the percentages of non-stressed control rats (53 ± 4%). In contrast, non-stressed control males spent a greater proportion of their total social interaction times (46 ± 4%, respectively) engaged in social interactions requiring physical contact than their prenatally stressed peers.
Figure 1b.
Comparison of contact and non-contact social interaction behaviors in adult male rats exposed to unpredictable prenatal stress. Adult male rats exposed to prenatal stress (n=6/group) spent a significantly larger percentage (*p < 0.001) of their social interaction time engaged in a non-contact behavior (following) with a male target as opposed to behaviors with obligate close physical proximity (anogential exploration or crawling over/under). The distribution for unstressed control rats (n=8) behavioral percentages revealed a significantly greater (*p < 0.001) preference for contact behaviors. Data shown are mean percent of total time spent engaged in social interaction ± SEM.
Social interaction in adolescent and adult rats following prenatal stress exposure (Figure 1c)
Figure 1c.
Comparison of social interaction behavior in prepubertal (35 days old) and young adult (56 days old) male rats following exposure to an unpredictable prenatal stress paradigm. Data shown are expressed as the mean percent of age-matched, non-stress exposed male rat social interaction time ± SEM. Six rats were tested in each experimental group. Statistical differences are designated by # (p<0.001 compared to control rats) and by ‡ (p<0.05 compared to 35 days old prenatally stressed rats).
The decrement in social interaction behavior is prominent in young adult male rats that are between 56 and 64 days of age (Figure 1a, b). In addition, exposure to an unpredictable series of stresses during gestation also produced social withdrawal in 35-day old prepubescent male rats. Although the magnitude of the social withdrawal is less in the prepubescent rats compared to 56-day old adult rats, these young rats still display about 50% less social interaction behavior than age-matched, non-stressed male rats (Figure 1c, t = 8.13; df = 12; p < 0.001).
Open field locomotion after prenatal stress exposure (Table 3)
Table 3.
Expression of OT, AVP, OTR and V1a mRNAs, and OTR and V1a receptor autoradiographic binding in distinct brain regions of adult male Sprague-Dawley rats not exposed to stress during pregnancy or exposed to a variable stress paradigm during the 3rd week of gestation.
| Region | No Stress Control (n=6) | Prenatal Stress (n=6) | |
|---|---|---|---|
| OT mRNA | PVN | 100 ± 3% | 78 ± 3%** |
| OTR mRNA | CeA | 100 ± 3% | 97 ± 6% |
| VMH | 100 ± 3% | 103 ± 5% | |
| OTR binding | CeA | 100 ± 3% | 177 ± 4%* |
| AVP mRNA | PVN | 100 ± 3% | 106 ± 4% |
| V1aR mRNA | CeA | 100 ± 4% | 93 ± 8% |
| V1aR binding | CeA | 100 ± 3% | 40 ± 3%** |
Data are mean percents ± SEM.
p < 0.05
p < 0.001
Though some investigators who have repeatedly exposed rats to restraint stress during the prenatal period have noted a decrease in open field exploration (Vallee et al., 1997, Lehmann et al., 2000), the unpredictable stress paradigm utilized in these studies did not appear to affect locomotor behaviors in a novel environment as there was no effect attributable to prenatal stress exposure (Repeated measures ANOVA, F(1,20) = 0.000005, p > 0.05) consistent with previous findings [50].
Light-Dark emergence after prenatal stress exposure
In a light-dark emergence test, all subjects emerged from the enclosure within the first 30 seconds of the test. The mean latency to emerge times for prenatal stress rats (n=5) was 2.4 ± 0.9 seconds; the mean latency for control unstressed males (n=5) was 5.0 ± 2.4 seconds. The mean times spent outside the enclosure for prenatal stress and control rats were 291.2 ± 1.9 seconds and 293.2 ± 1.2 seconds, respectively. A repeated measures ANOVA performed on these data revealed there was no effect attributable to prenatal stress treatment (F(1,16) = 1.82, p > 0.05).
Neuropeptide and Receptor Expression and Binding Studies
Oxytocin and oxytocin receptor mRNA expression (Figure 2, Table 4)
Figure 2.
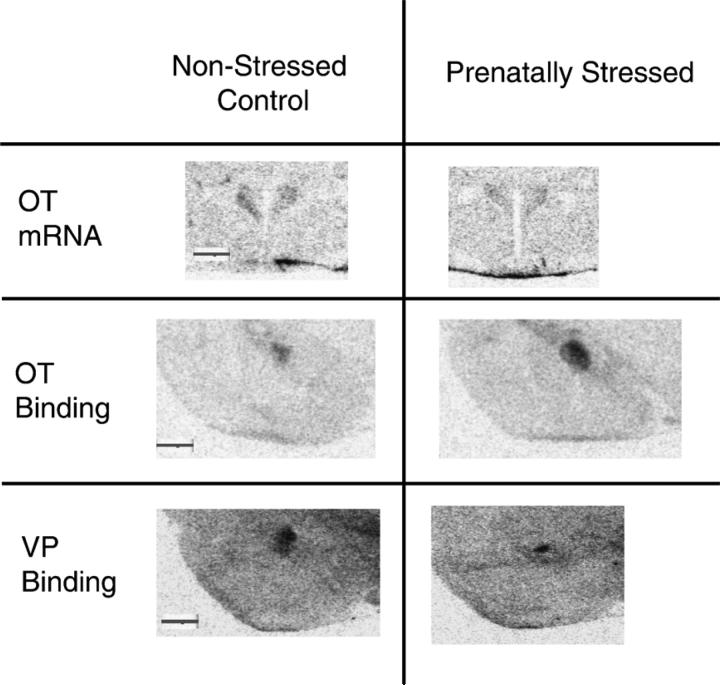
Representative autoradiographic images of PVN oxytocin (OT) mRNA expression, and OT and vasopressin (VP) receptor binding in the CeA of a non-stressed control male rats and a prenatally stressed male rats. All images are light-field images captured from film autoradiographs using a CCD camera. A 1 mm scale bar is included in the left-hand image of each image set for comparison purposes.
Table 4.
Open Field Locomotor Behavior of Adult Male Sprague-Dawley Rats Exposed to Repeated, Variable Prenatal Stress
| Total Peripheral Distance Traveled (cm) | Total Center Distance Traveled (cm) | |
|---|---|---|
| No Stress Control | 600 ± 323 | 141 ± 112 |
| Prenatal Stress | 517 ± 244 | 223 ± 138 |
Data are means ± SEM. Five non-stress control rats and five prenatally stressed rats from different litters were used in this experiment.
Oxytocin mRNA expression was evaluated in the PVN and supraoptic nucleus (SON) of prenatally stressed male rats and non-stressed control rats on post-natal day 64. Figure 2 depicts representative examples of the oxytocin mRNA signal in the PVN of a non-stressed control and a prenatally stressed rat. Oxytocin mRNA expression was significantly reduced in the PVN of adult prenatally stressed rats relative to non-stressed control rats (t-test, t = 5.43, p < 0.003, n = 6 per group). In contrast to the PVN, the expression of oxytocin mRNA in the SON was slightly increased in the adult prenatally stressed male rats but the increase was not statistical different (110.13 ± 7.67%, t = 1.54, p = 0.155, n = 6 per group). Oxytocin receptor mRNA expression was examined in the CeA and ventromedial nucleus of the hypothalamus (VMH). No significant differences in oxytocin receptor mRNA expression levels were noted for prenatally stressed rats in either of these regions.
Vasopressin and V1aR mRNA expression (Table 4)
Vasopressin mRNA was evaluated in the PVN and SON of prenatally stressed rats. No significant changes were noted in vasopressin mRNA expression in either hypothalamic nucleus following exposure to prenatal stress. Similarly, V1aR mRNA was found to be unchanged in the CeA of prenatally stressed rats.
Oxytocin receptor and V1aR binding autoradiography (Figure 2, Table 4)
Representative images depicting binding of a radiolabeled oxytocin receptor-specific ligand in the CeA of prenatally stressed and non-stressed control rats are shown in Figure 2. OTR binding was significantly increased by prenatal stress in the CeA (177 ± 4% of unstressed control binding, t = 4.36, n=6 per group, p < 0.001). No difference in oxytocin receptor binding was found in the lateral septum or ventromedial nucleus of rats in the prenatal stress model rats (97 ± 4% of non-stressed controls, t = 0.78, p > 0.05; 103 ± 5% of unstressed control, t = 0.318, p > 0.05, respectively) relative to their control condition peers.
Several representative autoradiographic images of V1aR binding in the CeA are shown in Figure 2. V1aR binding was decreased in the CeA of prenatally stressed rats relative to non-stressed control rats (Table 4). In adult male rats exposed to prenatal stress there was a significant (t = 6.01, p < 0.001, n=6 per group) reduction of approximately 60% in receptor binding.
The diminished expression of oxytocin mRNA in the PVN, increased CeA oxytocin receptor binding and lower CeA V1aR binding have been replicated in at least two independent groups of prenatally stress and non-stressed control rats (data not shown). In addition, the reduction of oxytocin mRNA expression in the PVN and the elevation of CeA oxytocin receptor binding have been found in rats exposed to social interaction testing and also in rats naïve to behavioral testing (data not shown).
EXPERIMENT 2. Effect of Cross-Fostering
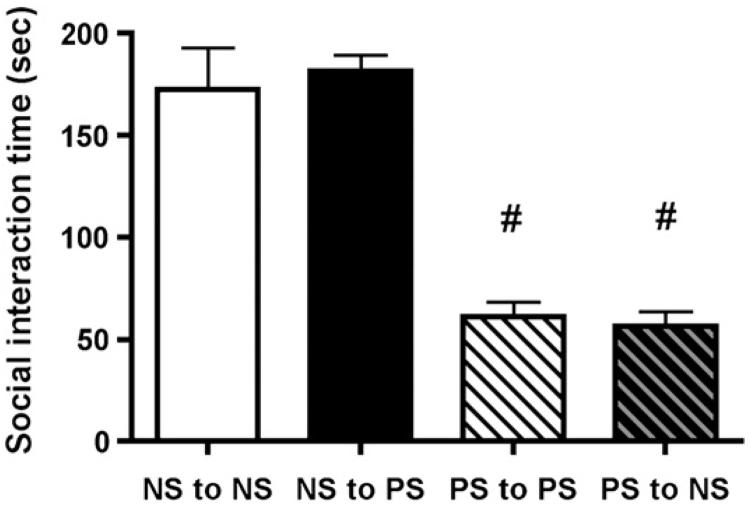
Litters of prenatally stressed pups and non-stressed pups were cross-fostered on day 1 of post-natal life to female rats stressed during pregnancy or female rats experiencing standard animal room procedures during pregnancy. There were no incidents of dams rejecting pups. Shown in Figure 3 are the data on social interaction behaviors in young adult prenatally stressed male rats and non-stressed rats raised by dams that experienced unpredictable stress exposure during pregnancy or no stress. ANOVA revealed a highly significant difference among the treatment groups in this experiment (F(3, 23) = 78.30, p< 0.001). Non-stressed male offspring, regardless of their fostered mother’s experience during pregnancy, were highly social and spent substantial time engaged in social behaviors when paired with a novel weight-matched male rat the social interaction test. Pups derived from dams experiencing stress during pregnancy, regardless of their mother’s stress history, exhibited significantly less time engaged in social behaviors compared to the non-stressed offspring (p < 0.001) suggesting that the in utero experience of the pups dictated the social behavior of the rats as adults.
Figure 3.
Effect of cross-fostering on social behavior in prenatally stressed and non-stressed male rats. Rat pups from non-stressed mothers were cross-fostered to other non-stressed mothers or prenatally stressed mothers within 24 hours of birth. Rat pups from prenatally stress mothers were also cross-fostered to other prenatally stressed or non-stressed mother rats. On postnatal day 56, social interaction behavior was determined in randomly selected cross-fostered male offspring. Prenatally stressed male rats, regardless of postnatal rearing, were less inclined to display social interaction behaviors (# - p < 0.001) compared to non-stressed male rats. Data shown are mean social interaction times (sec) ± SEM for each group (n = 6 in all groups).
EXPERIMENT 3
Social interaction after prenatal stress exposure followed by intra-amygdalar neuropeptide infusions (Figure 4)
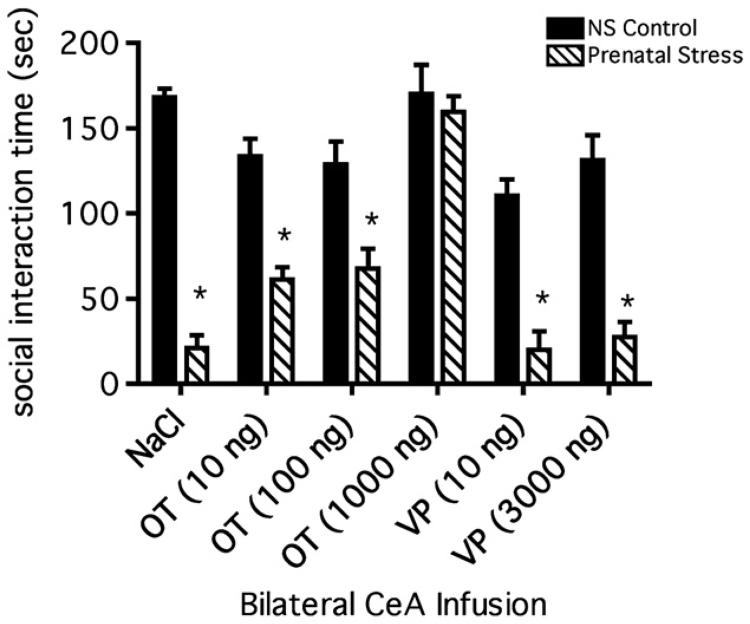
Figure 4.
Cumulative mean time spent engaged in social interaction behavior by prenatally stressed and unstressed control male rats after infusions into the CeA of VP, OT, and saline (vehicle). All post-infusion social interaction mean times for prenatally stressed rats were compared to the mean time of the unstressed, saline-infused control rats (n=8) except for the 1000 ng dose which was compared a 1000 ng dose of OT in unstressed controls. OT infusions (1000 ng) into unstressed control male rats (n=8) had no effect on social interaction. Bilateral infusions of 1000 ng of OT into prenatally stressed rats (n=8) increased social interaction to a level identical to that of unstressed control rats infused with saline or OT. Infusions of two lower doses of OT into prenatally stressed rats, 100 ng (n=8) and 10 ng (n=7), were associated with the same significant (*p < 0.001) reduction in social interaction found in saline-infused, prenatally stressed rats (n=5). Two infused doses of VP, 10 ng (n=7) and 3000 ng (n=8), were also unable to restore normal levels of social interaction in prenatally stressed rats. Data are shown as the mean total social interaction times (sec) ± SEM.
A two-way ANOVA revealed a significant effect of prenatal stress exposure (F(1,82) = 164, p < 0.001) on social interaction. As in previous experiments (see above), prenatal stress was associated with a significant decrease in social interaction relative to the interaction of unstressed control rats; the magnitude of this decrease was approximately 81% of chronic unstressed control rats in this study as compared to approximately 76% in previous prenatal stress experiments (Figure 1a, b). The relative decrease in social interaction was not altered by infusions of saline into the CeA. The two-way ANOVA also revealed a significant effect of infusion (F(5,82) = 19.60, p < 0.001, Figure 4) and a significant interaction between prenatal stress and infusion (F(5,82) = 8.72, p < 0.001). A post hoc Bonferroni test comparing all mean cumulative social interaction times revealed that the mean social interaction times of prenatally stressed rats following infusions of saline (Bonferroni, p < 0.001), 10 ng of oxytocin (Bonferroni, p < 0.001), 100 ng of oxytocin (Bonferroni, p < 0.001), 10 ng of VP (Bonferroni, p < 0.001), or 3000 ng of VP (Bonferroni, p < 0.001) were significantly lower than the cumulative mean time of unstressed control rats infused with saline or with 1000 ng of oxytocin (Figure 4). The cumulative social interaction times of unstressed control rats infused with 1000 ng of oxytocin and the cumulative mean time of prenatally stressed rats infused with 1000 ng of oxytocin were not significantly different (Bonferroni, p > 0.05) than the mean time of unstressed control, saline-infused rats. Interestingly, further post hoc statistical analysis using multiple comparisons testing demonstrated that the infusion of either 10 ng or 100 ng of oxytocin both elicited a small but significant improvement in the social function of the prenatally stressed rats (oxytocin 10 ng, t = 3.44, p < 0.05 and oxytocin 100 ng, t = 4.01, p < 0.01). Neither dose of vasopressin generated improvement in the social function of the prenatally stressed rats (10 ng, t = 0.0994, p > 0.05 and 3000 ng, t = 0.549, p > 0.05). Locomotor activity measures in all prenatally stressed and control rats failed to reveal any differences between the groups of animals (data not shown).
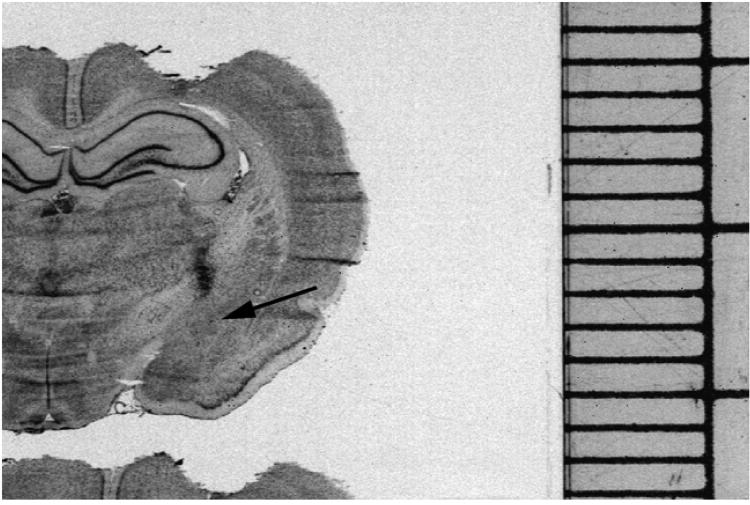
Following termination of the social interaction testing, animals were euthanized and brains were collected for confirmation of the cannulae locations. Figure 5 is a representative image of a cresyl-violet stained section through the CeA that was collected for verification of the cannulae location. The arrowhead shows the deposition of dye injected through a cannula at the conclusion of an experiment. Note the deposition of the dye immediately dorsal to the CeA (arrow) confirming the positioning of the cannula. The behavioral data obtained from rats with cannulae not located in a similar position were not included in the data analysis (n=2).
Figure 5.
Representative cresyl violet-stained rat brain section used in the oxytocin and vasopressin infusion experiments demonstrating a typical location of the injection cannulae above the CeA. Dye was injected to mark the cannula location and is depicted as the dark spot traversing the internal capsule referred to by the black arrow. The scale on the right is a millimeter scale for size comparison.
Previous investigations of prenatal stress’s effects (see above) on social interaction had demonstrated decreased time spent engaged in social interaction as well as a shift from contact to non-contact interactive behaviors. The proportionate time infused rats from the prenatal stress paradigm engaged in contact and non-contact behaviors was evaluated to ascertain whether the successful restoration of cumulative time noted at the 1000 ng dose of oxytocin heralded a parallel normalization of interest in physical contact with a male peer.
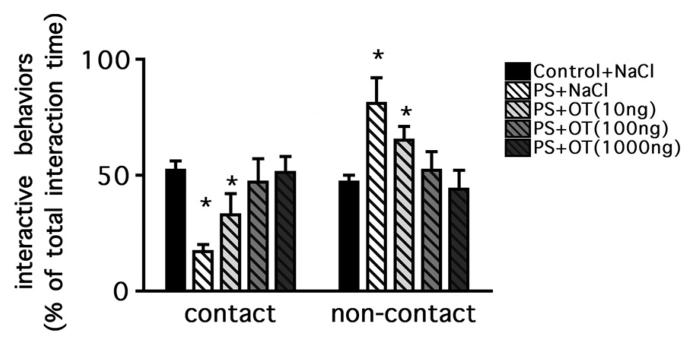
A two-way ANOVA confirmed a significant interaction between prenatal stress exposure and peptide infusion (F(4,70) = 8.28, p < 0.001, Figure 6). A post hoc analysis revealed that the previously observed shift from contact to non-contact behaviors was still present in prenatally stressed rats infused with saline as they spent a higher percentage of their interaction engaged in non-contact behaviors (Bonferroni, p < 0.01). One of the two infused doses of oxytocin that failed to elevate social interaction, 10 ng, also failed to alter the shift to more non-contact interactive behaviors in prenatally stressed animals (Bonferroni, p < 0.01). However, as shown in Figure 6, the 100 ng and 1000 ng doses of infused oxytocin restored the percentage of contact behaviors to a level similar to that of unstressed control subjects infused with saline (Bonferroni, p > 0.05).
Figure 6.
Contact and non-contact behaviors after OT or saline infusions into the CeA of prenatally stressed or unstressed control male rats expressed as a percentage of total social interaction time. The mean percents of contact and non-contact behaviors for each group were compared to those of saline-infused unstressed control rats (n=8). Bilateral infusions of 1000 ng of OT into prenatally stressed rats (n=8) increased contact behaviors (sniffing, crawling over/under) to a level identical to that of unstressed control rats infused with saline. Two other infused doses of OT, 100 ng (n=8) and 10 ng (n=8) were associated with the same significant (*p < 0.01) reduction in contact interactions found in saline-infused, prenatally stressed rats (n=8). Data are shown as the mean percent of total social interaction times ± SEM.
3. DISCUSSION
Using a verified epidemiological risk factor for schizophrenia, i.e. prenatal stress, to generate an animal model for the disease may present an opportunity to gain additional knowledge about the pathophysiology of schizophrenia’s negative symptom domain. Our studies revealed that male rats exposed in utero to a series of unpredictable stresses during the third week of gestation exhibited diminished social behaviors as adolescents and adults. Exposure to an unpredictable prenatal stress regimen, like neonatal ventral hippocampal lesioning, increases the locomotor response to stimulants and disrupts sensorimotor gating, as well as reducing social drive in the offspring (Sams-Dodd et al., 1997, Lipska and Weinberger, 2000, Le Pen and Moreau, 2002, Rueter et al., 2004, Koenig et al., 2005). Furthermore, cognitive disturbances (social recognition, novel object recognition) and changes in prefrontal markers of glutamatergic neurotransmission are also present in both of these animal preparations (Le Pen et al., 2000, Lipska et al., 2002, Kinnunen et al., 2003, Koenig, 2005). While some prenatal manipulations in rats, such as immune challenge, viral infection and protein malnutrition also recapitulate sensory gating abnormalities and cognitive disturbances, only unpredictable prenatal stress, hippocampal lesioning and prenatal immune challenge in mice generate social impairments (Borrell et al., 2002, Zuckerman et al., 2003, Palmer et al., 2004). Impaired social interaction behavior was observed in prepubertal prenatally stressed rats 35 days of age as well as in young adult prenatally stressed rats. One of the first clinical signs associated with schizophrenia is social withdrawal during adolescence (Kelley et al., 1992, Moller and Husby, 2000, Cornblatt, 2002). The emergence of social withdrawal in the adolescent prenatally stressed rats appears to be consistent with the clinical schizophrenia literature and further supports the relevance of this model to the schizophrenia phenotype.
The diminution in social interaction behaviors could reflect an increase in anxiety in the prenatally stressed rats (Weinstock, 2001). However, the equivalent open-field locomotor activity of the prenatally stressed and non-stressed rats, and the time spent in the center of the arena (Prut and Belzung, 2003), paired with the short latency of the rats to leave the enclosure in the light-dark emergence test do not support a generalized state of elevated anxiety (Gurtman et al., 2002, Prut and Belzung, 2003). The lack of change in locomotor activity or emergence from a dark environment do not reflect behaviors associated with schizophrenia’s negative symptoms but are intended to assess the anxiety state of the animal. The lack of increased anxiety in this unpredictable prenatal stress preparation differs from other prenatal stress preparations in which animals are repeatedly exposed to the same stress (Vallee et al., 1997, Ward et al., 2000, Weinstock, 2001). An intriguing, but speculative explanation for this difference may be related to the timing of the stress exposure, relative to critical developmental events in the fetal brain. Since pregnant rats exposed to a single stress repeatedly will adapt to the stressor (Bhatnagar and Dallman, 1998), the duration of fetal glucocorticoid exposure will be shorter. Since the amygdala develops earlier than other brain regions (Bayer et al., 1993), it may be that earlier stresses generate corticosterone changes that preferentially target the amygdala. However, in the unpredictable stress preparation longer periods of elevated glucocorticoid exposure could persist, modifying the developmental trajectory of other fetal brain regions that mature later than the amygdala. Thus, the prolonged exposure to glucocorticoids in the unpredictable prenatal stress paradigm might generate more complex behavioral changes than a single stress paradigm.
Postnatal stress models of psychiatric diseases, such as those employing the separation of a dam from her newborn pups, yield adult rats that exhibit increased anxiety-like (Caldji et al., 1998) or depression-like (Heim et al., 1997) behaviors. Maternal licking/grooming and arched back nursing appear to program offspring stress reactivity in Long-Evans rats as well as subsequent maternal behavior (Meaney, 2001). However, the data generated by the cross-fostering study eliminate maternal care and the post-natal environment as factors precipitating diminished social behavior in prenatally stressed Sprague-Dawley rats. Our use of a different rat strain and a different prenatal manipulation are likely factors accounting for these disparate results. The present data combined with our previous findings (Koenig et al., 2005) distinguish this unpredictable prenatal stress model for schizophrenia from models of depression and anxiety, and substantiate the importance of the in utero environment in the pups’ neurodevelopment and subsequent behavioral patterns.
Oxytocin is strongly associated with female affiliative and maternal behaviors (Pedersen et al., 1982, Insel, 1992, Carter et al., 1995, Choleris et al., 2003) however limited data are available on the behavioral role of this peptide in males. Centrally administered oxytocin can improve male rat social interaction behaviors (Witt et al., 1992) and exogenous oxytocin can rescue the reduction in social memory in male oxytocin knockout mice (Ferguson et al., 2000). Interestingly, several recent studies in humans corroborate the importance of oxytocin in non-sexual, social behaviors (Fries et al., 2005, Kosfeld et al., 2005, Zak et al., 2005). Support for the importance of oxytocin in male social behavior can be derived from the reduction in oxytocin mRNA production in the PVN and the ability of exogenous oxytocin to improve the social withdrawal in the prenatally stressed rats. While a relatively high dose of oxytocin was needed to observe the pro-social effect of oxytocin in this model, these concentrations are similar to other studies where behavioral effects have been observed (Witt et al., 1992, Consiglio et al., 2005). Without knowledge of the actual concentration of oxytocin that binds to the receptors in the amygdala, it is impossible to determine the physiological relevance of this effect but these findings nonetheless suggest a focal location, the CeA, as a final common pathway for male non-sexual interactive behaviors.
Vasopressin, which is structurally similar to oxytocin, surprisingly failed to improve the unpredictable prenatal stress-induced social deficit in the present investigations. Vasopressin in species such as the prairie vole, is a potent mediator of a variety of social behaviors (Witt et al., 1990, Young et al., 1997, Cho et al., 1999, Bales and Carter, 2003). In addition, vasopressin has been implicated in the social deficits in rats following repeated phencyclidine administration (Tanaka et al., 2003). How the present data integrate with the data from Tanaka and colleagues remains unclear. Interestingly, we find a reduction of V1a receptor binding in the CeA without changes in either vasopressin or V1a mRNA in the prenatally stressed rat preparation. However, vasopressin infusion into the CeA failed to improve the social impairment in the prenatally stressed rat. Unfortunately, there is no apparent explanation for these dichotomous findings. Changes in testosterone secretion during development have been reported in prenatally stressed rats (Ward et al., 2002) and it is known that the expression of the V1a receptor in some brain regions can be regulated by androgens (Young et al., 2000). However, V1a receptor binding in the CeA appears to be independent of circulating androgen levels in adult rats (Askew et al., 2006). It is conceivable that the reduced prenatal testosterone surge programs lower V1a receptor expression in the CeA, which then could change the balance between oxytocin and vasopressin effects in the CeA. This change would become apparent in situations where oxytocin was administered exogenously leading to enhance oxytocin-mediated excitation of the CeA efferents as opposed to less vasopressinergic inhibitory outputs. Consistent with this notion of differential actions of oxytocin and vasopressin in the CeA are the data provided by Huber et al. who investigated the electrophysiological signatures of CeA neurons. In these studies, neurons in the lateral aspect of the CeA were excited by oxytocin, while neurons in the medial aspect of the CeA were inhibited by vasopressin (Huber et al., 2005). Therefore, even if exogenous vasopressin were to bind to intrinsic CeA oxytocin receptors, these electrophysiological findings would predict that the activation of CeA V1a receptors would oppose any vasopressin-induced oxytocin receptor activation that would be working to enhance social behavior and would appear to support our experimental observations. Our experiments would appear to support different effects of oxytocin and vasopressin in the CeA on a behavioral level.
Studies on the role of oxytocin in schizophrenia have been inconclusive. Reduced numbers of nitric oxide synthase-containing oxytocin neurons in the PVN of human schizophrenic brains have been reported (Bernstein et al., 1998). There are also reports of lower concentrations of oxytocin in cerebrospinal fluid obtained from schizophrenic patients in some but not all studies (Linkowski et al., 1984, Beckmann et al., 1985, Mai et al., 1993, Glovinsky et al., 1994). Our results would predict that differences in oxytocin expression should be found in cases with primary but not secondary negative symptoms, which may account for the earlier ambiguous findings investigating oxytocin and schizophrenia. In fact, oxytocin infusion has been reported to have some beneficial effects in schizophrenic patients (Bujanow, 1974). Recent studies found that the atypical antipsychotic drug, clozapine, but not haloperidol increases plasma concentrations of oxytocin (Uvnas-Moberg et al., 1992) suggesting that some antipsychotic medications may have beneficial effects that could be mediated by the oxytocin neuropeptide system. In light of the present data and previous findings from our laboratory (Lee et al., 2005), it would appear that more emphasis needs to be directed toward re-evaluating this neuropeptide in human schizophrenic brain tissue.
Oxytocin receptor binding is dense in the amygdala and abundant data point to amygdala dysfunction in schizophrenic patients. Neuroimaging studies report reductions in amygdalar volumes in schizophrenic patients (Rajarethinam et al., 2001, Anderson et al., 2002). In addition, fMRI studies revealed reduced amygdalar activation in schizophrenic subjects when evaluating facial expressions for emotional salience (Gur et al., 2002, Hempel et al., 2003) and this impairment appears to be specific to patients with primary negative symptoms (Anderson et al., 2002). These studies in human subjects are consistent with published animal findings that amygdalar lesions decrease social behavior (Daenen et al., 2002). Based on these data collectively, we would posit that analyses of human post-mortem schizophrenic tissue for oxytocin receptor changes could yield informative data regarding schizophrenia’s pathophysiology. It is also interesting to note that oxytocin receptor binding was not changed by unpredictable prenatal stress in either the ventromedial hypothalamic nucleus or the lateral septum, brain regions that subserve other neurohypophysial peptide-mediated behaviors further supporting the specificity of our findings.
The negative symptoms of schizophrenia have only recently been accepted as a core feature of the disease (Kirkpatrick et al., 2006). Treatment with antipsychotic drugs that ameliorate positive symptoms can have an ancillary effect on secondary negative symptoms without improving primary negative symptoms (Buchanan et al., 1998, McGurk, 1999, Javitt, 2001, Bellack et al., 2004). Investigations into the origin and treatment of the primary negative symptoms have been hampered by this confusing, but critical, taxonomy. The paucity of animal preparations that recapitulate this aspect of the disease has also hampered progress in understanding primary negative symptoms. Recent studies compellingly demonstrated that animals can be useful in studying the social withdrawal component of the negative symptoms of schizophrenia (Sams-Dodd, 1995, Sams-Dodd, 1996, Sams-Dodd et al., 1997, Shi et al., 2003 3712, Rueter et al., 2004, Lee et al., 2005). Given the importance of both environmental and genetic factors in the etiology of schizophrenia, there may be a utility of these models in identifying factors contributing to the origins of the disease.
Therefore, our results indicate that oxytocin may be an important substrate for peer-peer social behavior in male rodents. In addition, the present findings provide evidence to support a distinction between the central roles of oxytocin and vasopressin in male rodents. Specifically, the oxytocinergic system appears to be crucial for the appropriate quality and quantity of social interaction between male rats. The fact that these studies were undertaken in a rodent model of the negative symptoms of schizophrenia is noteworthy, because it links a pathological state (a paucity of normal interactive behaviors) seen in both rats and humans to changes in oxytocin’s production and in its receptors. Further description of the male oxytocinergic system in both rats and humans is needed, particularly for neuropsychiatric illnesses with social pathology.
4. Experimental Procedure
Animals
Timed pregnant female Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). The pregnant rats arrived at our laboratory on gestational day 2. All rats were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) on a 12 hour light/dark schedule (lights on - 0700 hrs) with ad libitum access to food and water throughout the duration of the experiment (except as noted). The treatment of these rats was in accordance with the National Institutes of Health (NIH) guidelines for animal research, and all protocols were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Unpredictable Prenatal Stress Paradigm
Randomly selected pregnant female rats were exposed to a repeated unpredictable stress paradigm during the final eight days of gestation (embryonic days 14-22) (Kinnunen et al., 2003, Koenig et al., 2005). The stress paradigm consisted of 1-hour restraint in cylindrical plastic restrainers (Harvard Bioscience. Boston, MA); 6 hour exposure to a cold (4° C) environment; 18 hour food deprivation; 15 minute swim stress in room temperature water; reversal of light-dark cycle (lights on - 1900); 18 hour overcrowding during dark phase of light cycle. Stressors were applied in a randomized manner to prevent accommodation. A schedule for the application of the stressors is shown in Table 1. All stressed animals received exactly the same stressors. Control (no stress) dams remained in the animal room and were exposed to standard animal room cage maintenance procedures. Following birth, all dams and their pups were left undisturbed in their cages until weaning at 22 days of age at which time male and female offspring were separated and group housed in cages of 2 to 3 same sex littermates except as described below when rats pups were cross-fostered. Young adult male rats 56 - 64 days of age were selected randomly from each litter for the studies. In the majority of experiments, not more than one rat from each litter was used. However, in cases where more than one rat from a litter was subjected to the same experimental procedure, the values from these rats were averaged and this value was used to represent the response of that litter for that particular treatment condition.
Cross-fostering procedure
Timed pregnant female Sprague-Dawley rats (Charles River Laboratories, Wilmington MA) were obtained on day 2 of pregnancy. Randomly selected pregnant female rats were exposed either to the prenatal stress paradigm as described in Table 1 or to standard animal room procedures as described above. Within the first twenty-four hours following birth, all litters were culled to twelve pups (6 male and 6 female). These litters of twelve prenatally stressed pups or non-stressed pups were then transferred to novel mother rats who also had just given birth and had been exposed to either the prenatal stress procedure or standard animal room procedures from days 14-22 of pregnancy. Following the pup transfer, all the reconstituted litters were left undisturbed in their cages until weaning at 22 days of age at which time male and female offspring were separated and group housed in cages of 2 to 3 same sex littermates exposed to the same prenatal treatment (stress or no stress). The male offspring were tested as young adults between 56 - 63 days of age as described below.
Surgical Procedures
Stereotaxic implantation of bilateral CeA cannulae
Cannulae directed toward the CeA were implanted into the brain of anesthetized male rats using a stereotaxic apparatus (David Kopf, Inc., Tujunga, CA) as previously described [54]. Holes in the skull over the CeA were created using a dental drill. Guide cannulae (Plastics One, Inc., Roanoke, VA) constructed of 22 gauge stainless steel tubing, were placed bilaterally above the CeA using coordinates from Paxinos and Watson (Paxinos and Watson, 1986) (AP: - 2.3 mm relative to bregma, L: ±4.1 mm, DV: -7.0 mm, Plate 27). The guide cannulae were fixed to the rat’s skull using four self-tapping stainless steel screws and acrylic dental cement (Lang Dental, Wheeling, IL). The cannulae were capped with an obdurator constructed from 28-gauge stainless steel wire. During the seven-day period of recuperation, the animals were handled and weighed. Animals that had regained their pre-surgical body weight were used as experimental subjects. Rats failing to regain their body weight during the seven days after surgery were excluded from experimental use. As a part of the daily handling and acclimation routine, the obdurators were removed from the guide cannulae to acclimate animals to the manipulation of the cannulae that would occur during the testing phase of the studies. Cannula positions were histologically verified for all animals following behavioral testing using cresyl violet stained sections and in some cases, [125I]-d(CH2)5[Tyr(Me)2,Thr4,Orn8,Tyr9-NH2]-vasotocin (OVTA) was microinjected and autoradiographic images were compared with cresyl violet stained sections. Inclusion criteria for accepting the behavioral data consisted of a rat having two patent CeA cannulae and the placement of both cannulae had to be within 1 mm of the CeA. Animals with cannulae outside of these specifications were not included in the final analysis of the behavioral data.
Behavioral Analyses
Social interaction test
The social interaction procedure was performed as previously described (File and Hyde, 1979, Lee et al., 2005). Briefly, all male rats in these studies were tested at the same age, typically between 56 and 64 days of age. On each of the two days prior to testing experimental (no stress control or prenatally stressed rats) or their weight-matched male target rats were weighed and placed into a black plexiglass arena (dimensions: 65 cm length × 65 cm width × 47 cm height) individually for ten minutes in order to acclimate to the novel setting under dim lighting (150 lux). The bottom of the arena was lined with absorbent bedding. In rats bearing cannulae directed toward the amygdala, acclimation to the test apparatus took place at least 5 days after surgery to allow for recovery in all rats.
On the day of testing, an experimental rat received bilateral intracranial infusions as described above and was placed immediately into the arena alone for 10 minutes using the same lighting conditions as during training. When this 10-minute session ended, a weight-matched male target rat was introduced into the arena. This social interaction trial lasted 10 minutes. Experimental and target rats were not used in this paradigm more than one time. The arena was cleaned with 70% ethanol between each trial. All sessions were videotaped using a cordless video camera so the experimenter could remain outside the room while monitoring the entire testing session. Later, video tapes were scored in a blinded fashion for the time the experimental rat actively engaged in social interaction behaviors (e.g., sniffing, grooming, following, crawling over/under, or boxing/wrestling) with the target male.
Further analysis was performed on the videotaped behavior to assess the relative amounts of time experimental rats engaged in contact or non-contact social interaction with their novel peers. Contact behaviors were defined as the percentage of the total interacting behavior time during which the animals were in direct physical contact; anogenital exploration, sniffing with direct contact, crawling, grooming, and play behaviors met this criterion. Following and proximal (no contact) sniffing were considered non-contact social interactions and scored as such.
Open field testing
Locomotor activities of prenatally stressed and unstressed control rats were monitored using Coulbourn Instruments’ Tru Scan Activity Monitors (Allentown, PA). Activity was measured in a square Plexiglas arena (41 cm length × 41 cm width × 38 cm height) using the interpolation of photobeam interruptions located in two sensor rings. A single sensor ring supplied the X-Y floor plane coordinates. The photobeams were evenly spaced at 2.54 cm intervals in each ring. Photobeam interruptions were assessed every 250 milliseconds. Data were collected on a PC-compatible computer. All rats were tested on postnatal day 56. Prior to testing, the subjects were brought from the animal facility and placed in a holding room for a 60 minute acclimation period. After the acclimation period, each animal was placed in the activity monitor for a 30 minute baseline habituation period. After the 30 minute period data collection was automatically suspended.
Light-Dark emergence test
This procedure was adapted from that of Gurtman et al, (Gurtman et al., 2002). Male rats were placed inside of a familiar black cylindrical tube (dimensions: 19 cm length × 7.5 cm diameter) open at one end. The tube was positioned such that the opening faced the upper left corner of an open field (which was the same arena used in social interaction testing). The rats’ behaviors were videotaped for five minutes while the experimenter was outside the room. The arena and tube were cleaned with a 70% ethanol after each trial. The latency to emerge and times spent inside and outside the tube were scored from the videotape in a blinded fashion. This test was performed prior to social interaction testing so the rats were naïve to the arena.
Analytical Protocols
Oxytocin and vasopressin mRNA in situ hybridization histochemistry
These methods have been previously published (Lee et al., 2005). Rats designated for use in these biochemical studies were sacrificed by decapitation at 64 days of age, 24 hours after social interaction testing. A second group of behaviorally naïve rats was sacrificed to confirm that the mRNA and binding changes were independent of the animal’s experience in the behavioral testing arena. Brains from all rats were removed rapidly, placed into powdered dry ice with hypothalamus facing upwards, and stored at - 70° C until sectioned into coronal brain sections (12 micron) using a cryostat (Leica Instruments, Deerfield, IL). These sections were mounted consecutively on SupraFrost Plus slides (Fisher Scientific Co., Pittsburgh, PA) and stored at -70° C until thawing immediately prior to use. Every ninth section was mounted separately and stained with cresyl violet for anatomical verification. Sections from all similar experimental groups and their controls were run in the same in situ hybridization experiment. Thawed sections from subjects in all experimental groups were fixed in 4% paraformaldehyde, then acetylated with acetic anhydride (0.25%) in triethanolamine (0.1 M, pH 8) and finally were dehydrated through graded alcohols and delipidated in chloroform. The sections were coated with a hybridization buffer containing 50% formamide, 2x SSC, 10% dextran sulfate, 0.25% BSA, 0.25% PVP, 0.25% Ficoll 400, 250 mM Tris (pH 7.5), 0.5% SDS, 250 μg/ml single stranded salmon sperm DNA containing 1 × 106 cpm of the appropriate 35S-labeled cRNA probe (see below). Following hybridization (14 hrs. at 55° C in a humid chamber), the slides are washed in 4x SSC, incubated with RNase A (20 mg/ml) to reduce background, washed under high stringency conditions (0.1x SSC at 68° C) and dehydrated in graded ethanol solutions (70%-100%). After the slides had dried for a minimum of two hours, they were placed alongside calibrated 14C microscales (Amersham Pharmacia Biotech, Piscataway, NJ) into X-ray cassettes with Kodax BioMax MR film (Rochester, NY). Optimal exposure times were determined empirically for each probe (see below). The films were developed according to the manufacturer’s protocol.
Descriptions of mRNA probes
Bacterial plasmid vectors containing oxytocin and vasopressin coding sequences were obtained from Thomas Sherman Ph.D. (Georgetown University, Washington, DC, (Sherman et al., 1988)). Oxytocin receptor mRNA studies in CeA and ventromedial hypothalamus (VMH) were performed using a probe obtained from Dr. Dan Dorsa (Oregon Health Sciences University, Portland, Oregon). The oxytocin receptor and the V1aR DNA templates have been described elsewhere (Szot et al., 1994, Bale and Dorsa, 1995). Radiolabeled antisense cRNA probes were produced by in vitro transcription of appropriately linearized plasmid vectors using T7 or SP6 RNA polymerase as described by the manufacuter (Promega). Probes were labeled by incorporating 35S-CTP into the reaction mixture (Amersham Pharmacia Biotech, Piscataway, NJ). Background hybridization was determined using radiolabeled sense cRNA sequences that were also generated from appropriately linearized plasmids. Exposure times were based on the radioactive content of the applied probes (oxytocin mRNA - 24 hrs; vasopressin mRNA - 45 minute; oxytocin receptor mRNA - 14-15 days; V1aR mRNA - 10 days).
Oxytocin and vasopressin receptor binding assay: Receptor Autoradiography
Oxytocin and vasopressin receptor autoradiography were performed according to the protocol of Francis et al (Francis et al., 2000). Briefly, fresh-frozen rat brain tissue sections prepared identically to those used in the in situ hybridization studies described above were used. Slides were thawed at room temperature for 10 minute; a Pap pen (Sigma-Aldrich, St Louis, MO) was used to encircle the outer perimeter of the tissue sections. Next, the tissue was fixed in 4% paraformaldehyde for 2 minutes and rinsed in three washes of 50 mM Tris-buffered saline (TBS, pH 7.4) for 5 minutes at room temperature. Immediately following the third wash, tissue sections were incubated for 1 hour with a 500 microliter aliquot of TBS containing 10 mM MgCl2, 0.1% bovine serum albumin, 0.05% bacitracin and 50 pM of the [125I]-labeled receptor-specific ligand. For oxytocin receptor binding, the ligand was [125I]-d(CH2)5[Tyr(Me)2,Thr4,Orn8,Tyr9-NH2]-vasotocin (OVTA) (2200 Ci/mmol, Amersham Pharmacia Biotech, Piscataway, NJ); for V1a receptor the ligand was 125I-lin-vasopressin (2200 Ci/mmol, Amersham Pharmacia Biotech, Piscataway, NJ). Non-specific binding was determined by adding 50 mM unlabeled Thr4, Gly7 oxytocin or [1-(β-mercapto-β,β-cyclo-pentamethylene propionic acid),2-(O-methyl)-tyrosine]-arg8-vasopressin (Bachem, San Carlos, CA, USA) to the incubation mixture. After incubation, slides were rinsed in four cold (4° C) 50 mM TBS washes containing 10 mM MgCl2 for five minutes followed by a fifth wash in the same chilled buffer for 30 minutes. Finally, slides were rapidly dried under a stream of cool air. When slides were dry, they were placed into autoradiographic cassettes with Kodak BioMax MR film (Rochester, NY). [125I]-microscale standards (Amersham Biosciences, Piscataway, NJ, USA) were placed into all X-ray cassettes. Film was developed after 6-7 days of exposure.
Data analysis
The Paxinos and Watson brain atlas (Paxinos and Watson, 1986) was used to define the locations and boundaries of the structures of interest (CeA, PVN, SON, VMH and LS) on cresyl violet-stained sections taken at approximately 100 micron intervals from each brain. Densitometric analyses were performed on the autoradiographic films using NIH Image software (version 1.62) running on a Power Macintosh computer. Measurements were obtained in at least three consecutive tissue sections (except PVN, which was performed on at least two consecutive sections) containing the desired structure. For in situ hybridization histochemistry, background levels of hybridization were obtained from readings in white matter structures such as the corpus callosum where minimal binding would be expected to occur and subtracted from the mean reading of the area of interest using the semiquantitation methodology and from analyzing sections incubated with radiolabeled sense-strand probes (Bowers et al., 1998). For receptor binding, the amount of radioactivity within the tissue sections (as a dpm/mg equivalent) were determined by creating a best-fit curve (a Rodbard fit) to the image of the known [125I] microscale standard. Non-specific binding in an adjacent section was subtracted from the final estimate of bound ligand to determine the specific tissue binding.
Statistical Analysis
Data from the social interaction and light-dark emergence tests are reported as mean time in seconds ± standard error of the mean (SEM) or as percent of total time ± SEM. Open field data are reported as mean total cumulative distances (in cm) ± SEM. Statistical significance for social interaction comparisons between prenatally stressed and no stress control rats was determined by an independent samples t-test using Graphpad Prism version 4 software (San Diego, CA). A repeated measures ANOVA was used to analyze the contact vs. non-contact comparisons, light-dark emergence data, and locomotor behaviors of experimental rats and their matched controls. Two-way ANOVA (Graphpad Prism 4.0, San Diego, CA) was used to determine the effect of prenatal stress exposure and oxytocin infusions on social interaction behaviors. Bonferroni multiple comparisons test was used to assess differences between individual groups in this experiment also (Graphpad Prism 4.0, San Diego, CA).
The in situ hybridization histochemistry data are reported as the mean percent of the no stress control subjects’ relative optical density readings ± SEM. Receptor binding data are expressed as the mean percent of control radioactive ligand content ± SEM. Optical density readings of mRNA and receptor binding present in no stress control tissue samples were established as 100% for purposes of relative comparison. Statistical significance for mRNA expression and receptor binding was determined by an independent samples t-test using Graphpad Prism version 4 software (San Diego, CA). In all instances, a p-value < 0.05 was considered significant.
Specific Experimental Protocols
EXPERIMENT 1
Twelve timed-pregnant female Sprague-Dawley rats (Charles River Labs., Wilmington, MA) arrived on day 2 of gestation. The rats were individually housed in animal quarters maintained on a constant light-dark cycle (lights on at 0700-1900) with controlled humidity. The rats were randomly assigned to the non-stressed group that received normal animal husbandry daily (n=6) or the prenatal stress group that was exposed to the unpredictable prenatal stress protocol outlined in Table 1 from gestational days 14-21 (n=6). Rats were born on day 22 and the litters were left undisturbed until the pups were weaned on post-natal day 22. At weaning the pups were weighed and group-housed with 1-2 same sex littermates. On post-natal day 33, a randomly selected male pup from the litters exposed to unpredictable prenatal stress (n=6) or the control litters (n=6) were brought to the laboratory. After being weighed, the rats were placed in an open-field arena for 10 minutes. At the end of the 10-minute period, the rats were returned to their cages and the walls of the arena were cleansed with 70% ethanol before placing the next rat into the arena. At the end of day, the rats were returned to the animal quarters. This procedure was repeated the following day. On post-natal day 35, the same rats were brought to the laboratory again and were weighed. On this day, however, when the pups were placed in the arena, a weight-matched, novel, non-stressed male Sprague-Dawley rat (the target rat) was already present in the arena. The target rat had been exposed to the arena on two previous occasions also and its fur had been dyed with non-sweetened food coloring at least 24 hours prior to being paired with the experimental rat. The rats were videotaped in the arena for 10 minutes and removed. The walls of the arena were cleaned with 70% ethanol between rats. The videotapes were scored for time spent in social behaviors generated by the experimental animal toward the marked target by a trained rater who was blind to the group of the experimental subject. The social interaction times for each prenatally stressed rat (n=6) or non-stressed control rat (n=6) were used to generate the mean group social interaction times (sec) ± SEM. Rats were only used one time for the social interaction test because of the potential for carry-over effects from this exposure.
On post-natal day 54, which is post-puberty in male Sprague-Dawley rats, other rats from each litter were randomly selected for social interaction testing. The rats were exposed to the novel, open field arena for two days as above. On post-natal day 56, the rats were brought to the laboratory again and were weighed. On this day, however, when the rats were placed in the arena, a weight-matched, novel, non-stressed male Sprague-Dawley rat (the target rat) was already present in the arena. The target rat had been exposed to the arena on two previous occasions also and its fur had been dyed with non-sweetened food coloring at least 24 hours prior to being paired with the experimental rat. The rats were videotaped in the arena for 10 minutes and removed. The walls of the arena were washed with 70% ethanol between rats. The videotapes were scored for time spent in social behaviors generated by the experimental animal toward the marked target by a trained rater who was blind to the group of the experimental subject. The social interaction times for each prenatally stressed rat (n=6) or non-stressed control rat (n=6) were used to generate the mean group social interaction times (sec) ± SEM. Immediately after exposure to the arena, the experimental rats were replaced in their home cage and returned to the animal quarters. Twenty-four hours after behavioral testing the experimental rats were euthanized by decapitation. The brains were collected on dry ice and were stored at -70°C. The brains were cut into 12 micron sections on a cryostat and placed onto Suprfrost Plus slides. These sections were used to determine oxytocin and vasopressin mRNA expression as well as oxytocin and vasopressin V1A receptor binding. Rats were only used for the social interaction test one time because of the potential for carry-over effects from this exposure.
Other rats from these pregnant dams were used to assess anxiety-related behaviors using open-field testing with specific attention to center time and light-dark emergence testing. The rats used in these tests had not been exposed to behavioral testing prior to post-natal day 56. One randomly selected male rat from each litter was used to populate the test subjects for these analyses with an overall n of 6 rats from 6 different litters in each experimental group (prenatally stressed and non-stressed control).
EXPERIMENT 2
Forty-eight timed-pregnant female Sprague-Dawley rats (Charles River Labs., Wilmington, MA) arrived on day 2 of gestation. The rats were individually housed in animal quarters maintained on a constant light-dark cycle (lights on at 0700-1900) with controlled humidity. The rats were randomly assigned to the non-stressed group that received normal animal husbandry daily (n=24) or the prenatal stress group that was exposed to the unpredictable prenatal stress protocol outlined in Table 1 from gestational days 14-21 (n=24). Rats were born on day 22. Within the first twenty-four hours following birth, all litters were culled to twelve pups (6 male and 6 female). These litters of twelve prenatally stressed pups or non-stressed pups were transferred to novel mother rats who also had just given birth and had been exposed to either the prenatal stress procedure or standard animal room procedures from days 14-22 of pregnancy. Following the pup transfer, all the reconstituted litters were left undisturbed in their cages until weaning at 22 days of age at which time male and female offspring were separated and housed in groups of 2 to 3 rats with same sex peers exposed to the same prenatal treatment (stress or no stress). On post-natal day 54, male rats from each litter were randomly selected for social interaction testing. The rats were exposed to the novel, open field arena for two days, 10 minutes per day. On post-natal day 56, the same rats were brought to the laboratory again and were weighed. On this day, however, when the rats were placed in the arena, a weight-matched, novel, non-stressed male Sprague-Dawley rat (the target rat) was already present in the arena. The target rat had been exposed to the arena on two previous occasions also and its fur had been dyed with non-sweetened food coloring at least 24 hours prior to being paired with the experimental rat. The rats were videotaped in the arena for 10 minutes and removed. The walls of the arena were washed with 70% ethanol between rats. The videotapes were scored for social behaviors generated by the experimental animal toward the marked target rat by a trained rater blind to the experimental group. The individual social interaction times (sec) were used to generate group mean social interaction times ± SEM for the prenatally stressed rats (n=6) fostered by non-stressed dams or prenatally stressed dams (n=6) and non-stressed control rats (n=6) fostered by either non-stressed or prenatally stress dams (n=6).
EXPERIMENT 3
Sixteen timed-pregnant female Sprague-Dawley rats (Charles River Labs., Wilmington, MA) arrived on day 2 of gestation. The rats were individually housed in animal quarters maintained on a constant light-dark cycle (lights on at 0700-1900) with controlled humidity. The rats were randomly assigned to the non-stressed group which received normal animal husbandry daily (n=8) or the prenatal stress group that was exposed to the unpredictable stress protocol outlined in Table 1 from gestational days 14-21 (n=8). Rats were born vaginally on day 22 and the litters were left undisturbed until the pups were weaned on post-natal day 22. The rat pups were group-housed with 1-2 same sex littermates. On post-natal day 56, male rats from each litter were randomly selected for surgical implantation of CeA-directed cannulae using the procedure described above. Following cannula implantation, the rats were individually housed and each day they were weighed and handled for at least 5 minutes to acclimate the animals to manipulation. Beginning three days after surgery while the rats were being handled, the obdurators of the CeA-directed cannulae were removed and replaced to acclimate the rats to this procedure. Beginning on post-natal day 61, the rats were placed in the open-field arena for 10 minutes as above. On post-natal day 63, the rats were brought to the laboratory again and weighed. On this day, however, each rat received an infusion of saline vehicle, oxytocin (10 - 1000 ng) or vasopressin (10 or 3000 ng) into the CeA cannulae. There were 8 rats in each treatment group and no more than one rat from a litter received each treatment. The rats were then placed into the arena for 10 minutes. At the end of this period, a weight-matched, novel, non-stressed male Sprague-Dawley rat (target rat) was placed into the arena. The target rat had been exposed to the arena on two previous occasions also and its fur had been dyed with green food coloring at least 24 hours prior to being paired with the experimental rat. The rats were videotaped in the arena for 10 minutes and removed. The walls of the arena were washed with 70% ethanol between rats. The videotapes were scored for social behaviors generated by the experimental animal toward the marked target by a trained rater who was not aware of the experimental groups. The social interaction times for each individual prenatally stressed rat or non-stressed control rat were used to generate the mean group social interaction times (sec) ± SEM. Twenty-four hours after behavioral testing, the experimental rats were euthanized by decapitation. The brains were collected on dry ice and were stored at -70°C in order to verify the location of the CeA-directed cannulae histologically. Behavioral data obtained from rats with cannulae not located within 2 mm of the CeA were not included in the data analysis, nor were any subjects in whom only one cannula was optimally positioned or functional at the time of infusion.
Acknowledgements
Support for this work was provided by NIH grant MH073826 (JIK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson JE, Wible CG, McCarley RW, Jakab M, Kasai K, Shenton ME. An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophr Res. 2002;58:123–134. doi: 10.1016/s0920-9964(01)00372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Carpenter WT. Diagnosis and classification of schizophrenia. Schiz Bull. 1993;19:199–214. doi: 10.1093/schbul/19.2.199. [DOI] [PubMed] [Google Scholar]
- Askew A, Gonzalez FA, Stahl JM, Karom MC. Food competition and social experience effects on V1a receptor binding in the forebrain of male Long-Evans hooded rats. Horm Behav. 2006;49:328–336. doi: 10.1016/j.yhbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM. Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology. 1995;136:5135–5138. doi: 10.1210/endo.136.11.7588251. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Beckmann H, Lang RE, Gattaz WF. Vasopressin--oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10:187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Schooler NR, Marder SR, Kane JM, Brown CH, Yang Y. Do clozapine and risperidone affect social competence and problem solving? Am J Psychiatry. 2004;161:364–367. doi: 10.1176/appi.ajp.161.2.364. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83:867–875. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Boksa P, El-Khodor BF. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev. 2003;27:91–101. doi: 10.1016/s0149-7634(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT., Jr. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Brixey SN, Gallagher BJ, 3rd, McFalls JA, Jr., Parmelee LF. Gestational and neonatal factors in the etiology of schizophrenia. J Clin Psychol. 1993;49:447–456. doi: 10.1002/1097-4679(199305)49:3<447::aid-jclp2270490321>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr. Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Immunocytochemical demonstration of vasopressin and oxytocin in the rat brain by light and electron microscopy. J Histochem Cytochem. 1980;28:357–360. doi: 10.1177/28.4.6989899. [DOI] [PubMed] [Google Scholar]
- Bujanow W. Letter: Is oxytocin an anti-schizophrenic hormone? Can Psychiatr Assoc J. 1974;19:323. doi: 10.1177/070674377401900323. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998:95. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT, Jr., Buchanan RW. Schizophrenia. N Engl J Med. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehavioral Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav. 2005;85:354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA. The New York high risk project to the Hillside recognition and prevention (RAP) program. Am J Med Genet. 2002;114:956–966. doi: 10.1002/ajmg.b.10520. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res. 2002;136:571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. A test of anxiety that distinguishes between the actions of benzodiazepines and those of other minor tranquilisers and of stimulants. Pharmacol Biochem Behav. 1979;11:65–69. doi: 10.1016/0091-3057(79)90298-3. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Fride E, Dan Y, Feldon J, Halevy G, Weinstock M. Effects of prenatal stress on vulnerability to stress on prepubertal and adult rats. Physiol Beh. 1986;37:681–687. doi: 10.1016/0031-9384(86)90172-1. [DOI] [PubMed] [Google Scholar]
- Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, Wyatt RJ. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res. 1994;11:273–276. doi: 10.1016/0920-9964(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Gurtman CG, Morley KC, Li KM, Hunt GE, McGregor IS. Increased anxiety in rats after 3, 4-methylenedioxymethamphetamine: association with serotonin depletion. Eur J Pharmacol. 2002;446:89–96. doi: 10.1016/s0014-2999(02)01820-4. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. I. Endocrine factors in the pathophysiology of mental disorders. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull. 1997:33. [PubMed] [Google Scholar]
- Hempel A, Hempel E, Schonknecht P, Stippich C, Schroder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Res. 2003;122:115–124. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, LeMoal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Management of negative symptoms of schizophrenia. Curr Psychiatry Rep. 2001;3:413–417. doi: 10.1007/s11920-996-0036-9. [DOI] [PubMed] [Google Scholar]
- Kelley ME, Gilbertson M, Mouton A, van Kammen DP. Deterioration in premorbid functioning in schizophrenia: a developmental model of negative symptoms in drug-free patients. Am J Psychiatry. 1992;149:1543–1548. doi: 10.1176/ajp.149.11.1543. [DOI] [PubMed] [Google Scholar]
- Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J Neurochem. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr., Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Koenig JI. Hippocampal-dependent cognitive function and nicotinic receptor expression is compromised by prenatal stress exposure in the rat. Soc Neurosci Abstr. 2005;35:556–515. [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Martin JR, Jenck F, Moreau JL. Spatial and associative learning deficits induced by neonatal excitotoxic hippocampal damage in rats: further evaluation of an animal model of schizophrenia. Behav Pharmacol. 2000;11:257–268. doi: 10.1097/00008877-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady D, Shapiro RA, Dorsa DM, Koenig JI.Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin Neuropsychopharmacology 2005301883–1894.(published online 1882/1816/1805) [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Stohr T, Feldon J. Long-term effects of prenatal stress experience and postnatal maternal separation on emotionality and attentional processes. Beh Brain Res. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ. Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci. 1984;234:162–165. doi: 10.1007/BF00461555. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Mai JK, Berger K, Sofroniew MV. Morphometric evaluation of neurophysin-immunoreactivity in the human brain: pronounced inter-individual variability and evidence for altered staining patterns in schizophrenia. J Hirnforsch. 1993;34:133–154. [PubMed] [Google Scholar]
- McGurk SR. The effects of clozapine on cognitive functioning in schizophrenia. J Clin Psychiatry. 1999;60(Suppl 12):24–29. [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Moller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull. 2000;26:217–232. doi: 10.1093/oxfordjournals.schbul.a033442. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Printz DJ, Butler PD, Dulawa SC, Printz MP. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193–201. doi: 10.1016/j.brainres.2003.09.077. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando, Fl: 1986. [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, DeQuardo JR, Miedler J, Arndt S, Kirbat R, Brunberg JA, Tandon R. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res. 2001;108:79–87. doi: 10.1016/s0925-4927(01)00120-2. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Ballard ME, Gallagher KB, Basso AM, Curzon P, Kohlhaas KL. Chronic low dose risperidone and clozapine alleviate positive but not negative symptoms in the rat neonatal ventral hippocampal lesion model of schizophrenia. Psychopharmacology (Berl) 2004;176:312–319. doi: 10.1007/s00213-004-1897-4. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Distinct effects of d-amphetamine and phencyclidine on the social behaviour of rats. Behav Pharmacol. 1995;6:55–65. [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol. 1996;7:3–23. [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Sherman TG, Day R, Civelli O, Douglass J, Herbert E, Akil H, Watson SJ.1988. Regulation of hypothalamic magnocellular neuropeptides and their mRNAs in the brattleboro rat: coordinate responses to further osmotic challenge [DOI] [PMC free article] [PubMed]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. The genetics of schizophrenia. PLoS Med. 2005;2:e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Mol Brain Res. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Haglin C, Kalin NH. Prenatal stress potentiates stress-induced behavior and reduces the propensity to play in juvenile rats. Physiol Behav. 1992;51:319–323. doi: 10.1016/0031-9384(92)90147-t. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Suzuki M, Sumiyoshi T, Murata M, Tsunoda M, Kurachi M. Subchronic phencyclidine administration alters central vasopressin receptor binding and social interaction in the rat. Brain Res. 2003;992:239–245. doi: 10.1016/j.brainres.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl) 1992;109:473–476. doi: 10.1007/BF02247726. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, LeMoal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. The Journal of Neuroscience. 1997:17. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav. 2000;70:359–366. doi: 10.1016/s0031-9384(00)00270-5. [DOI] [PubMed] [Google Scholar]
- Ward OB, Ward IL, Denning JH, Hendricks SE, French JA. Hormonal mechanisms underlying aberrant sexual differentiation in male rats prenatally exposed to alcohol, stress, or both. Arch Sex Behav. 2002;31:9–16. doi: 10.1023/a:1014018931977. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs and schizophrenia: a search for common ground. Schizophrenia Research. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behavior of the offspring. Progress in Neurobiology. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Walton DM. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster) Pharmacol Biochem Behav. 1990;37:63–69. doi: 10.1016/0091-3057(90)90042-g. [DOI] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
- Young LJ. Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Cooper TT, Albers HE. Vasopressin (V1a) receptor binding, mRNA expression and transcriptional regulation by androgen in the Syrian hamster brain. J Neuroendocrinol. 2000;12:1179–1185. doi: 10.1046/j.1365-2826.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Wang Z, Gingrich B, Guo Q, Matzuk MM, Insel TR. Gene targeting approaches to neuroendocrinology: oxytocin, maternal behavior, and affiliation. Horm Behav. 1997;31:221–231. doi: 10.1006/hbeh.1997.1377. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopamergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]