Abstract
Aim
The aim of this study was to evaluate whether maternal circulating adrenomedullin (AM) values in patients with preeclampsia are different from those in normotensive pregnant women at different gestational ages.
Subjects and Methods
In a prospective clinical study, 90 women aged 17 to 40 years old, were divided into 4 main groups: group I (45 women): Normotensive pregnant women at first trimester (15 women), second trimester (15 women), and third trimester (15 women) of pregnancies. Group II (15 women): Pregnant women with preeclampsia at 25 to 38 weeks of gestation. Group III (15 women): Normotensive healthy nonpregnant women. Group IV (15 women): Hypertensive nonpregnant women. The plasma AM concentration was measured in all women by using enzyme immunoassay kits.
Results
Plasma AM levels in pregnant women with normal blood pressure at different gestational ages (first, second, and third trimesters) were statistically significantly higher than those detected in nonpregnant normotensive women and significantly increased with increasing gestational age (P < .001). Moreover, there was significant positive correlation between plasma AM levels and increasing gestational age (r = 0.915, P < .001). Preeclamptic patients had the highest mean plasma AM levels compared with all other groups, which is statistically significant (P < .001) and there was a significant positive correlation between plasma AM levels and systolic blood pressure, diastolic blood pressure, severity of preeclampsia, and proteinuria in pregnant patients with preeclampsia.
Conclusion
Maternal plasma AM concentration increases throughout pregnancy and increases as gestational age progresses. AM production starts very early in gestation, suggesting that it may have an important role in human reproduction, from implantation to delivery. Maternal plasma AM level in preeclampsia appears to be higher than that in normal pregnancy.
Introduction
Adrenomedullin (AM) is a 52-amino acid peptide originally isolated in 1993 by Kitamura and colleagues from a human pheochromocytoma. It is a multifunctional peptide produced by many cells and tissue systems. AM was observed to stimulate adenylyl cyclase activity in a platelet bioassay and shares a slight homology with the calcitonin gene-related peptide, a potent hypotensive peptide,[1] reviewed by Hinson and colleagues.[2] AM has been proposed early as an important hormone in circulation control and maintenance of vascular tone because it regulates endothelial permeability[3] and contributes to the differentiation of bone marrow-derived mononuclear cells into endothelial progenitor cells.[4] AM gene is expressed in most organs including the reproductive system; however, the main source of plasma AM is considered to be vascular endothelial cells and vascular smooth muscle cells.[2,5] The plasma level of AM is elevated in various diseases that are often associated with pathologic processes of the vasculature. Furthermore, culture studies on vascular smooth muscle cells or endothelial cells have shown that oxidative stress increases AM production. Collectively, a body of evidence suggests that AM participates in the mechanism against progression of vascular damage and remodeling, thereby alleviating the ischemia of tissues and organs.[6]
In normal pregnancy, fetoplacental perfusion is regulated by the local and systemic effects of the different vasoactive compounds produced by the endothelium and consequently the systemic blood pressure, so maintenance of adequate blood supply to the fetus is ensured. In preeclampsia, abnormal function of the endothelium could contribute to the changes in the peripheral resistance and fetoplacental perfusion.[7–9] In the first trimester, improper implantation of the placenta occurs, which predisposes to vasospasm and ischemia of the spiral arteries. By production of vasoactive compounds such as prostaglandins, nitric oxide, and AM, the placenta interacts with the maternal physiology during preeclampsia.[10–12] The roles of AM in the pathogenesis of preeclampsia and in the regulation of fetoplacental perfusion are complex.[13] The aim of this study was to evaluate whether maternal circulating AM values in patients with preeclampsia are different from those in normotensive pregnant women at different gestational ages.
Subjects and Methods
This prospective clinical study was carried out on 90 women who were 17 to 40 years old, attending the antenatal clinic in Banha University Hospitals. Women were divided into 4 main groups according to a statistical basis: Group I (45 women): Normotensive pregnant women at first trimester (15 women), second trimester (15 women), and third trimester (15 women) of pregnancies. Gestational age was calculated from the date of the last normal menstrual cycle and confirmed by conventional ultrasound. Group II (15 women): Pregnant women with preeclampsia at 25 to 38 weeks of gestation with an elevated blood pressure (>/= 140/90 mm Hg) plus proteinuria 2+ or more in a dipstick test. Women were excluded from the study if any of the following were present: Women were in labor or experienced uterine contractions, evidence of intrapartum infection, evidence of other pregnancy complications (multiple gestation, intrauterine growth restriction, diabetes or premature rupture of membranes), prepregnancy medical diseases (chronic hypertension, renal disease, liver disease, or collagen vascular disease), and maternal smoking. Group III (15 women): Normotensive healthy nonpregnant women. Group IV (15 women): Hypertensive nonpregnant women. No woman was known to have a history of liver, renal, or metabolic disease.
All women in the present study were subjected to full history-taking; complete general and abdominal examination; a mid-stream, clean-catch urine sample was examined for proteins; and transabdominal ultrasound for pregnant women by using a New Sonic HQ-LC 2000, plus Doppler with a 3.5-MHz curvilinear probe. Informed consent from all women about the aim of the work and the tests to be done on a protocol approved by the University of Banha, Clinical Pathology Department and the local Ethics Committee. The plasma AM concentration was measured for all women by using enzyme immunoassay kits (EIA-3418, DRG International). Blood samples, anticoagulated with EDTA and aprotinin (0.6 TIU / mL of blood), were kept on ice and gently rocked several times to inhibit the activity of proteinases. Centrifugation of the blood was done at 1600 × g for 15 minutes at 4 C and the plasma kept at −70 C until assayed.
Principle of Enzyme Immunoassay
The immunoplate in this kit is precoated with secondary antibody and the nonspecific binding sites are blocked. The secondary antibody can bind to the Fc fragment of the primary antibody (peptide antibody) whose Fab fragment will be competitively bound by both biotinylated peptide and peptide standard or targeted peptide in sample. The biotinylated peptide is able to interact with streptavidin-horseradish peroxidase (SA-HRP), which catalyzes the substrate solution composed of 3,3′,5,5′-tetramethylbenzidine (TMB) and hydrogen peroxidase to produce a blue solution. The enzyme-substrate reaction is stopped by hydrogen chloride (HCI) and the solution turns yellow. The intensity of the yellow is directly proportional to the amount of biotinylated peptide-SA-HRP complex but inversely proportional to the amount of the peptide in standard solutions or samples. The intra- and interassay coefficient of variation of the results obtained using this kit did not exceed 5% and 14%, respectively.[14] Statistical package for social sciences version 10.0 (SPSS version 10.0) was used for data analysis. The results were analyzed by the suitable statistical methods that include: mean ± SD, student's t-test (t), correlation coefficient “r,” and P < .05 is considered significant.[15]
Results
The results of the present study showed that all groups were matched regarding the mean age and mean parity except in the hypertensive nonpregnant group who had significantly higher age and parity compared with normotensive nonpregnant women, pregnant women with normal blood pressure at different gestational ages and preeclampsia (37.5 ± 1.7, 24.4 ± 2.9, 25.1 ± 5.3, 25.8 ± 4.1, 25.0 ± 4.2, and 24.7 ± 4.8, respectively for age, < .001 and 4.66 ± 1.23, 2.0 ± 0.65, 1.26 ± 1.53, 1.33 ± 1.13, 1.27 ± 1.12, and 0.8 ± 1.14, respectively for parity, P < .001).
Regarding the mean systolic and diastolic blood pressure among the studied groups, preeclamptic patients had the highest mean systolic and diastolic blood pressure (162.7 ± 13.47 and 104.66 ± 8.12, respectively), which were statistically significant compared with normotensive nonpregnant women (114.0 ± 7.36 and 70.66 ± 7.98, respectively) and pregnant women with normal blood pressure at different gestational ages (111.0 ± 8.70, 70.66 ± 7.28 for first trimester; 110.0 ± 9.81, 70.67 ± 7.76 for second trimester; and 108.0 ± 8.54, 70.33 ± 6.39 for third trimester) and nonpregnant women with hypertension (153.6 ± 8.33, 95.0 ± 5.0, P < .001. Significant proteinuria was observed only in patients with preeclampsia (1.47 g/dL ±1.125, P< .001).
Regarding AM, preeclamptic patients had the highest plasma levels compared with all other groups, which is statistically significant (P < .001). Nonpregnant women with hypertension had statistically significantly higher plasma AM levels compared with normotensive nonpregnant women and pregnant women with normal blood pressure at first trimester (P < .001). However, plasma AM levels in pregnant women with normal blood pressure at different gestational ages (first, second and third trimester) were statistically significantly higher than those detected in nonpregnant normotensive women (P < .001). Also, in pregnant women with normal blood pressure at third trimester AM levels were significantly higher compared with nonpregnant women with hypertension. Moreover, plasma AM levels were significantly increased with increasing gestational age in pregnant women with normal blood pressure (P < .001) (Figure 1). Also, there was significant positive correlation between plasma AM levels and increasing gestational age in normotensive pregnant women (first, second, and third trimester), (r = 0.915, P < .001), (Figure 2). There was a significant positive correlation between plasma AM levels and systolic blood pressure, diastolic blood pressure, severity of preeclampsia, and proteinuria (P < .05, P < .05, P < .01, and P = .01, respectively) in pregnant patients with preeclampsia (Table 1). Also, there was a significant positive correlation between plasma AM levels and both systolic and diastolic blood pressure (r = 0.740, P = .002; and r = 0.675, P = .006, respectively) in nonpregnant women with hypertension (Table 2).
Figure 1.
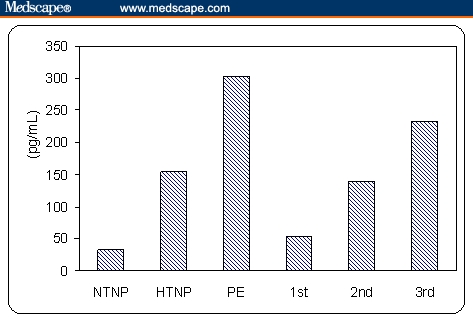
Mean value of AM in the studied groups (NTNP: normotensive nonpregnant, HTNP: hypertensive nonpregnant; PE: preeclampsia, 1st: normotensive pregnant 1st trimester, 2nd: normotensive pregnant 2nd trimester, 3rd: normotensive pregnant 3rd trimester).
Figure 2.
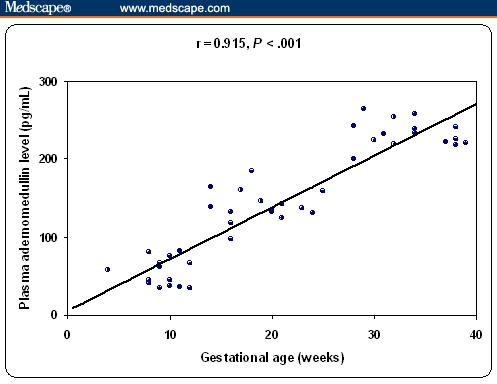
Correlation between plasma AM levels (pg/mL) and gestational age in normotensive pregnant women (first, second, and third trimesters).
Table 1.
Correlation Between Plasma Adrenomedullin Levels (pg/mL) and Other Parameters in Preeclampsia Group
| Parameters | Plasma adrenomedullin levels (pg/mL) | |
|---|---|---|
| Correlation Coefficient (r) | P Value | |
| Age (y) | −0.121 | .667 |
| Parity (n) | −0.152 | .589 |
| Gestational age (wk) | −0.019 | .946 |
| Systolic blood pressure (mm Hg) | 0.551 | .033* |
| Diastolic blood pressure (mm Hg) | 0.529 | .043* |
| Proteinuria (g/dL) | 0.678 | .005** |
| Severity of preeclampsia | 0.643 | .01** |
Correlation is significant at the .05 level (2-tailed);
correlation is significant at the .01 level (2-tailed)
Table 2.
Correlation Between Plasma Adrenomedullin Levels (pg/mL) and Other Parameters in Hypertensive Nonpregnant Group
| Parameters | Plasma Adrenomedullin Levels (pg/mL) | |
|---|---|---|
| Correlation Coefficient (r) | P Value | |
| Age (y) | −0.003 | .991 |
| Parity (n) | −0.020 | .943 |
| Systolic blood pressure (mm Hg) | 0.740 | .002* |
| Diastolic blood pressure (mm Hg) | 0.675 | .006* |
| Proteinuria (g/dL) | 0.260 | .350 |
Correlation is significant at the .01 level (2-tailed)
Discussion
The results of this study showed that plasma AM levels in pregnant women with normal blood pressure at different gestational ages (first, second, and third trimester) were statistically significantly higher than those detected in nonpregnant normotensive women (53.4 ± 17.3, 139.8 ± 20.9, and 232.6 ± 17.2 vs 32.9 ± 8.7 pg/mL, P < .001). Also plasma AM levels were significantly increased with increasing gestational age in pregnant women with normal blood pressure in the first, second and third trimester respectively (P < .001). Also, there was significant positive correlation between plasma AM levels and increasing gestational age in normotensive pregnant women (r = 0.915, P < .001). These results agree with those reported by Di Iorio and colleagues[16] and Hoshimoto and colleagues,[17] who observed a gradual increase in plasma AM as pregnancy progressed and there was a correlation to the gestational age.
Kobayashi and colleagues[18] provided evidence that the placenta produces AM and secretes it into the maternal circulation, particularly during the third trimester of pregnancy. The finding of increased concentrations in late pregnancy, followed by a significant decrease in the puerperium, and the demonstration of AM mRNA in placenta and decidua, suggested that the placenta is a source of AM during pregnancy. All the previous studies, together with the present work, partially disagree with the study done by Di Iorio and colleagues,[19] who reported that, while plasma AM was increased on average 5-fold in pregnant women compared with nonpregnant women, there was no correlation with gestational age and that increased plasma AM was associated with increased blood volume during pregnancy.[20]
Successful pregnancy outcome depends on maintenance of efficient placental perfusion; this allows the exchange of nutrients and oxygenation of fetal blood. These important adaptations occur through the peptide AM via its profound effects on the vasculature including vasodilatation and subsequent promotion of angiogenesis.[21]
In our study preeclamptic patients had the highest mean plasma AM levels compared with normotensive pregnant women at different gestational ages (first, second, and third trimester), normotensive nonpregnant women and hypertensive nonpregnant women, which is statistically significant (302.1 ± 46.0 vs 53.4 ± 17.3, 139.8 ± 20.9, 232.6 ± 17.2, 32.9 ± 8.7, and 153.8 ± 31.4 pg/mL, respectively, P < .001). These results agree with Lauria and colleagues,[22] who showed that women with preeclampsia had significantly elevated AM levels when compared with normotensive controls and disagree with those reported by Hata and colleagues,[27] who suggested that production of AM increases during pregnancy and after delivery, whereas that in preeclampsia decreases and with Jerat and colleagues,[28] who showed that there were no significant differences in maternal plasma AM levels among women with normal pregnancies, gestational hypertension, and preeclampsia.
Gratton and colleagues[13] found an increase in regional localization and cellular expression of AM mRNA in the placenta of preeclamptic women; however, others suggested that fetal membranes, but not placenta were the main source of increased AM during preeclampsia in preterm and term tissues.[23–25] The autocrine effect of the different vasoconstrictors that act on the placental vasculature in preeclampsia is counteracted by the overproduction of AM so fetal circulation is maintained at a physiologic level.[11]
In general, it appears that elevated AM in preeclampsia is a consequence rather than a cause of the pathology. It is unclear whether systemic increases in AM reflect an overflow from local sites of production and action or whether in certain conditions increased AM has a hormonal function causing a general decrease in vascular resistance and fall in blood pressure.
Our study showed significant positive correlation between plasma AM levels and systolic blood pressure, diastolic blood pressure, severity of preeclampsia, and proteinuria (r = 0.551, P < .05; r = 0.529, P < .05; r = 0.643, P < .01; and r = 0.678, P < .01, respectively) in pregnant patients with preeclampsia.
These results are in agreement with those reported by Lu and colleagues,[33] who reported that there was a significant difference between AM levels in patients with severe, moderate, and mild preeclampsia. Also the author found a significant positive correlation between AM levels and systolic blood pressure, diasolic blood pressure, and proteinuria.
The results of the present study showed that nonpregnant women with hypertension had significantly higher plasma AM concentrations (153.8 ± 31.4 pg/mL) compared with normotensive nonpregnant women (32.9 ± 8.7 pg/mL) (P < .001). Also, there was a significant positive correlation between plasma AM levels and both systolic and diastolic blood pressure (r = 0.740, P = .002; and r = 0.675, P = .006, respectively), these results agree with those reported by some authors.[11–13, 21, 29]
AM is a potent and long-lasting vasodilator, is becoming increasingly attractive as a potential key mediator of blood pressure homeostasis. In addition, plasma AM levels are increased in cardiovascular diseases, such as heart failure, hypertension, and septic shock, where AM seems to play a protective role.[30–32]
Conclusions
The presence of significantly elevated AM plasma levels both in healthy and preeclamptic pregnant women (as compared with respective values from nonpregnant women) may suggest that AM is involved in altered endocrine status triggered by pregnancy.
The presence of the highest AM level in the preeclamptic pregnant women (as compared with all other groups including the hypertensive nonpregnant group), in addition to the significant positive correlation between AM plasma levels and systolic blood pressure, diastolic blood pressure, severity of preeclampsia, and proteinuria make participation of AM in the pathogenesis of hypertension in preeclampsia likely.
Footnotes
Reader Comments on: Study of Plasma Adrenomedullin Level in Normal Pregnancy and Preeclampsia See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at Azza_abosenna@yahoo.com or to George Lundberg, MD, Editor in Chief of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: glundberg@medscape.net
Contributor Information
Azza Abo Senna, Banha University, Faculty of Medicine, Banha, Egypt Author's email: Azza_abosenna@yahoo.com.
Magda Zedan, Banha University, Faculty of Medicine, Banha, Egypt.
Gamal E. Abd El Salam, Banha University, Faculty of Medicine, Banha, Egypt.
Ashraf I. El Mashad, Banha University, Faculty of Medicine, Banha, Egypt.
References
- 1.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 2.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatorypeptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 3.Hippentiel S, Witzenrath M, Schmeck B, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91:618–625. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- 4.Iwase T, Nagaya N, Fujii T, et al. Adrenomedullin enhances angiogenic potency of bone marrow transplantation in a rat model of hindlimb ischemia. Circulation. 2005;111:356–362. doi: 10.1161/01.CIR.0000153352.29335.B9. [DOI] [PubMed] [Google Scholar]
- 5.Charles CJ, Nicholls MG, Rademaker MT, et al. Comparative actions of adrenomedullin and nitroprusside: interactions with ANG II and norepinephrine. AmJ Physiol. 2001;281:R1887–R1894. doi: 10.1152/ajpregu.2001.281.6.R1887. [DOI] [PubMed] [Google Scholar]
- 6.Kato J, Tsuruda T, Kita T, et al. Adrenomedullin: a protective factor for blood vessels. Arterioscl Thromb Vasc Biol. 2005;25:2480–2485. doi: 10.1161/01.ATV.0000184759.91369.f8. [DOI] [PubMed] [Google Scholar]
- 7.Walters WAW, Lim YL. Blood volume and haemodynamics in pregnancy. Clin Obstet Gynecol. 1975;2:301–320. [Google Scholar]
- 8.Macara LM, Kingdom JC, Kaufimann P, et al. Control of fetoplacental circulation. Fetal Maternal Med Rev. 1993;5:167–179. [Google Scholar]
- 9.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 10.Apodaca CC, Moore KH, Rossignol TM, et al. Localization of messenger ribonucleic acid for adrenomedullin and adrenomedullin receptor in the human placenta in normal pregnancies and pregnancies complicated by oligohydramnios. Am J Obstet Gynecol. 2000;183:1213–1219. doi: 10.1067/mob.2000.109038. [DOI] [PubMed] [Google Scholar]
- 11.Di Iorio R, Marinoni E, Letizia C, et al. Adrenomedullin is increased in the fetoplacental circulation in intrauterine growth restriction with abnormal umbilical artery waveforms. Am J Obstet Gynecol. 2000;182:650–654. doi: 10.1067/mob.2000.103944. [DOI] [PubMed] [Google Scholar]
- 12.Di Iorio R, Marinoni E, Urban G, et al. Fetomaternal adrenomedullin levels in diabetic pregnancy. Horm Metab Res. 2001;33:486–490. doi: 10.1055/s-2001-16942. [DOI] [PubMed] [Google Scholar]
- 13.Gratton RJ, Gluszynski M, Mazzuca M, et al. Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2003;88:6048–6055. doi: 10.1210/jc.2003-030323. [DOI] [PubMed] [Google Scholar]
- 14.Porstmann T, Kiessig ST. Enzyme immunoassay techniques, an overview. J Immunol Meth. 1992;150:5–21. doi: 10.1016/0022-1759(92)90061-w. [DOI] [PubMed] [Google Scholar]
- 15.Knapp RG, Miller MC. Clinical epidemiology and biostatistics. Malvern, Penn: National Medical series from Williams & Wilkins; 1992. pp. P209–213. [Google Scholar]
- 16.Di Iorio R, Marinoni E, Letizia C, et al. Adrenomedulin production is increased in normal pregnancy. Eur J Endocrinol. 1999;140:201–206. doi: 10.1530/eje.0.1400201. [DOI] [PubMed] [Google Scholar]
- 17.Hoshimoto K, Hayashi M, Ohkura T. Mature adrenomedullin concentrations in plasma during pregnancy. J Matern Fetal Neonatal Med. 2002;11:126–129. doi: 10.1080/jmf.11.2.126.129. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Kubota T, Aso T, Hirata Y, et al. Immunoreactive adrenomedullin (AM) concentration in maternal plasma during pregnancy and AM expression in placenta. Eur J Endocrinol. 2000;142:683–687. doi: 10.1530/eje.0.1420683. [DOI] [PubMed] [Google Scholar]
- 19.Di Iorio R, Marinoni E, Cosmi EV. Adrenomedullin in pre-eclampsia. Lancet. 1998;351:676–676. doi: 10.1016/S0140-6736(05)78463-4. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Ueyama H, Mashimo T, et al. Circulating mature adrenomedullin is related to blood volume in full-term pregnancy. Anesth Analg. 2005;101:1816–1820. doi: 10.1213/01.ANE.0000182329.02880.83. [DOI] [PubMed] [Google Scholar]
- 21.Wilson C, Nikitenko LL, Sargent IL, et al. Adrenomedullin: multiple functions in human pregnancy. Angiogenesis. 2004;7:203–212. doi: 10.1007/s10456-004-4183-5. [DOI] [PubMed] [Google Scholar]
- 22.Lauria MR, Standley CA, Sorokin Y, et al. Adrenomedullin levels in normal and preeclamptic pregnancy at term. J Soc Gynecol Invest. 1999;6:318–321. doi: 10.1016/s1071-5576(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 23.Sugo S, Minamino N, Kangawa K, et al. Endothelial cells actively synthesize adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160–1165. doi: 10.1006/bbrc.1994.1827. [DOI] [PubMed] [Google Scholar]
- 24.Kato H, Shichiri M, Marumo F, et al. Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology. 1997;138:2615–2620. doi: 10.1210/endo.138.6.5197. [DOI] [PubMed] [Google Scholar]
- 25.Al-Ghafra A, Gude NM, Brennecke SP, et al. Increased adrenomedullin protein content and mRNA expression in human fetal membranes but not placental tissues in preeclampsia. Mol Hum Reprod. 2006;12:181–186. doi: 10.1093/molehr/gal016. [DOI] [PubMed] [Google Scholar]
- 26.Di Iorio R, Marinoni E, Scavo D, et al. Adrenomedullin in pregnancy. Lancet. 1997;349:328–335. doi: 10.1016/s0140-6736(05)62827-9. [DOI] [PubMed] [Google Scholar]
- 27.Hata T, Miyazaki K, Matsui K. Decreased circulating adrenomedullin in pre-eclampsia. Lancet. 1997;350:1600–1605. doi: 10.1016/S0140-6736(05)64016-0. [DOI] [PubMed] [Google Scholar]
- 28.Jerat S, Morrish DW, Davidge ST, et al. Effect of adrenomedullin on placental arteries in normal and preeclamptic pregnancies. Hypertension. 2001;37:227–231. doi: 10.1161/01.hyp.37.2.227. [DOI] [PubMed] [Google Scholar]
- 29.Kato J, Kitamura K, Eto T. Plasma adrenomedullin level and development of hypertension. J Hum Hypertens. 2006;20:566–570. doi: 10.1038/sj.jhh.1002033. [DOI] [PubMed] [Google Scholar]
- 30.Eto T. A review of the biological properties and clinical properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides. 2001;22:1693–1771. doi: 10.1016/s0196-9781(01)00513-7. [DOI] [PubMed] [Google Scholar]
- 31.Lopez J, Martnez A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. Int Rev Cytol. 2002;221:1–92. doi: 10.1016/s0074-7696(02)21010-4. [DOI] [PubMed] [Google Scholar]
- 32.Martinez A, Vos M, Guedez L, et al. The effects of adrenomedullin overexpression in breast tumor cells. J National Cancer Instit. 2002;94:1226–1237. doi: 10.1093/jnci/94.16.1226. [DOI] [PubMed] [Google Scholar]
- 33.Lu B, Wang A, Fei Y. Study of adrenomedullin value in pregnancy induced hypertension patients. Zhoonghua Fu Chan Ke Za Zhi. 1999;34:17–19. [PubMed] [Google Scholar]


