Abstract
Advanced glycation end products (AGEs) are thought to contribute to the abnormal lipoprotein profiles and increased risk of cardiovascular disease of patients with diabetes and renal failure, in part by preventing apolipoprotein B (apoB)-mediated cellular uptake of low density lipoproteins (LDL) by LDL receptors (LDLr). It has been proposed that AGE modification at one site in apoB, almost 1,800 residues from the putative apoB LDLr-binding domain, may be sufficient to induce an apoB conformational change that prevents binding to the LDLr. To further explore this hypothesis, we used 29 anti-human apoB mAbs to identify other potential sites on apoB that may be modified by in vitro advanced glycation of LDL. Glycation of LDL caused a time-dependent decrease in its ability to bind to the LDLr and in the immunoreactivity of six distinct apoB epitopes, including two that flank the apoB LDLr-binding domain. ApoB appears to be modified at multiple sites by these criteria, as the loss of glycation-sensitive epitopes was detected on both native glycated LDL and denatured, delipidated glycated apoB. Moreover, residues directly within the putative apoB LDLr-binding site are not apparently modified in glycated LDL. We propose that the inability of LDL modified by AGEs to bind to the LDLr is caused by modification of residues adjacent to the putative LDLr-binding site that were undetected by previous immunochemical studies. AGE modification either eliminates the direct participation of the residues in LDLr binding or indirectly alters the conformation of the apoB LDLr-binding site.
Nonenzymatic protein glycation by glucose is a physiological process that proceeds through a complex cascade of reactions which generate a heterogeneous mixture of products termed advanced glycation end products (AGEs) (1, 2). AGEs are believed to contribute to the pathogenesis of diabetes (3, 4) and neurodegenerative amyloidal diseases such as Alzheimer’s disease (5, 6). As nonenzymatic glycation is thought to also occur in normoglycemic individuals, albeit at a slower rate than in diabetic subjects, AGEs have also been proposed to contribute to the pathogenesis of aging (7, 8). The generation of AGE-modified proteins in the circulation is thought to result only, in part, from a direct interaction of glucose with serum proteins. Serum proteins can also be modified by low molecular weight, highly reactive AGE peptides that are present in the circulation, particularly under conditions of impaired renal function (9, 10). These are degradation products of AGE-modified proteins that are released into the blood stream and are normally cleared by the kidneys. AGE-modified serum proteins prepared in vitro have been shown to be toxic, immunogenic, and capable of triggering cellular injury responses after uptake by specific cellular receptors. (11, 12). In vivo, AGEs can cause alterations to the extracellular matrix including cross-linking of collagen, thickening of basement membranes, and the covalent binding of plasma proteins including low density lipoproteins (LDL) and Ig (13).
It is well recognized that individuals with diabetes and renal insufficiency are at an increased risk for the development of atherosclerosis (14). Plasma lipoprotein profiles are frequently abnormal in these conditions with an elevation in the level of the apolipoprotein B (apoB)-containing lipoproteins, including LDL. Defective lipoprotein uptake and metabolism in diabetic patients have been demonstrated (15), and the uptake and degradation of LDL isolated from diabetic subjects by normal fibroblasts has been shown to be impaired (16). LDL modified by in vitro incubation with glucose shows retarded intravascular clearance in humans (17) and animals (18), and reduced LDL receptor (LDLr)-mediated binding and uptake is also shown by cultured human fibroblasts (17, 18). Similarly, in transgenic mice that express the human LDLr, there is impaired clearance of LDL that had been pre-exposed to AGE peptides (19). Although there is reduced uptake of AGE-modified proteins via the LDLr, cell surface receptors for the AGE moiety are present on a number of cell types including monocytes, macrophages, and endothelial cells (9). Two receptors have recently been identified that can mediate the uptake of AGE-modified proteins, the class A scavenger receptor (20) and the receptor for AGE (RAGE) (21). Binding of AGE-modified proteins to the AGE receptors triggers a number of cellular responses that could contribute to AGE-associated pathogenesis (13).
A major site for AGE modification within the apoB primary structure has recently been identified (22). Although this site is distant from the putative apoB LDLr-binding domain, it has been proposed that AGE modification at this site provokes a change in the conformation of apoB that prevents its binding to the LDLr. In the present study we have used a panel of 29 well-characterized anti-apoB mAbs to demonstrate that in vitro glycation of LDL results in modification at multiple sites in apoB, including two that lie in close proximity to the apoB LDLr-binding domain.
MATERIALS AND METHODS
Preparation of AGE-LDL and Reductively Methylated LDL.
Plasma from healthy donors was collected and supplemented immediately with 1 mM EDTA, 20 μM butylated hydroxytulene (BHT), 0.5 mM phenylmethanesulfonal fluoride, and 0.02% sodium azide. LDL (density 1.019–1.063 g/ml) was isolated by sequential ultracentrifugation at 40.000 rpm for 18 h (23). AGE-LDL was prepared by incubating LDL (2 mg/ml) with 200 mM glucose at 37°C for up to 2 weeks in PBS containing 1 mM EDTA and 20 μM BHT with or without 300 mM aminoguanidine (22). Control LDL was incubated under the same conditions without glucose or aminoguanidine. After incubation, the LDL was dialyzed against PBS containing 1 mM EDTA and 0.02% NaN3. For the time course of glycation, aliquots of LDL were incubated at 37°C, and glucose was added to individual samples at t0 or after 4, 8, or 12 days to the final concentration of 200 mM. After 16 days of incubation all LDL were dialyzed as above. LDL was reductively methylated by the method of Weisgraber et al. (24).
LDLr-Binding Assay.
Glycated or control LDL were tested for their ability to compete with 125I-native LDL (25) for binding to the LDLr on the surface of cultured human fibroblasts as described (26). In short, 125I-LDL (3 μg/ml) and the appropriately diluted competitor LDL, in a total volume of 1 ml, were incubated for 3 h at 4°C with cultured human fibroblasts. Bound 125I-LDL was determined, and the ratio of bound radioactivity in the presence of competitor to that in the absence of competitor was plotted as a function of the concentration of the competitor.
mAbs.
The characterization of anti-human apoB (refs. 26 and 27; X.W. and R.M., unpublished data) and anti-AGE (22) mAbs have been described elsewhere. The rabbit antipeptide antiserum specific for apo B residues 3,352–3,371 was kindly provided by Tom Innerarity (Gladstone Institute of Cardiovascular Disease) (28).
Competitive Radioimmunometric Assay (RIA).
The competitive apoB RIA has been described previously (29). Immulon II Removawells (Dynatech) were coated by an overnight incubation with 200 μl of reference LDL (30 μg/ml in 5 mM glycine, pH 9.2). Plates were washed with a solution of 0.15 M NaCl containing 0.025% Tween 20 (Tween-saline) and then subsequently saturated by incubation for 1 h with 250 μl of 1% BSA in PBS, pH 7.4 (PBS-BSA). Serial dilutions (150 μl) of test LDL were prepared in separate microtiter plates to which was added 150 μl of anti-apoB mAb appropriately diluted in PBS-BSA. After a 2-h incubation, 200 μl of the LDL-mAb mixture was transferred to the LDL-coated Removawells. The plates were incubated overnight and again washed with the Tween-saline solution. Two hundred microliters of 125I-goat anti-mouse IgG diluted to 70 ng/ml in PBS-BSA was added to each well and incubated overnight. The wells then were washed with the Tween-saline solution as above and counted for bound radioactivity.
Western Blotting Analysis.
LDL samples were subjected to SDS/PAGE (3–10% gradient) and were electrophoretically transferred to nitrocellulose paper (30). The nitrocellulose membranes were exposed to diluted anti-human apoB or anti-AGE mAbs or polyclonal anti-apoB peptide antibodies and then to the appropriate affinity-purified, horseradish peroxidase-conjugated anti-Ig secondary antibody. Bound Ig was detected by using an ECL kit (Amersham).
Other Analytical Methods.
Protein concentration of the LDL was measured by the method of Lowry by using BSA as a standard (31). Free amino groups on LDL were estimated by the trinitrobenzenesulfonic acid (TNBS) method (32) by using valine as a standard. The relative LDL surface charge was assessed by agarose gel electrophoresis at 150 V for 30 min (Beckman).
RESULTS
To study the structural and functional alterations that result from LDL glycation, freshly isolated LDL was incubated for 14 days at 37°C under sterile conditions in the presence of 200 mM glucose, anti-oxidants, and protease inhibitors. LDL was also similarly incubated in the absence of glucose and in the presence of both 200 mM glucose and 300 mM aminoguanidine, an inhibitor of advanced glycation (33). Although a glucose concentration of 200 mM is nonphysiological, it has been shown to generate an AGE modification of LDL that is immunochemically related and comparable in concentration to that observed in patients with diabetes or renal insufficiency (19, 34). In three experiments the TNBS reactivities of LDL incubated with glucose or with both glucose and aminoguanidine were reduced by 42 ± 6% and 8 ± 4%, respectively, compared to LDL incubated in the absence of glucose. Even at the highest concentration tested (160 μg/ml), LDL that had been incubated with glucose could not compete with 125I-LDL for binding to the LDLr of cultured human fibroblasts (data not shown). LDL that had been subjected to the glycation conditions in the presence of aminoguanidine retained about 50% of the LDLr-binding activity of LDL incubated in the absence of glucose. These results are similar to those previously reported (22).
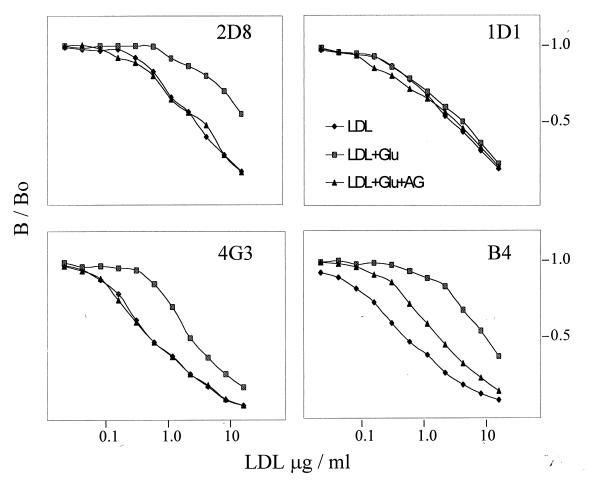
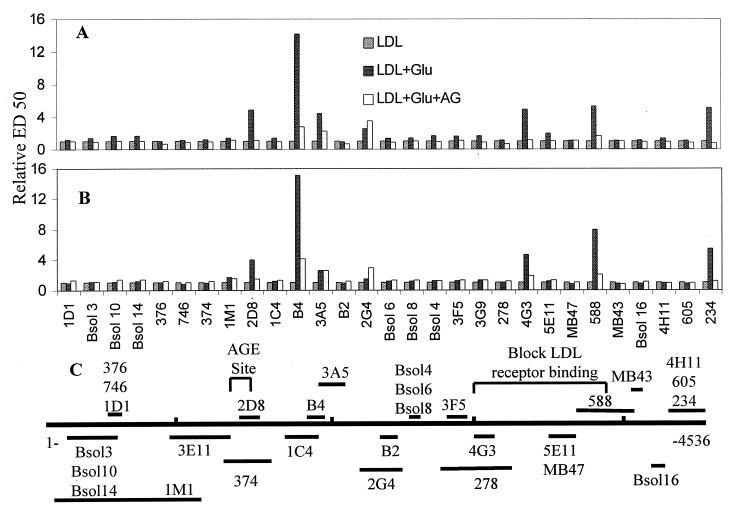
It has been proposed that AGE modification of one or more Lys residues between apoB residues 1,388 and 1,454 induces a conformational change in the apoB LDLr-binding site that impairs its interaction with the LDLr (22). To define the immunochemical changes of apoB that accompany LDL glycation, we tested LDL that had been subjected to glycation in the presence or absence of aminoguanidine in a solid-phase RIA by using a panel of 29 well-characterized anti-apoB mAbs. Complete competition curves for four of the mAbs from one experiment are presented in Fig. 1, and the ED50 values for all antibodies with LDL obtained from two normal subjects are shown in Fig. 2, together with an apoB epitope map. Whereas glycated LDL are as reactive as LDL that had been incubated in the absence of glucose with certain mAbs (e.g., 1D1), for other mAbs they are much less reactive than nonglycated LDL (e.g., 2D8, B4, and 4G3). Interestingly, one of the epitopes that is particularly sensitive to glycation, 2D8, has been mapped to a region (residues 1,438–1,481) that overlaps the major site of AGE modification of apoB. Other epitopes, 4G3 and 588, that are glycation-sensitive are close to the putative apoB LDLr-binding site. Aminoguanidine completely or partially prevented the loss of immunoreactivity of most epitopes that are sensitive to glycation. In contrast to other glycation-sensitive epitopes, however, the 2G4 epitope could not be protected by aminoguanidine.
Figure 1.
The immunoreactivity of glycated LDL. The immunoreactivity of LDL that had been incubated for 14 days at 37°C without glucose (LDL), with 200 mM glucose (LDL + Glu), or with 200 mM glucose and 300 mM aminoguanidine (AG) (LDL + Glu + AG) was determined by a solid phase RIA by using a panel of 29 anti-apoB mAbs. Competition curves for mAbs 1D1, 2D8, 4G3, and B4 are presented.
Figure 2.
The immunoreactivity of glycated LDL. The relative concentrations of LDL, LDL + Glu, and LDL + Glu + aminoguanidine (AG) that were required to obtain 50% of maximum binding (ED50) in the solid-phase RIA. LDL from two subjects (A and B) were modified under the conditions described in the legend to Fig. 1. The ED50 for LDL that had been incubated in the absence of glucose was normalized to a value of 1 for each mAb. The positions of the epitopes within apoB primary structure recognized by the 29 mAbs are shown (27) (C).
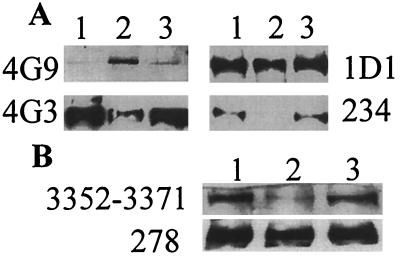
The loss of immunoreactivity of epitopes close to the apoB LDLr-binding site could result from either glucose-mediated modification of residues that comprise the respective epitopes themselves or from changes in the conformation of these epitopes that are secondary to AGE modification of Lys residues between apoB residues 1,388–1,454. To distinguish between these two possibilities we analyzed the samples for their reactivity with the mAbs on Western blots. As seen in Fig. 3A, antibodies that showed a decreased reactivity with glycated LDL in the solid phase RIA also showed decreased reactivity with glycated apoB on Western blots. As it would be improbable that changes in epitope expression that are secondary to changes in apoB conformation could be detected by antibodies after SDS electrophoresis of LDL, the loss of reactivity of the mAbs most likely reflects direct modification of these epitopes. A mAb specific for the AGE moiety, 4G9 (22), also was found to react strongly with glycated apoB and very weakly with LDL that had been incubated without glucose or in presence of aminoguanidine.
Figure 3.
Immunoreactivity of glycated LDL after SDS/PAGE. (A) Equal quantities of LDL (lane 1), LDL + Glu (lane 2), or LDL + Glu + aminoguanidine (AG) (lane 3), modified under the conditions described in the legend to Fig. 1, were subjected to SDS/PAGE and transferred to nitrocellulose membranes. Immunoreactivity of the transferred proteins was tested with the panel of anti-apoB mAbs and mAb 4G9 that is specific for AGE adducts. Results with three anti-apoB mAbs and mAb 4G9 are presented. (B) Western blot analysis of LDL (lane 1), reductively methylated LDL (lane 2), and LDL + Glu (lane 3) with an antiserum specific for apoB residues 3,352–3,371 and anti-apoB mAb 278.
ApoB residues 3,359–3,367 are believed to participate directly in binding to the LDLr (35, 36). Accordingly, we tested the reactivity of an antiserum prepared against a synthetic peptide that represents apoB residues 3,352–3,371 with glycated LDL and LDL that had been modified by reductive methylation (Fig. 3B). The antibody recognizes glycated but not reductively methylated LDL. Therefore, although lysine residues contribute to the epitope(s) recognized by this antiserum, they are not modified by glycation. In contrast, the 278 epitope is insensitive to both glycation and reductive methylation.
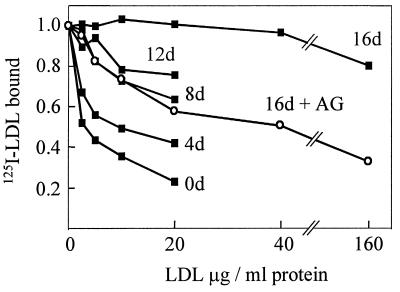
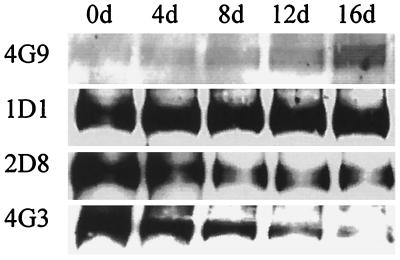
To study the time course of modification of epitopes of apoB and the loss of LDLr-binding capacity by glycation, we incubated LDL with 200 mM glucose for 4, 8, 12, and 16 days. A decrease in the ability of glycated LDL to compete with 125I-LDL for binding to the LDLr was detectable after only 4 days of glycation and showed a further progressive loss of activity over the subsequent 12 days (Fig. 4). There was also a time-dependent loss of immunoreactivity of glycation-sensitive epitopes as detected by either Western blotting (Fig. 5) or RIA (data not shown).
Figure 4.
The ability of LDL to bind to the LDLr as a function of the time of glycation. LDL incubated with 200 mM glucose, with or without aminoguanidine (AG), for 0, 4, 8, 12, or 16 days (see Materials and Methods) were tested for their ability to compete with 125I-LDL for binding to the LDLr on cultured human fibroblasts. Only the 16-day sample for LDL incubated with glucose and AG is shown. Data points represent duplicate values.
Figure 5.
Immunoreactivity of LDL as a function of the time of glycation. The reactivity of LDL that had been modified under the conditions described in Fig. 4 with anti-apoB and anti-AGE mAbs was determined by Western blotting analysis. Results with the anti-AGE mAb, 4G9, and anti-apoB mAbs 1D1, 2D8, and 4G3 are presented.
DISCUSSION
Basic residues of apoB are known to be critical for binding to the LDLr. This result was originally deduced from the observation that chemical modification of either Arg or Lys residues in apoB prevents binding of LDL to the LDLr (24). This finding could reflect modification of basic residues within the apoB LDLr-binding site or a change in apoB conformation caused by modification of residues elsewhere in the molecule. As Lys residues are the primary targets of AGE modification, either mechanism could also be responsible for the inability of glycated LDL to bind to the LDLr. Consistent with an indirect effect of AGE modification on apoB-mediated binding to the LDLr, it has been demonstrated recently that a major site of apoB modification by AGE is located between residues 1,388 and 1,455, some 1,791 residues upstream of the putative apoB LDLr-binding domain (22). Based on this observation, it was proposed that AGE modification in the region of residues 1,388–1,455 induces a change in conformation of the apoB LDLr binding site. In the case of apoB there are precedents for such a mechanism. A natural apoB variant that is characterized by an Arg-3500 → Gln substitution is defective in its binding to the LDLr and is responsible for the condition known as familial defective apoB (37). Although Arg-3500 does not apparently form part of the apoB LDLr-binding site, the replacement of Arg-3500 by Gln is thought to induce a conformational change that perturbs apoB-mediated binding to the LDLr (38).
The results presented here show that the expression of a number of apoB epitopes is altered in glycated apoB. These include both epitopes that are both close to and distant from the major site of apoB AGE modification. The 2D8 epitope (residues 1,438–1,481), which has been mapped to a region that overlaps the major AGE modification site on apoB, is sensitive to advanced glycation. In spite of this proximity, the 2D8 epitope and the major site of apoB AGE modification do appear to be distinct. We have observed that inclusion of the 2D8 mAb during the glycation of LDL could protect the 2D8 epitope from modification but could prevent neither the acquisition of AGE immunoreactivity nor the loss of LDLr-binding activity of the apoB (X.W. and R.M., unpublished observation). Two of the mAbs, 4G3 and 588, that show reduced affinity for glycated apoB are specific for epitopes (residues 2,980–3,084 and 3,687–4,081, respectively) that flank the putative apoB LDLr-binding site and can block apoB-mediated binding to the LDLr. Other glycation-sensitive epitopes are located between residues 1,854–1,878 (B4), 1,880–2,100 (3A5), 2,148–2,375 (2G4), and 4,342–4,536 (234). In all cases the loss of expression of these epitopes in glycated LDL is apparent both in RIA and after SDS electrophoresis. The loss of reactivity of the epitopes when tested under both native and denaturing conditions therefore indicates that glycation directly modifies residues that compose the epitopes and that the loss of epitope expression is not caused by a change in apoB conformation. This result would imply that the region of apoB comprised by residues 1,388–1,455 is not the only target of modification during in vitro glycation. This finding would be consistent with the respective magnitudes of the loss of TNBS-active Lys residues and the increase in LDL electrophoretic mobility that were observed following glycation (data not shown).
The nonenzymatic glycation of proteins is a complex cascade of reactions that gives rise to a mixture of AGEs that are heterogeneous and poorly characterized. As the identification of residues 1,388–1,455 as a major site of apoB AGE modification in the previous study (22) was based on the reactivity of an AGE-specific mAb, modification of residues elsewhere in apoB may involve AGE adducts that are not recognized by this mAb and/or by non-AGE modifications. It is notable that the epitope recognized by the anti-apoB mAb 2G4 could not be protected during glycation by aminoguanidine, an AGE inhibitor that is thought to block an early step in advanced glycation. It is possible that reactive AGE-forming intermediates can arise from oxidative reactions (glycoxidation) or from initial Schiff base condensation products with protein amino groups rather than from Amadori rearrangement products (39). It also has been shown that in vitro glycation of LDL can promote lipid oxidation (40). In spite of the inclusion of antioxidants to the LDL and our inability to detect an increase in lipid peroxides in the glycated LDL, we cannot exclude the possibility that the loss of expression of certain of the epitopes resulted from oxidative modification of apoB.
Although our studies did identify glycation-sensitive epitopes in the proximity of the apoB LDLr-binding site, it is notable that the glycation of LDL did not affect its reactivity with an antiserum specific for apoB residues 3,352–3,371. ApoB residues 3,359–3,367 were initially identified as being potentially implicated in binding to the LDLr by their enrichment in basic amino acids and their homology to the LDLr-binding domain of apoE (35). The importance of this sequence in binding to the LDLr is now supported by strong experimental evidence (36). It is possible that glycation-sensitive Lys residues that are situated outside of the sequence 3,357–3,367, directly participate in binding to the LDLr. Alternatively, as was previously proposed (22), the inability of glycated LDL to bind to the LDLr may result from an apoB conformational change that is secondary to the modification of residues outside of the apoB LDLr-binding site. As we have demonstrated that the expression of epitopes distributed throughout apoB primary structure is decreased in glycated LDL, however, it is no longer necessary to attribute this putative conformational change only to the modification of residues distant from the LDLr-binding site. Modification of Lys residues in close proximity to the apoB LDLr-binding site may, in fact, be primarily responsible for the decreased uptake of LDL modified by advanced glycation.
Acknowledgments
We thank Drs. Linda Curtiss, Stephen Young, and Joseph Witztum for providing anti-apoB mAbs MB43 and MB47; Dr. Jean-Charles Fruchart for anti-apoB mAbs B2 and B4; and Drs. Tom Innerarity and Kay Arnold for the rabbit anti-human apoB antiserum. This work was supported by Grant PG-11471 from the Medical Research Council of Canada.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: apo, apolipoprotein; AGE, advanced glycation end product; LDL, low density lipoprotein; LDLr, low density lipoprotein receptor; RIA, radioimmunoassay; AGE-LDL, AGE-modified LDL; TNBS, trinitrobenzenesulfonic acid.
References
- 1.Means, G. E. & Chang, M. K. (1982) Diabetes 31, Suppl. 3, 1–4.
- 2.Bucala R, Cerami A. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M, Cerami A, Vlassara H. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 4.Vlassara H, Bucala R, Striker L. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- 5.Vitek M P, Bhattacharya K, Glendening J M, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A. Proc Natl Acad Sci USA. 1994;91:4776–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington C R, Colaco C A L S. Nature (London) 1994;370:247–248. doi: 10.1038/370247a0. [DOI] [PubMed] [Google Scholar]
- 7.Monnier V M, Cerami A. Science. 1981;211:491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 8.Dyer D G, Dunn J A, Thorpe S R, Bailie K E, Lyons T J, McCance D R, Baynes J W. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makita Z, Radoff S, Rayfield E J, Yang Z, Skolnik E, Delaney V, Friedman E A, Cerami A, Vlassara H. N Engl J Med. 1991;325:836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 10.Makita Z, Bucala R, Rayfield E J, Friedman E A, Kaufman A M, Korbet S M, Barth R H, Winston J A, Fuh H, Manogue K, Vlassara H. Lancet. 1994;343:1519–1522. doi: 10.1016/s0140-6736(94)92935-1. [DOI] [PubMed] [Google Scholar]
- 11.Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Proc Natl Acad Sci USA. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan S D, Chen X, Schmidt A M, Brett J, Godman G, Zou Y S, Scott C W, Caputo C, Frappier T, Smith M A, et al. Proc Natl Acad Sci USA. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlassara, H. & Bucala, R. (1996) Diabetes 45 (Suppl. 3), S65–S66. [DOI] [PubMed]
- 14.Kannel W B, McGee D L. J Am Med Assoc. 1978;241:2035–2038. [Google Scholar]
- 15.Hiramatsu K, Bierman E L, Chait A. Diabetes. 1985;34:8–14. doi: 10.2337/diab.34.1.8. [DOI] [PubMed] [Google Scholar]
- 16.Lopes-Virella M F, Sherer G K, Lees A M, Wohlmann H, Mayfield R, Sagel J, Le Roy E C, Coldwell J A. Diabetologia. 1982;22:430–436. doi: 10.1007/BF00282585. [DOI] [PubMed] [Google Scholar]
- 17.Kesaniemi Y A, Witztum J L, Steinbrecher U P. J Clin Invest. 1983;71:950–959. doi: 10.1172/JCI110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbrecher U, Witztum J. Diabetes. 1984;33:130–134. doi: 10.2337/diab.33.2.130. [DOI] [PubMed] [Google Scholar]
- 19.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Proc Natl Acad Sci USA. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Khoury J, Thomas C A, Loike J, Cao L, Silverstein S C. J Biol Chem. 1994;269:10197–10200. [PubMed] [Google Scholar]
- 21.Neeper M, Schmidt A M, Brett J, Yan SD, Wang F, Pan Y C, Elliston K, Stern D, Shaw A. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 22.Bucala R, Mitchell R, Arnold K, Innerarity T, Vlassara H, Cerami A. J Biol Chem. 1995;270:10828–10832. doi: 10.1074/jbc.270.18.10828. [DOI] [PubMed] [Google Scholar]
- 23.Havel R J, Eder H A, Bragdon J H. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisgraber K H, Innerarity T L, Mahley R W. J Biol Chem. 1978;253:9053–9062. [PubMed] [Google Scholar]
- 25.Bilheimer D W, Eisenberg S, Levy R I. Biochim Biophys Acta. 1972;260:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- 26.Milne R W, Théolis R, Jr, Verdery R B, Marcel Y L. Arteriosclerosis. 1983;3:23–30. doi: 10.1161/01.atv.3.1.23. [DOI] [PubMed] [Google Scholar]
- 27.Pease R J, Milne R W, Jessup W, Law A, Provost P, Fruchart J C, Dean R T, Marcel Y L, Scott J. J Biol Chem. 1990;265:553–568. [PubMed] [Google Scholar]
- 28.Innerarity T L, Young S G, Poksay K S, Mahley R W, Smith R S, Milne R W, Marcel Y L, Weisgraber K H. J Clin Invest. 1987;80:1794–1798. doi: 10.1172/JCI113273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcel Y L, Hogue M, Weech P K, Milne R W. J Biol Chem. 1984;259:6952–6957. [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Habeeb A F S A. Anal Biochem. 1966;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 33.Brownlee M, Vlassara H, Kooney T, Ulrich P, Cerami A. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 34.Makita S, Vlassara H, Cerami A, Bucala R. J Biol Chem. 1992;267:5133–5138. [PubMed] [Google Scholar]
- 35.Knott T J, Rall S C, Innerarity T L, Jacobson S F, Urdea M S, Levy-Wilson B, Powell L M, Pease R J, Eddy R, Nakai H, et al. Science. 1985;230:37–43. doi: 10.1126/science.2994225. [DOI] [PubMed] [Google Scholar]
- 36.Borén J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity T L. J Clin Invest. 1998;101:1084–1093. doi: 10.1172/JCI1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Innerarity T L, Mahley R W, Weisgraber K H, Bersot T P, Krauss R M, Vega G L, Grundy S M, Friedl W, Davignon J, McCarthy B J. J Lipid Res. 1990;31:1337–1349. [PubMed] [Google Scholar]
- 38.Lund-Katz S, Innerarity T L, Arnold K S, Curtiss L K, Philips M C. J Biol Chem. 1991;266:2701–2704. [PubMed] [Google Scholar]
- 39.Booth A A, Khalifah R A, Todd P, Hudson B G. J Biol Chem. 1997;272:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 40.Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Proc Natl Acad Sci USA. 1993;90:6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]