Abstract
Orysa sativa pathogenesis-related protein 10a (OsPR10a) was induced by pathogens, salicylic acid (SA), jasmonic acid (JA), ethephon, abscisic acid (ABA), and NaCl. We tried to analyze the OsPR10a promoter to investigate the transcriptional regulation of OsPR10a by SA. We demonstrated the inducibility of OsPR10a promoter by SA using transgenic Arabidopsis carrying OsPR10a:GFP as well as by transient expression assays in rice. To further identify the promoter region responsible for its induction by SA, four different deletions of the OsPR10a promoter were made, and their activities were measured by transient assays. The construct containing 687-bp OsPR10a promoter from its start codon exhibited a six-fold increase of induction compared to the control in response to SA. Mutation in the W-box like element 1 (WLE 1) between 687 and 637-bp from TGACA to TGAAA completely abolished induction of the OsPR10a promoter by SA, indicating that the WLE 1 between −687 and −637 of OsPR10a promoter is important in SA-mediated OsPR10a expression. We show for the first time that the W-box like element plays a role in SA mediated PR gene expression.
Keywords: cis-Acting element, OsPR10a promoter, Salicylic acid, Salicylic acid induction
Introduction
Plants have developed defense mechanisms to recognize pathogens and subsequently activate defense-related genes, such as pathogenesis-related proteins (PR proteins). The major families of PR proteins have been grouped into at least 14 different classes, primarily on the basis of their amino acid sequences (Van Loon and Van Strien 1999). Although the biological and/or biochemical functions of many PR proteins remain unclear, PR2 (β-1, 3-glucanase activity) and PR3 (chitinase) proteins have been shown to inhibit fungal growth (Woloshuk et al. 1991; Sela-Buurlarge et al. 1993). These responses are not limited to pathogen attack and can be induced by defense signaling molecules such as SA, JA and ET (Dempsey et al. 1999; Pieterse and van Loon 1999). To study the defense signaling in plants, many groups have isolated promoters of PR proteins in several plant species, such as Arabidopsis, tobacco, pepper, and rice (Malnoy et al. 2003; Hong et al. 2005; Li et al. 2005; Liu et al. 2005a; Lee and Hwang 2006).
Expression profiles of PR proteins, such as OsPR1, OsPR10 and OsPR1b were reported. Originally, OsPR10a was known to be induced by probenazole and thus, was called a probenazole-inducible gene, PBZ1 (Midoh and Iwata 1996). Later, PBZ1 was renamed as OsPR10a because it shares a similar sequence with (has sequence similarity to) PR-10 proteins. The investigators reported that OsPR10a is only induced by probenazole but not by ethephon, NAA, SA, NaCl or mannitol in rice leaves. In contrast, Rakwal et al. (2001) reported that OsPR10a is induced by JA, SA, and ABA but not by IAA or GA in light. Ryu et al. (2006) found similar results using RT-PCR. Chen et al. (2006) reported that the elicitor derived from Magnaporthe grisea induces OsPR10a. However, there has been only one study on OsPR10a and OsCHNIII promoters, even though many reports are available for the expression profile of PR genes in rice (Rakwal et al. 2001; Hashimoto et al. 2004; Chen et al. 2006; Ryu et al. 2006). The authors reported that OsPR10a and OsCHNIII promoters are induced by an elicitor derived from Magnaporthe grisea by a transient assay in vitro. However, the cis elements were not analyzed.
Most promoters induced by pathogens or SA contain the W-box, GCC box, RAV1 AAT, or ASF1 motif, etc. (Li et al. 2005; Lee and Hwang 2006; Sohn et al. 2006). Their cis-elements have been identified by series deletion of the promoter and site directed mutagenesis of its plausible site. Maleck et al. (2000) analyzed the transcriptome of Arabidopsis under defense inducing conditions, and they studied induced promoters such as PR1. The W-boxes ((T)TGACC/T) are enriched in the PR1 regulon promoter. They also described that the W-box like element ((T)TGACA) is also enriched in PR-1 regulon promoters even though there is no evidence that WRKYs bind to this motif (Maleck et al. 2000).
Transcription factors that can recognize the cognate cis element were identified by methods such as the gel-mobility shift assay, yeast-one hybrid, transient assay in plant. WRKY, ERF, RAV, bZIP, MYB, etc. have been shown to be involved in the defense signaling (Rushton et al. 1996, 2002; Eulgem et al. 1999; Kirsch et al. 2001; Heise et al. 2002). The interaction of a transcription factor to its cognate cis element is a key step in the process of defense signaling. Among transcription factors, WRKY proteins are the most extensively studied in defense signaling (Eulgem et al. 1999; Robatzek and Somssich 2001; Shimono et al. 2007). Asai et al. (2002) reported that AtWRKY22 and AtWRKY29 regulate FLS2-mediated defense signaling. The complex of TGA factor and NPR1 binds to the LS7 in the PR-1 promoter of Arabidopsis (Johnson et al. 2003). Furthermore, the TGA/NPR1 complex is as well conserved in rice as in Arabidopsis (Fitzgerald et al. 2005). Recently, there are three reports that OsWRKY45, OsWRKY71 and OsWRKY03 regulate the defense signaling in rice, respectively (Liu et al. 2005b, 2006; Shimono et al. 2007), implicating that WRKYs also binds to the W boxes in rice as it does in Arabidopsis.
In this study, we analyzed the expression profile of OsPR10a. We isolated its promoter and analyzed its cis-elements. We also identified the WLE1 (TGACA) controlling induction of OsPR10a promoter by SA. This is the first report that the W-box like element actually plays a role in SA-mediated defense signaling.
Material and methods
Plant materials
Rice seedlings (Oryza sativa cv. Hwachung; seeds from Dr. Wan-He Ye, NIAST, Suwon, South Korea) were grown in a greenhouse at 28°C for 3 weeks. Three-week-old rice seedlings were washed, incubated in tap water for 2 days, and then treated with SA, JA, ethephon, ABA, or NaCl at 1 mM, 100 μM, 100 μM, 100 μM, and 200 mM, respectively. Rice leaves were harvested at the times indicated in the figures. For bacterial inoculations, a strain of Xanthomonas oryzae pv oryzae KXO98 (Xoo; obtained from Korean Agricultural Culture Collection, KACC, Suwon, South Korea) incompatible to O. sativa cv. Hwachung was grown in PSA medium (10 g peptone, 10 g sucrose, 1 g sodium–glutamate, and 15 g agar per L) for 2 days and then resuspended in 1 mM MgCl2 to a final OD600 of 0.5. Xoo was sprayed on 3-week-old rice seedlings. After inoculation, plants were incubated in a humidity chamber for 24 h. Samples were taken at the times indicated in the figures and were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
Isolation of OsPR10a promoter
Based on an annotation of the rice genome, a −1000 bp fragment of the OsPR10a promoter was obtained by PCR from rice genomic DNA using an OsPR10a gene-specific primer sets. These primers were designed from the Genbank sequence AL845342. PCR was performed for 30 cycles under the following condition: 94°C for 30 s, 53°C for 1 min, and 72°C for 1 min, followed by a final extension at 72°C for 7 min. The primer sets are as follows:
5′-AAAAAGCAGGCTTGTTTTGAATGCTGGAATGATAA-3′, and 5′-AGAAAGCTGGGTCACTGAAGATATAATCTA-3′.
The underlined sequences match the attB1 and attB2 sites for the Gateway cloning system (Invitrogen, Carlsbad, CA, USA). A 1000 bp amplified PCR product was cloned into pDONR221 to make an entry clone by BP clonase (Invitrogen); successful insertion was confirmed by sequencing.
Promoter-LUC constructs
The reporter constructs used in the transient expression assays in this study were prepared according to the following procedure. For a 1.0 kb OsPR10a promoter, 1000-bp upstream from the start codon of OsPR10a was cloned by BP reaction into pDONR221 to make the −1000 bp PR10a promoter entry clone described in the previous section. −1000-OsPR10a:LUC was created by LR reaction with the −1000-PR10a entry clone and promoter destination vector (attB1-ccdB-Cmr-attB2-LUC, unpublished results; Invitrogen). On the basis of W-boxes involved in the activation of defense genes in plants to construct the deleted OsPR10a:LUC construct, we amplified the OsPR10a promoter region using these sense primers:
−818: 5′-AAAAAGCAGGCTCGTGACATCAGATTGAGTAT-3′
−687: 5′-AAAAAGCAGGCTCGATAAAGGGTATTTGTTTA-3′
−637: 5′-AAAAAGCAGGCTACCTATCATCTAAAAGCATT-3′.
We also used the antisense primer 5′-AGAAAGCTGGGTCACTGAAGATATAATCTA-3′. PCR was performed for 30 cycles under the following condition: 94°C for 30 s, 53–55°C for 1 min, and 72°C for 1 min, followed by a final extension at 72°C for 7 min.
The sequences underlined match attB1 and attB2 sites in the Gateway cloning system. These −818, −687, and −637 bp amplified PCR products were cloned into pDONR221 to make entry clones by BP clonase and confirmed by sequencing. 818-, 687-, and 637-OsPR10a:LUC were created by LR reaction with the 818-, 687-, and 637-OsPR10a entry clones and the promoter destination vector (attB1-ccdB-Cmr-attB2-LUC, unpublished results).
Site directed mutagenesis
The mutagenized reporter constructs used in the transient expression assays in this study were prepared according to the manufacturer’s instruction (Stratagene, La Jolla, CA, USA). For the mutagenized 687 bp-OsPR10a promoter, −1000-OsPR10a entry clone (20 μg) was added to 1 μL of 10× reaction buffer, 1 μL (1 ρmole) phosphorylated specific mutagenic primer sets, 0.5 μL 10 mM dNTPs, 0.6 μL Quick solution; and the volume was adjusted to 10 μL with sterile deionized water. The solution was added to 0.3 μL of Pfu Turbo DNA polymerase (2.5 units/μL). The reaction matrix mix was used for subsequent PCR. Specific PCRs were performed for 18 cycles under the following conditions: 95°C for 50 s, 60°C for 50 s, and 68°C for 1 min, followed by a final extension at 68°C for 7 min. After generation of the mutgenic double stranded plasmid containing staggered nicks, the product was treated with Dpn I and incubated at 42°C for 60 min. The nicked plasmid incorporating the desired mutations was purified with phenol and chloroform extraction and ligated with T4 DNA Ligase at 16°C for overnight. Five microliters of the mutated plasmid was transformed into E.coli (DH5α) cells. After transformation, the plasmid DNA was isolated from the mutagenic transformant and confirmed by sequencing. For PCR of mutant strand synthesis reaction, the following mutagenic primer pairs were used: 5′-TGAAATGTAGTCGTACCTATCA-3′ and 5′-TGCTCTGAGATGGGTCTAAACA-3′.
Particle bombardment and transient expression assays
Leaf bombardments were performed in a Biolistic PDS-1000/He particle delivery system using 1100-p.s.i. rupture disks (BioRad, Hercules, CA, USA). Plasmid DNAs for particle bombardment were prepared as described by the manufacturer’s instructions. For reporters, −1000-bp OsPR10a:LUC and 818-, 687-, 637-OsPR10a:LUC and m687-OsPR10a:LUC were used; 35S:RLUC was used as an internal control to normalize LUC activities between samples after bombardments. About 2 cm lengths of one-week old rice seedlings grown in the dark were cut and incubated on a plate in 1/2 MS medium overnight (Murashige and Skoog 1962). Tungsten particles coated with 1000-, 818-, 687-, 637-OsPR10a:LUC, or m687-OsPR10a:LUC, and the internal control were delivered into leaf segments by the particle delivery system (BioRad). Leaf segments were incubated at 28°C for 24 h with buffer (1/2 MS medium) or with 1 mM SA and then harvested. Leaf segments were ground in liquid nitrogen and dissociated in 1× passive buffer. The luciferase activities from protein extracts were measured by a dual luciferase system (Promega, Madison, WI, USA) with a luminometer (Aureon Biosystems, Vienna, Austria).
RT-PCR analysis
Leaf samples were ground to powder in liquid nitrogen, and total RNA was extracted using the Trizol reagent according to the manufacturer’s instructions (Invitrogen). For reverse transcription, total RNA (1 μg) was added to 1 μL of oligo (dT)16 and 1 μL of gene specific primer sets (0.5 ρmole); and the volume was adjusted to 15 μL with sterile deionized water. The solution was incubated at 70°C for 5 min, then immediately transferred to ice before the addition of 35 μL of reverse transcriptase master mix containing 10 μL 5× buffer, 3 μL 0.1 M DTT, 5 μL 10 mM dNTPs, 1 μL (200 units/μL) M-MLV RTase (Promega) and 0.2 μL (40 units/μL) RNasin (Promega). The reaction was incubated at 42°C for 90 min before heat inactivation at 65°C for 10 min. Two microliters of each reverse transcriptase reaction was used for subsequent PCR. Gene specific PCRs were performed for 35 cycles under the following conditions: 94°C for 30 s, 53°C for 1 min, and 72°C for 1 min, followed by a final extension at 72°C for 7 min. Samples were visualized on 1.2% agarose gels. For RT-PCR analysis of OsPR10a genes in rice, the following primer pairs were used:
OsPR10a (D38170)
5′-GCTACAGGCATCAGTGGTCA-3′ and 5′-GACTCAAACGCCACGAGAAT-3′,
OsActin (XM469569)
5′-TCCATCTTGGCATCTCTCAG-3′and 5′-GTACCCGCATCAGGCATCTG-3′.
Generation of transgenic Arabidopsis, induction with SA, and fluorescence microscopy
OsPR10a:GFP was constructed by LR reaction with pBGWFS7 (Gateway™; Department of Plant Systems Biology, VIB-Ghent University, Belgium) and the OsPR10a promoter entry clone described in the previous section, and then transformed into Agrobacterium tumefaciens GV3101 for Arabidopsis. Arabidopsis (Columbia ecotype) was transformed with A. tumefaciens GV3101 carrying OsPR10a:GFP::GUS and 35S:GFP(35S:pBGWFS7::GUS) as an internal control. A bacterial suspension of A. tumefaciens GV3101 carrying OsPR10a:GFP::GUS and 35S:GFP::GUS was sprayed on the unopened flowers of Arabidopsis. T1 plants were screened by 0.3% Barstar spray (Misung, Daejeon, South Korea). Samples were taken from independent T1 plants for RT-PCR analysis. T2 plants were also screened by 0.3% Barstar spray. Three individual T2 plants in each line were used for induction with 1 mM SA at 28°C for 72 h. After induction, transgenic plants carrying OsPR10a:GFP and 35S:GFP were examined by fluorescence microscopy using an Olympus SZX-RFL3 (Olympus Optical Co., LTD, Tokyo, Japan). Excitation and emission filters SZX-FGFP and SZX-FGFPA were used for GFP and GFPA (Ex 460–490/Em510- for GFP and Ex460–490/Em510–550 for GFPA). Images were captured with a JP/FV300 camera (Olympus).
Results
Expression patterns of OsPR10a in response to different stimuli
Several research groups have reported some discrepancy for the expression patterns of OsPR10a to some stimuli. Here we looked at the expression of OsPR10a by RT-PCR, which is a more sensitive method than reported previously. First, we tested whether OsPR10a is induced by a pathogen, as reported previously (Midoh and Iwata 1996; Ryu et al. 2006; Fig. 1a). Induction of OsPR10a started at 6 h and reached a maximum at 48 h after Xoo infection, as reported previously (Ryu et al. 2006). We also determined whether OsPR10a was induced by biotic elicitors such as SA, JA, and ethephon. For the fist time, we show that OsPR10a is induced by ethephon (Fig. 1b). Witzh regard to abiotic stress treatments, OsPR10a was induced by NaCl and ABA (Fig. 1c). Taken together, we conclude that OsPR10a is induced by the pathogen Xoo, SA, JA, ethephon, NaCl, and ABA.
Fig. 1.
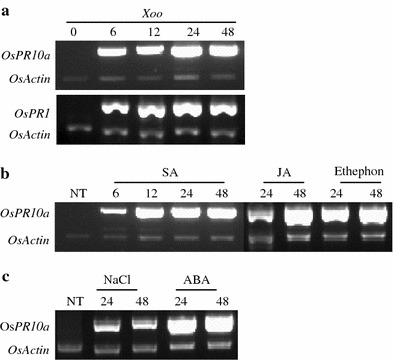
Expression pattern of OsPR10a in rice leaves treated with Xoo and five compounds. a Three-week-old rice seedlings were infected with Xoo and were harvested at 0, 6, 12, 24, and 48 h. b, c Three-week-old rice seedlings were treated with SA, JA, ethephon, ABA, or NaCl and were harvested at 0, 6, 12, 24, and 48 h. Total RNA was isolated from each sample, and RT-PCR was performed using OsPR10a specific primer pair. Transcript levels of OsActin show that equal amounts of RNA were used in the RT-PCR samples
The OsPR10a promoter is induced by SA treatment as shown in transient-assay system
We analyzed the expression of the OsPR10a gene to pathogens and various phytohormones. We focused on the SA-mediated response of the OsPR10a gene. To investigate how the OsPR10a gene was transcriptionally regulated by SA, we isolated the OsPR10a promoter in a 1.0 kb genomic DNA fragment upstream from the start codon of the OsPR10a gene by PCR. To analyze whether the 1.0 kb OsPR10a promoter was activated by SA as expected by its expression pattern, we carried out a transient assay using particle bombardment. The 1.0 kb fragment of OsPR10a promoter was used to make a reporter construct (OsPR10a:LUC). Its schematic diagram is shown in Fig. 2a. OsPR10a:LUC was introduced into rice leaves by particle bombardment; leaf segments were then treated with either buffer or SA. Protein extracts were prepared from samples after 24 h post-treatment, and their relative luciferase activities were measured. OsPR10a promoter activities were expressed as relative luciferase activities. Figure 2b shows a representative graph out of more than three independent experiments. The absolute values from each experiment were different, but the relative ratios from each sample were similar. Luciferase activity in the SA-treated sample was about two-fold higher than in non-treated (control) samples (Fig. 2b). This result indicates that the OsPR10a promoter is activated by SA, based on its expression profile.
Fig. 2.
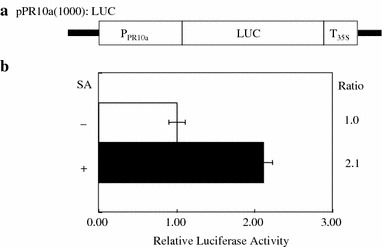
OsPR10a promoter activity in response to SA: a schematic representation of the OsPR10a promoter in the reporter construct. b A transient assay showing the OsPR10a promoter in response to SA. OsPR10a:LUC was bombarded into rice leaves, which were then incubated in MS medium or MS medium containing 1 mM SA at 28°C for 24 h. Protein extracts were made by dissociation in passive lysis buffer as described in “Materials and methods”. Relative luciferase activity is the ratio of the value obtained with the SA-treated OsPR10a:LUC divided by the value obtained with the buffer-treated OsPR10a:LUC. Bars indicate the standard error of three replicates
The OsPR10a promoter is induced by SA treatment in stably transformed Arabidopsis
We further investigated whether SA, as seen in the transient assay, activates the OsPR10a promoter using a transgenic approach. A 1.0 kb fragment of the OsPR10a promoter was cloned into a promoter-less GFP::GUS expression vector to make a OsPR10a:GFP::GUS construct, and was then introduced into Arabidopsis by Agrobacterium-mediated transformation (Fig. 3a). Transgenic Arabidopsis plants (T1) were screened by spraying with 0.3% Barstar and then with self-crossing. Induction of the OsPR10a promoter by SA was analyzed by GFP fluorescence in T2 transgenic Arabidopsis seedlings treated with either SA or buffer (Fig. 3b). In these GFP filter images (>510 nm), transgenic Arabidopsis carrying OsPR10a:GFP::GUS exhibited an orange fluorescence in the SA-treated sample because the green fluorescence from GFP was mixed with the red fluorescence from plants themselves (Fig. 3b, middle panel). The green fluorescence from GFP is shown more clearly using a GFPA filter (510–550 nm; bottom panel of Fig. 3b). As shown in Fig. 3b, the OsPR10a promoter was clearly activated by SA in transgenic Arabidopsis.
Fig. 3.
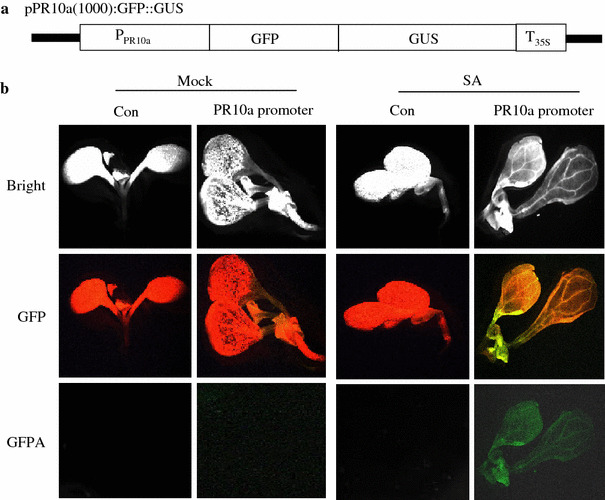
Fluorescence images of Arabidopsis transgenic plants carrying OsPR10a:GFP: a schematic diagram of OsPR10a:GFP::GUS fusion construct. b Induction of OsPR10a promoter by SA. OsPR10a:GFP::GUS was introduced into Arabidopsis by Agrobacterium-mediated transformation. Transgenic Arabidopsis seedlings carrying the OsPR10a:GFP::GUS was examined using fluorescence microscopy after SA treatment at 72 h. Non-transgenic Arabidopsis seedling was used as a control (left panel at mock and SA treatments). Shown are the bright-field images (upper panel Bright), the green fluorescent images using GFP filter (middle panel GFP) and the GFPA filter (bottom panel GFPA). Images are representatives from two independent experiments. The experiments were repeated at least twice
Analysis of cis-elements of OsPR10a promoter
In order to find a cis-acting element of the promoter in response to SA, an analysis was done using the PLACE program (a database for PLAnt Cis-acting Elements located at http://www.dna.affrc.go.jp/cDNA/place) (Fig. 4). Among many putative cis elements, we only indicated cis-elements in boxes known to be related to defense inducers, such as the pathogens, SA, JA, and ethephon, of the OsPR10a gene shown in Fig. 1 (Shinshi et al. 1995; Eulgem et al. 1999; Kagaya et al. 1999). The OsPR10a promoter analyzed by the PLACE program contains four W-boxes, whose detail sequences are different; there are one canonical W-box ((T)TGACC/T) and three W-box like elements (WLE 1) containing TGAC core (TGACA). There would be more W-box like elements in defense gene regulon promoters. Therefore, we decided to name TGACA as the W-box like element 1 (WLE1). In addition, there are three RAV1AAT elements, and one ASF1 motif element (Fig. 4). The W-box, RAV1AAT, and ASF1 motif are known to be cis-elements of the WRKY, RAV1, and bZIP proteins, respectively (Abe et al. 1997; Chen and Chen 2002; Yamamoto et al. 2004). The WRKY, RAV1, or bZIP proteins might be involved in the response of the OsPR10a promoter to SA. In addition to them, there are many cis-elements involved in ABA responsiveness, even though they are not indicated in Fig. 4. These elements might be involved in the induction of OsPR10a by ABA as shown in Fig. 1c. Interestingly, there is no cis-element, such as the JA responsive element (JERE) (AGACCGCC) or the ethylene response element (ERE) (AGCCGCC), which is the binding site for ethylene response element binding proteins (EREBP), despite the fact that OsPR10a was induced by JA and ethephon.
Fig. 4.

Putative cis-acting elements in 1.0 kb OsPR10a promoter. The putative cis-elements are indicated in boxes and its name is given above each element. Arrows indicate the direction of the cis-element. W-box WRKY transcription factor binding site; RAV1AAT RAV transcription factor binding site; ASF1 motif bZIP factor binding site; WLE1 putative WRKY transcription factor binding site
Deletion analysis of the OsPR10a promoter to identify the regions responsible for the induction by SA
To identify the region of the OsPR10a promoter involved in the response to SA, we made serial deletions of the OsPR10a promoter by PCR (Fig. 5a). Deletions, beginning with the locations −818, −687, and −637, were fused to the LUC coding sequences and 3′ nopaline synthase gene terminator (Fig. 5a). These four constructs were tested for SA inducibility of the OsPR10a promoter by introducing them into rice leaves using particle bombardment and then treating them with either buffer or SA for 24 h. Protein extracts were made from the bombarded leaves and their luciferase activities were measured (Fig. 5b). In the case of the 1.0 kb OsPR10a:LUC construct, luciferase activity was increased up to two fold over the control with SA treatment but not in the 818:LUC construct, indicating that there is a weak positive cis-element in region I between −1000 and −818 bp of OsPR10a promoter (Fig. 5a). One ASF1 motif was found in region I. The exact positive element in this region has not yet been identified. Luciferase activity in the 687:LUC construct was increased up to sixfold with SA treatment, indicating that there is a negative element in region II between −818 and −687 bp of the OsPR10a promoter. There is only one WLE1 with the TGAC core (TGACA) and one RAV1AAT element in region II that is known to be bound by transcription factors associated with the defense signaling (Fig. 4). Besides this, there are many putative cis-elements in region II (data not shown). Therefore, the exact negative element has not yet been determined. In the 637:LUC construct, there was only about a two-fold increase in luciferase activity with SA treatment, indicating that there is at least one positive element between −687 and −637 bp (region III) and another one between −637 and 1 bp (region IV) of the OsPR10a promoter. There is only one WLE1 containing the TGAC core (TGACA) in region III, suggesting that this element may play an important role in the strong inducibility of the 687:LUC construct by SA (Table 1). The W-box, RAV1AAT, and WLE1 are found in region IV of the OsPR10a promoter, and at least one of them can act as a weak positive element.
Fig. 5.
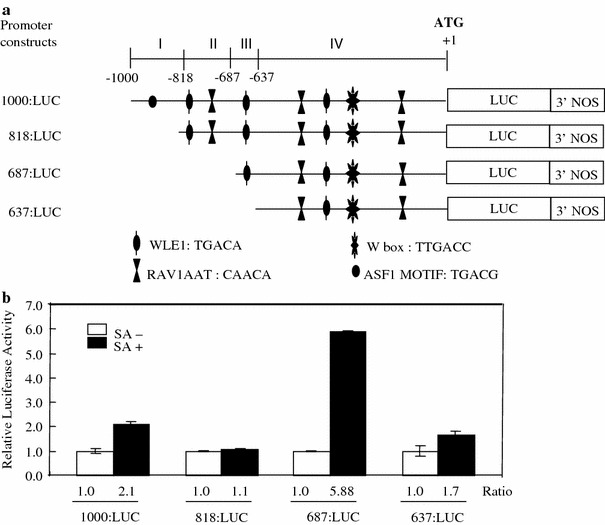
Deletion analysis of OsPR10a promoter: a schematic diagrams of serial deletion constructs of OsPR10a promoter. The numbers to the left of each construct indicate the distance from the start codon ATG. The predicted cis-elements ( W-box, RAV1AAT, and ASF1motif) are indicated by their respective abbreviations. The start codon, ATG, is written in bold. b Luciferase activity in deletion constructs of the OsPR10a promoter. Each deletion construct OsPR10a:LUC was bombarded into rice leaves, which were incubated in MS liquid medium or MS medium containing 1 mM SA at 28°C for 24 h. Protein extracts were made by dissociation in passive lysis buffer as described in “Materials and methods”. Bars indicate the standard error of three replicates. The values are the ratio of the value obtained from each deletion constructs of OsPR10a promoter treated with SA or buffer divided by the value obtained from 1.0 kb OsPR10a promoter construct treated with buffer
Table 1.
The list of putative cis-acting elements of the OsPR10 a promoter in region III
| Regiona | Positionb | cis-Elements (#)/putative factor c |
|---|---|---|
| III | −687 to −637 | GT1CONSENSUS(1)/GT-1, WLE1 (1)/WRKY |
a OsPR10a promoter was divided into four regions depending on the presence of W-box. The region III between −687 and −637 bp of OsPR10a promoter
bindicates the distance of upstream from the start codon of OsPR10a
cPutative cis-acting elements in region III of OsPR10a promoter were analyzed using PLACE (a database for PLAnt Cis-acting Elements located at the web site (http://www.dna.affrc.go.jp/cDNA/place)
Mutation of a W-box like element in OsPR10a promoter abolished its SA inducibility
SA inducibility of the 687:LUC construct is the highest among deletion constructs, and only one WLE1 with the TGAC core is present in region III. To further verify this, the WLE1 in region III was mutagenized from TGAC to TGAA (Fig. 6a). Eulgem et al. (1999) reported that the WRKY protein couldn’t bind to a TGAA sequence; therefore, this mutation prevents the association of WRKY to the WLE1 of the OsPR10a promoter. Interestingly, SA inducibility of 687 bp-OsPR10a promoter was completely abolished in the mutagenized 687 bp-OsPR10a promoter, indicating that this WLE1 is involved in the SA inducibility of OsPR10a.
Fig. 6.
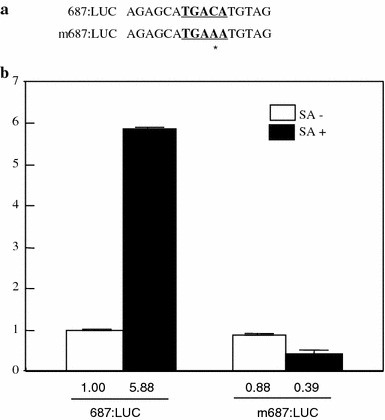
The effect of the mutation in the WLE1 of OsPR10a promoter region III. a Sequences of the WLE1 (the −659 to −644 bp) in the OsPR10a promoter and the mWLE1 with the TGAAA instead of TGACA. The WLE1 sequence is underlined and bolded. The asterisk represents the mutated base in the WLE1. b Luciferase activity in 687:LUC and m687:LUC in rice leaves. Bar indicates the standard error of the three replicates
Discussion
Plant defense mechanisms to pathogen attack have been extensively studied in Arabidopsis; however, it is not well studied in rice. To study the defense mechanisms in rice, we tried to understand the transcriptional regulation of OsPR10a because OsPR10a has been used as a marker of induction for the defense response in rice (Ryu et al. 2006; Chen et al. 2006). OsPR10a was originally cloned by Midoh and Iwata (1996). They reported that OsPR10a was induced by Magnaporthe grisea and probenazole but not by ethephon, NAA, SA, NaCl, mannitol, and wound. More recently, Rakwal et al. (2001) reported that OsPR10a was induced by various phytohormones, such as SA, JA, and ABA, but not by IAA and GA. There were discrepancies between these two reports. In our study, we have shown that OsPR10a was induced by Xanthomonas oryzae pv. oryzae, phytohormones, such as JA, SA, ethephon, ABA and NaCl, but not by IAA and GA. In the case of JA, our result is consistent with the previous report (Rakwal et al. 2001). However, in the case of SA, our result is consistent with the findings by Rakwal et al. (2001), but not with that one by Midoh and Iwata (1996). There might be some differences in the method of SA treatment. We treated rice seedlings with SA by the soil drenching method because our previous result, based on the expression of OsPR1 gene, indicated that spraying rice leaves with SA does not reliably induce the defense response. In the case of ethephon, our data are also not consistent with the results from Midoh and Iwata (1996). Their data on the expression of OsPR10a were generated by Northern blots, whereas our results were generated by a more sensitive method, RT-PCR. Our result is the first report on the response of OsPR10a to ethephon. Our data suggest that OsPR10a is induced by three different defense signaling transducers (SA, JA, and ethephon). For abiotic stress treatments, OsPR10a was induced by NaCl and ABA. In the case of NaCl, our result also differs from the data shown by Midoh and Iwata (1996). We think that there is a sensitivity difference due to the detection methods of OsPR10a mRNA between RT-PCR and Northern hybridization as in the case of ethephon. In the case of ABA, our result is consistent with a report by Rakwal et al. (2001). Taken together, we conclude that OsPR10a is induced by the pathogens, SA, JA, ethephon, NaCl, and ABA.
In this study, we focused on SA mediated induction of OsPR10a because SA mediated defense signaling is the most well studied in Arabidopsis. The OsPR10a promoter was isolated to study the transcriptional regulation of OsPR10a gene. Gene activity was induced by SA in a transient assay system as expected by its expression profile. Chen et al. (2006) reported that it was induced by an elicitor derived from Magnaporthe grisea as shown in a transient assay system. SA might be involved in elicitor-mediated defense signaling, yet there was no report on the activity of OsPR10a promoter in plants. Our data now have shown that the OsPR10a promoter was also activated by SA in stably transformed plants, as we have seen in a transient-assay system.
The cis-acting elements of the OsPR10a promoter were analyzed to find the elements responsible for its induction by SA. It resulted in many putative cis-acting elements. The transcription factors which play an important role in defense signaling are WRKY, ERF, bZIP, MYB, RAV1, etc. (Ruston and Somssich 1998; Singh et al. 2002; Sohn et al. 2006). Therefore, we searched binding sites in the OsPR10a promoter for WRKY, ERF, bZIP, MYB, and RAV1. We found W-box, RAV1AAT element, and ASF1 motif element. The W-box, RAV1AAT, and ASF1 motif are known to be the binding sites of the WRKY, RAV1, and bZIP proteins, respectively (Abe et al. 1997; Chen and Chen 2002; Yamamoto et al. 2004). This suggests that the WRKY, RAV1, or bZIP proteins might be responsible for the induction of OsPR10a promoter by SA. We also found several ABRE sequences that are known to be responsible for ABA responsiveness of the gene (Shinozaki and Yamaguchi-Shinozaki 1996). This element might be involved in the induction of OsPR10a by ABA. There is no cis-element, such as JERE or ERE even-though OsPR10a was induced by JA and ethephon. Induction of OsPR10a by JA and ethephon appears to occur indirectly through some other transcription factors bound to the OsPR10a promoter.
Based on cis-elements found in OsPR10a promoter, three different deletion constructs (818:LUC, 687:LUC, and 637:LUC) were made. Induction of the OsPR10a promoter by SA was completely abolished using the 818:LUC construct, indicating that at least one weak positive element exists in region I. In the 687:LUC construct, there was approximately a sixfold increase compared to the 1.0 kb OsPR10a promoter construct. This suggests that at least one negative element exists in region II. In the 637:LUC construct, its activity was dramatically reduced compared to the 687:LUC construct, suggesting that there is a positive element in region III. Induction of the promoter by SA was also maintained in the 637:LUC construct, suggesting a positive element is present in region IV. In region III, there were a number of available cis elements in the OsPR10a promoter (Table 1). However, only one WLE1 with the TGAC core was present in region III. Its nucleotide sequence is different from the canonical W-boxes ((T)TGACC/T) (Maleck et al. 2000). However, they also described that the WLE1 (TGACA) is enriched in PR-1 regulon promoters. To verify involvement of the WLE1 in response to SA, its sequences were mutagenized (Eulgem et al. 1999). The mutation of the WLE1 from TGAC to TGAA in region III completely abolished the induction of the 687:LUC construct by SA. This suggests that the WLE1 is important in the expression of the OsPR10a gene in response to SA. This is the first finding that the WLE1 (TGACA) is important in SA mediated PR gene expression. Interaction of transcription factors and cis-acting elements constitute a key step in the defense signaling. The OsTGA factor interacts with OsNPR1 as reported in Arabidopsis (Chern et al. 2001; Yu et al. 2001). These authors suggest that NPR1-mediated defense signaling in Arabidopsis is conserved in rice. However, they did not report the identity of the target gene of this complex. Liu et al. (2005b) reported that OsWRKY12 induces the expression of OsNPR1 and OsPR1b; however, they did not show evidence that OsWRKY12 directly regulates the expression of OsNPR1 and OsPR1b since their experiments utilized transgenic plants over-expressing OsWRKY12. Here, we suggest that WRKY may play a major role in SA-mediated OsPR10a expression in rice. However, we cannot exclude involvement of other transcription factors in SA-mediated expression of OsPR10a. In the near-future, we will carry out electrophoretic mobility assays of the WRKY proteins to the WLE1 described in this study. We will further address what kinds of WRKY proteins regulate the OsPR10a promoter and identify the different partners required for SA-mediated OsPR10a expression.
Acknowledgments
This work was supported in part by the grant CG3134-1 from twenty-first century frontier crop functional genomics and the two grants from National Institute of Agricultural Biotechnology (NIAB), Rural Development Adminstration (RDA) to Dr. Duk-Ju Hwang. In Ah Lee received the fellowship from Korea research foundation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ABA
Abscisic acid
- EREBP
Ethylene responsive element binding protein
- ET
Ethylene
- GA
Gibberellic acid
- IAA
Indole acetic acid
- JA
Jasmonic acid
- NAA
Alpha-napthalene acetic acid
- PR proteins
Pathogenesis-related proteins
- SA
Salicylic acid
- WLE
W-Box like element 1
- Xoo
Xanthomonas oryzae pv oryzae
References
- Abe H, Yamaguchi-Shinizaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell. 1997;9:1859–1868. doi: 10.1105/tpc.9.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gómez-Gómez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002;129:706–716. doi: 10.1104/pp.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang G-L. A highly efficient transient protoplast system for analyzing defense gene expression and protein-protein interactions in rice. Mol Plant Pathol. 2006;7:417–427. doi: 10.1111/j.1364-3703.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- Chern MS, Fitzgerald HA, Yadav RC, Canlas PE, Dong X, Ronald PC. Evidence for a disease resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis . Plant J. 2001;27:101–113. doi: 10.1046/j.1365-313x.2001.01070.x. [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Crit Rev Plant Sci. 1999;18:547–575. doi: 10.1080/07352689991309397. [DOI] [Google Scholar]
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE. Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J. 1999;18:4689–4699. doi: 10.1093/emboj/18.17.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald HA, Canlas PE, Chern MS, Ronald PC. Alteration of TGA factor activity in rice results in enhanced tolerance to Xanthomonas oryzae pv. oryzae . Plant J. 2005;43:335–347. doi: 10.1111/j.1365-313X.2005.02457.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T. A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol. 2004;45:550–559. doi: 10.1093/pcp/pch063. [DOI] [PubMed] [Google Scholar]
- Heise A, Lippok B, Kirsh C, Hahlbrock K. Two immediate-early pathogen-responsive members of the AtCMPG gene family in Arabidopsis thaliana and the W-box-containing elicitor-responsive element AtCMPG1. Proc Natl Acad Sci USA. 2002;99:9049–9054. doi: 10.1073/pnas.132277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JK, Lee SC, Hwang BK. Activation of pepper basic PR-1 gene promoter during defense signaling to pathogen, abiotic and environmental stresses. Gene. 2005;356:169–180. doi: 10.1016/j.gene.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Johnson C, Boden E, Arias J. Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense genes promoter in Arabidopsis . Plant Cell. 2003;15:1846–1858. doi: 10.1105/tpc.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya Y, Ohmiya K, Hattori T. RAV1 a novel DNA-binding protein, binds to bipartite recognition sequence through two distinct DNA-binding domains uniquely found in higher plants. Nucleic Acids Res. 1999;27:470–478. doi: 10.1093/nar/27.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch C, Logemann E, Lippok IB, Schmelzer E, Hahlbrock K. A highly specific pathogen-responsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum . Plant J. 2001;26:217–227. doi: 10.1046/j.1365-313x.2001.01015.x. [DOI] [PubMed] [Google Scholar]
- Lee SC, Hwang BK. Identification and deletion analysis of the promoter of the pepper SAR8.2 gene activated by bacterial infection and abiotic stresses. Planta. 2006;224:255–267. doi: 10.1007/s00425-005-0210-z. [DOI] [PubMed] [Google Scholar]
- Li Y-F, Zhu R, Xu P. Activation of the gene promoter of barley β-1,3-glucanase isoenzyme GIII is salicylic acid (SA)-dependent in transgenic rice plants. J Plant Res. 2005;118:215–221. doi: 10.1007/s10265-005-0213-7. [DOI] [PubMed] [Google Scholar]
- Liu J-J, Ekramoddoullah AKM, Piggoyy N, Zamani A. Molecular cloning of a pathogen/wound-inducible PR10 promoter from Pinus monticola and characterization in transgenic Arabidopsis plants. Planta. 2005;221:159–169. doi: 10.1007/s00425-004-1428-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Bai X, Qian Q, Wang X, Chen M, Chu C. OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res. 2005;15:593–603. doi: 10.1038/sj.cr.7290329. [DOI] [PubMed] [Google Scholar]
- Liu X, Bai X, Wang X, Chu C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol. 2006;164:969–979. doi: 10.1016/j.jplph.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Jürg S, Lawton KA, Dangle JL, Dietrich RA. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet. 2000;26:403–409. doi: 10.1038/82521. [DOI] [PubMed] [Google Scholar]
- Malnoy M, Venisse J-S, Reynoird JP, Chevreau E. Activation of three pathogen-inducible promoters of tobacco in transgenic pear (Pyrus communis L.) after abiotic and biotic elicitation. Planta. 2003;216:802–814. doi: 10.1007/s00425-002-0932-0. [DOI] [PubMed] [Google Scholar]
- Midoh N, Iwata M. Cloning and characterization of a probenazole-inducible gene for an intracellular pathogenesis-related protein in rice. Plant Cell Physiol. 1996;37:9–18. doi: 10.1093/oxfordjournals.pcp.a028918. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Pieterse CM, van Loon LC. Salicylic acid-independent plant defense pathways. Trends Plant Sci. 1999;4:52–58. doi: 10.1016/S1360-1385(98)01364-8. [DOI] [PubMed] [Google Scholar]
- Rakwal R, Agrawal GK, Yonekura M. Light-dependent induction of OsPR10 in rice (Oryza sativa L.) seedlings by the global stress signaling molecule jasmonic acid and protein phosphatase 2A inhibitors. Plant Sci. 2001;161:469–479. doi: 10.1016/S0168-9452(01)00433-2. [DOI] [Google Scholar]
- Robatzek S, Somssich IE. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defense-related processes. Plant J. 2001;28:123–133. doi: 10.1046/j.1365-313X.2001.01131.x. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996;15:5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Ruston PJ, Somssich IE. Transcriptional control of plant genes responsive to pathogens. Curr Opin Plant Biol. 1998;1:311–315. doi: 10.1016/1369-5266(88)80052-9. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Reinstadler A, Lipka V, Lippok B, Somssich IE. Synthetic plant promoters containing defined regulatory elements provides novel insights into pathogen- and wound-induced signaling. Plant Cell. 2002;14:749–762. doi: 10.1105/tpc.010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu HS, Han M, Lee SK, Cho JI, Ryoo N, Heu S, Lee YH, Bhoo SH, Wang G, Hahn TR, Jeon JS. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 2006;25:836–847. doi: 10.1007/s00299-006-0138-1. [DOI] [PubMed] [Google Scholar]
- Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA, Melchers LS, Van den Elzen PJM, Cornelissen BJC. Only specific tobacco (Nicotiana tabacum) chitinases and β-1, 3-glucanases exhibit antifungal activity. Plant Physiol. 1993;101:857–863. doi: 10.1104/pp.101.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono M, Sugano S, Nakayama A, Jiang C-J, Ono K, Toki S, Takatsuji H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–2076. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki Molecular responses to drought and cold stress. Curr Opin Biotechnol. 1996;7:161–167. doi: 10.1016/S0958-1669(96)80007-3. [DOI] [PubMed] [Google Scholar]
- Shinshi H, Usami S, Ohme-Takagi M. Identification of an ethylene-responsive region in the promoter of a tobacco class I chitinase gene. Plant Mol Biol. 1995;27:923–932. doi: 10.1007/BF00037020. [DOI] [PubMed] [Google Scholar]
- Singh K, Foley RC, Onate-Sanchez L. Transcription factors in plant defense and stress responses. Curr Opin Plant Biol. 2002;5:430–436. doi: 10.1016/S1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- Sohn KH, Lee SC, Jung HW, Hong JK, Hwang BK. Overexpression of the pepper CARAV1 pathogen-induced gene encoding a RAV transcription factor induces pathogenesis-related genes and enhances resistance to bacterial pathogen in Arabidopsis . Plant Mol Biol. 2006;61:897–915. doi: 10.1007/s11103-006-0057-0. [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comprehensive analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55:65–97. doi: 10.1006/pmpp.1999.0213. [DOI] [Google Scholar]
- Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, Van den Elyzen PJM, Cornelissen BJC. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans . Plant Cell. 1991;3:619–628. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nakano T, Suzuki K, Shinshi H. Elicitor-induced activation of transcription via W box-related cis-acting elements from a basic chitinase gene by WRKY transcription factors in tobacco. Biochim Biophys Acta. 2004;1679:279–287. doi: 10.1016/j.bbaexp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Yu D, Chen C, Chen Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell. 2001;13:1527–1540. doi: 10.1105/tpc.13.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]


