Abstract
While some prey species possess an innate recognition of their predators, others require learning to recognize their predators. The specific characteristics of the predators that prey learn and whether prey can generalize this learning to similar predatory threats have been virtually ignored. Here, we investigated whether fathead minnows that learned to chemically recognize a specific predator species as a threat has the ability to generalize their recognition to closely related predators. We found that minnows trained to recognize the odour of a lake trout as a threat (the reference predator) generalized their responses to brook trout (same genus as lake trout) and rainbow trout (same family), but did not generalize to a distantly related predatory pike or non-predatory suckers. We also found that the intensity of antipredator responses to the other species was correlated with the phylogenetic distance to the reference predator; minnows responded with a higher intensity response to brook trout than rainbow trout. This is the first study showing that prey have the ability to exhibit generalization of predator odour recognition. We discuss these results and provide a theoretical framework for future studies of generalization of predator recognition.
Keywords: predator recognition, generalization, antipredator behaviour, predator odours, fathead minnow, Pimephales promelas
1. Introduction
The majority of animal species are susceptible to predation by several species of predators. Thus, prey species are often under intense selection pressure to respond adaptively to predators. However, the nature and intensity of predation risk experienced by a given prey individual will probably vary both spatially and temporally (Lima & Dill 1990; Lima & Bednekoff 1999). For example, predation risk may change as the individual grows and shifts prey guilds, habitat or activity level (Sih et al. 2000). Likewise, over evolutionary time, predator communities change, exposing prey to new predation pressures.
A prerequisite for prey to respond adaptively to predation risk is to recognize threats posed by potential predators. The first alternative for prey is to possess an innate recognition of at least some of their potential predators (e.g. birds, Goth 2001, Wiebe 2004; fishes, Berejikian et al. 2003; mammals, Fendt 2006). Some other species, however, require experience to respond to predation (learning). Learned predator recognition has been demonstrated in a wide variety of taxa, for both invertebrates (Rochette et al. 1997) and vertebrates (birds, Curio et al. 1978; mammals, McLean et al. 1996, Griffin et al. 2001; fishes, Mathis & Smith 1993, Chivers & Smith 1994, Chivers & Smith 1998; amphibians, Woody & Mathis 1998, Mirza et al. 2006).
For many aquatic species, one mode of learning is through the pairing of cues (either chemical or visual cues) from a novel predator with the odour of injured conspecifics (Wisenden 2003). For many species of fishes, chemicals present in the epidermis, commonly referred to as ‘alarm cues’, have been demonstrated to elicit a dramatic increase in antipredator responses upon detection. Those chemicals are usually released upon the damage of their skin, which usually occurs when a fish is either captured or injured by a predator (Chivers & Smith 1998).
From a phylogenetic perspective, predators that are closely related would generally share similar foraging habits. For example, carnivorous species will require specific behavioural, morphological and physiological adaptations to capture, handle, eat and digest their prey. While these adaptations are diverse among taxa, closely related species will usually share similar adaptations. Thus, prey should have an advantage if they can generalize the recognition of a specific predator to closely related novel predators. This phenomenon, which we refer to as ‘generalization of predator recognition’ has surprisingly not received much attention from behavioural ecologists. Only two studies have empirically tested for visual generalization of predator recognition. In a landmark study, Griffin et al. (2001) demonstrated that tammar wallabies (Macropus eugenii) conditioned to recognize a red fox (Vulpes vulpes), subsequently displayed an antipredator response when exposed to a red fox and generalized their antipredator response to a feral cat (Felis catus) but not to a juvenile goat (Capra hircus). Chivers & Smith (1994) conditioned fathead minnows (Pimephales promelas) to visually recognize either a northern pike (Esox lucius) or a goldfish (Carassius auratus) as a predatory threat. Subsequent testing demonstrated that minnows displayed an antipredator response to the fishes they were conditioned to, but did not generalize the fear response to other species. Perhaps this is not surprising, given the considerable differences in the appearance of pike and goldfish. Only one study indirectly tested for the possibility of chemical generalization of predator recognition. Darwish et al. (2005) conditioned juvenile glowlight tetras (Hemigrammus erythrozonus) to recognize a cocktail of odours containing cues from largemouth bass (Micropterus salmoides), convict cichlids (Archocentrus nigrofasciatus) and comet goldfish (C. auratus). The tetras displayed an antipredator response when subsequently exposed to each of the predator odours separately, but not when exposed to the novel odour of yellow perch (Perca flavescens). Again, this may not be surprising given that perch belong to a different family (Percidae) than all the other fishes (Centrarchidae, Cichlidae and Cyprinidae). The studies completed so far indicated the generalization of predator recognition via visual cues by mammals but not by other vertebrates, and no generalization of predator recognition by chemical cues for any species. These results raise the questions of whether generalization is an ability that is restricted to the most advanced vertebrates and whether it is restricted to visual modalities.
Here, we test whether a prey fish has the ability to generalize its antipredator response to predator odours of closely related predator species. We conditioned fathead minnows to recognize the odour of lake trout (Salvenilus namaycush) as a predation threat and subsequently tested them for a response to lake trout (reference predator), brook trout (Salvelinus fontinalis—same genus as the reference predator), rainbow trout (Oncorhynchus mykiss—same family but different genus), northern pike (E. lucius—distantly related predatory fish) or white sucker (Catostomus commersoni—distantly related non-predatory fish). An underlying assumption of our work is that taxonomic relatedness will be reflected in the odour signatures of the fishes.
2. Material and methods
To investigate whether fathead minnows could generalize the recognition of potential predators based on predator odours, we first conditioned naive fathead minnows to recognize the odour of lake trout as a predatory threat. Naive minnows learned to recognize the odour of a novel predator (including other salmonid fishes) based on the pairing of alarm cues and predator odour (Chivers & Smith 1993; Ferrari et al. 2005; Ferrari & Chivers 2006a). Thus, we exposed naive minnows to lake trout odour paired with either (i) alarm cues (to obtain a group of minnows displaying a fright response when exposed to lake trout odour) or (ii) water (control—to obtain a group of minnows solely exposed to lake trout odour without any risk association). The second phase consisted of recording the intensity of antipredator responses displayed by the minnows when subsequently exposed to the ‘reference predator’ (lake trout) odour or to the odour of one of the other four fishes (table 1).
Table 1.
Simplified representation of the taxonomic relationship between the five fish species used in the experiment.
| division Teleostei |
| subdivision Euteleostei |
| superorder Ostariophysi |
| order Cypriniformes |
| family Catostomidae—white sucker |
| superorder Protacanthopterygii |
| order Salmoniformes |
| family Salmonidae |
| genus Salvelinus—lake trout, brook trout |
| genus Oncorhynchus—rainbow trout |
| order Esociformes |
| family Esocidae—northern pike |
(a) Predictions
The minnows used in this experiment were collected from a body of water lacking other fish species. Fathead minnows are known to lack innate predator recognition of the predators used in this experiment (pike, Chivers & Smith 1994; trout, Ferrari et al. 2005, Ferrari & Chivers 2006a). Consequently, we predicted that water-conditioned minnows should fail to exhibit antipredator responses to any of the five fishes.
Several predictions are made regarding the responses of alarm cue-conditioned minnows. First, since minnows have been conditioned to recognize the odour of lake trout as a threat, we predict that minnows should display their highest intensity response to lake trout odour. We could not standardize the diet of all the fishes we used, as pike are exclusively piscivorous and do not eat trout pellets, the food which was provided to the four other fishes. We tried to minimize potential diet effects by eliminating the remnants of the last meal of all fishes (see below). If the generalization of the response of minnows was based on the diet of the reference predator (i.e. the lake trout's diet), we predict that minnows should show an antipredator response to all the fishes but pike (scenario 1). If the antipredator response of minnows to lake trout odour is not generalized to the odour of other fishes, we predict that minnows should show an antipredator response when exposed to the odour of lake trout only, and not when exposed to the odours of other salmonids, pike or suckers (scenario 2). However, it is possible that minnows display partial or total generalization to other salmonid fishes. As brook trout belong to the same genus as lake trout, we predict that if generalization occurs, minnows should generalize their antipredator responses to brook trout more than to rainbow trout (scenario 3). It might be possible that minnows generalize their response to all predatory fishes and would display an antipredator response to the odour of all fishes but suckers (scenario 4), or they might even generalize their responses to all large fishes (scenario 5). The last two scenarios are less probable, given the knowledge of fathead minnow's response to the odour of unknown predators.
(b) Test fish
Fathead minnows were captured from a local pond in Saskatoon (SK, Canada), using minnow traps in September 2006. They were housed in a 6000 l flow-through pool filled with dechlorinated tap water at 11°C and fed ad libitum commercial fish flakes (Nutrafin basix, Rolf C. Hagen, Inc., Montreal, Quebec, Canada).
The brook trout and rainbow trout were obtained from the Fort Qu'Appelle fish hatchery (SK, Canada) in October 2004, and the lake trout were obtained from the same place in April 2006. The three species were housed separately in 6000 l flow-through pools filled with dechlorinated tap water and fed daily with commercial trout pellets (Martin's, Elmira, Ontario, Canada). The three species were kept under the same conditions for at least five months. Juvenile pike were captured from Pike Lake (SK, Canada) in October 2005 using a seine net. They were housed in a 6000 l flow-through pool and fed live minnows and dace. The white suckers were caught using a seine net in Katepwa Lake (SK, Canada) in April 2006, kept in a 6000 l pool and fed trout pellets. All the fishes were kept under a 14 : 10 h light : dark cycle.
(c) Stimulus collection
(i) Minnow skin extract
We collected skin extract from five fathead minnows (fork length, FL: mean±s.d.=5.66±0.46 cm). Minnows were killed by a blow to the head (in accordance with the Canadian Council on Animal Care) and skin fillets were removed from both sides of the body and placed in chilled distilled water. The fillets were then homogenized using a tissue homogenizer and filtered through glass wool to remove any remaining tissues. We collected a total of 25.9 cm2 of skin in a total of 518 ml of distilled water. This solution was diluted to obtain a final solution containing approximately 1 cm2 of skin per 40 l. This concentration has been shown to elicit overt antipredator responses in fathead minnows (Ferrari et al. 2005, 2006). Skin extracts were frozen into 20 ml aliquots at −20°C until required.
(ii) Fish odour
The three species of trout and the suckers were kept on a diet of trout pellets. However, pike are strictly piscivorous and thus could not be fed trout pellets. Furthermore, fishes can respond to predators based on the presence of conspecific alarm cues in the diet of the predator (Chivers & Mirza 2001), thus we had to remove any remnants of fathead minnow or dace alarm cues in the diet of the pike. According to Bevelhimer et al. (1985), the gut evacuation of juvenile pike takes 5 days at 5°C. Thus, 8 days prior to stimulus collection, two arbitrarily chosen juvenile pike (FL=32 and 38 cm) were transferred into their own 74 l tanks, containing a corner filter and an air stone and maintained at 18°C. The pike were not fed for 4 days and each pike received two adult green swordtails (Xiphophorus helleri, approx. 4.5 cm standard length) per day for the next 2 days. Swordtails were fed to the pike as they are known to lack the alarm substances recognized by fathead minnows (Mathis & Smith 1993; Brown et al. 1995).
Three days prior to stimulus collection, two lake trout (FL=25 and 26 cm), two brook trout (FL=34 and 35 cm), two rainbow trout (FL=39 and 40 cm), two suckers (FL=38 and 38 cm) and the two juvenile pike were placed individually in tanks containing 74 l of clean dechlorinated tap water. The fishes were arbitrarily chosen so as to minimize the size difference between all the five species. The fishes were kept in these individual tanks to allow the elimination of remnants of their last meal, to minimize the potential effect of diet and maximize the effect of species' odour on the response of minnows.
For stimulus collection, two fishes from each species were placed in a 74 l tank containing 50 l of dechlorinated tap water and were left to soak for 24 h. The fishes were then removed, returned to their original holding facility and fed. The fish-conditioned water was stirred and frozen in 60 ml aliquots until required.
(d) Experimental procedure
(i) Conditioning phase
Twenty-four hours prior to being conditioned, groups of three fathead minnows were placed in 37 l tanks (50×25×30 cm) containing 30 l of dechlorinated tap water and a gravel substrate. The tanks were also equipped with an air stone to which was attached a 2 m long piece of tubing used to inject the stimuli into the tanks. Minnows were fed after being transferred and also 1 h prior to being conditioned the next day. Prior to injecting the stimuli in the tank, we withdrew and discarded 60 ml of water from the injection tubes to remove any stagnant water, and an additional 60 ml of water were withdrawn and retained to flush the stimuli into the tank. The conditioning consisted of injecting sequentially 5 ml of either alarm cues or dechlorinated tap water and 20 ml of lake trout odour, followed by 60 ml of the retained tank water. On each conditioning day, half the tanks received the alarm cue treatment and the other half the water treatment, and the treatments were randomly assigned to the conditioning tanks within the experimental room. At least 1 h after being conditioned, the groups of three minnows were randomly transferred to identical 37 l tanks (used for testing) containing clean dechlorinated tap water and were fed.
(ii) Testing phase
The testing phase took place 24 h after the conditioning phase. Minnows were fed 1 h prior to being tested. During this phase, groups of minnows were randomly exposed to 20 ml of the odour of either lake trout, brook trout, rainbow trout, pike or sucker, and their behaviour was recorded. The protocol for the stimulus injection followed the same protocol as used in the conditioning phase.
(iii) Behavioural assay
The behaviour of the group of minnows was recorded for 8 min preceding the stimulus injection to obtain the baseline level of the minnow's activity and for 8 min immediately following the injection of the stimulus. The difference in activity between the pre- and post-stimulus injection periods represents the change in activity resulting from the stimulus injection. We used a well-established protocol to quantify the antipredator behaviour of the minnows (e.g. Mathis & Smith 1993; Ferrari et al. 2005; Ferrari & Chivers 2006a) based on shoaling index (the shoaling index of the three fish every 15 s; 1, no fish within a body length of another; 2, two fish within a body length of each other; and 3, all the three fishes within a body length of each other) and line crosses (the number of line crosses, using the 3×3 grid pattern drawn on the side of the tank, made by one of the three minnows, randomly chosen, during the observation period, using a click counter). An increase in shoaling and a reduction in movement are well-established antipredator responses in fathead minnows (Ferrari et al. 2005; Ferrari & Chivers 2006a).
(e) Statistical analysis
The data used for the analysis were the difference in behavioural measures between the pre- and post-injection periods. The data were normally distributed but the variance was not homogenous among treatments.
We first investigated potential interactions between the effect of fish species and conditioning on the responses of minnows by performing a two-way Scheirer–Ray–Hare extension of the Kruskal–Wallis test (Sokal & Rohlf 2003, pp. 446–447), a multiway ANOVA design for ranked data. We then investigated the effect of conditioning on the responses of minnows to each fish odour by performing five independent Welch's t-tests (Zar 1999, pp. 128–129) on the five odour treatments (the alpha level was not modified as the five tests use 10 different samples). We then analysed the effect of fishes separately by performing two Kruskal–Wallis tests on the responses of minnows conditioned with water, and minnows conditioned with alarm cues, followed by Mann–Whitney post hoc tests to investigate the difference between the groups of interests. Owing to drastic loss of power related to the number of comparisons, only three Mann–Whitney tests were performed to compare the difference in response of minnows exposed to lake trout, brook trout and rainbow trout odour (the comparisons of interests). For these tests, the alpha level was set to 0.016 following the Bonferroni correction to minimize the likelihood of type I error (Higgins 2004, pp. 93).
3. Results
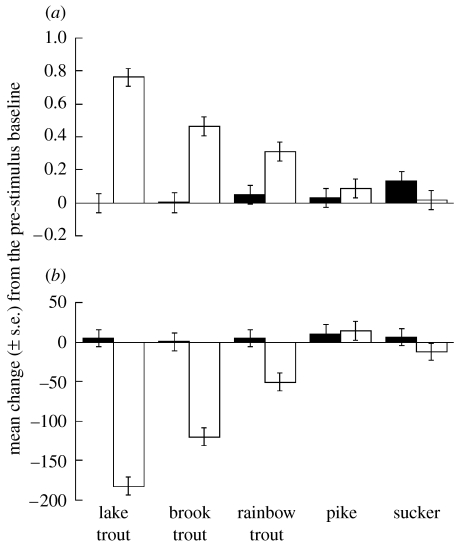
The results of the multifactorial ANOVA revealed a significant interaction between fishes and conditioning for both shoaling index (H4,188=7.8, p<0.001, figure 1a) and line crosses (H4,188=5.8, p<0.001, figure 1b). The t-tests showed no significant differences in the intensity of response of minnows conditioned with water or alarm cues when the minnows were exposed to the odour of either pike or sucker (shoaling index: both p>0.15, line crosses: both p>0.23). However, minnows conditioned with alarm cues displayed significantly higher antipredator responses than water-conditioned minnows, when exposed to the odour of lake trout, brook trout or rainbow trout (shoaling index: all p<0.039, line crosses: all p<0.002). The Kruskal–Wallis test on the responses of water-conditioned minnows revealed no significant effect of fishes on either change in shoaling index (Χ42=3.7, p=0.44) or line crosses (Χ42=0.5, p=0.97). However, the Kruskal–Wallis test on the responses of minnows conditioned with alarm cues revealed a significant effect of fishes on both shoaling index (Χ42=28.5, p<0.001) and line crosses (Χ42=39.3, p<0.001). These results, in conjunction with the results of the t-tests, show that minnows conditioned with alarm cues display antipredator responses when exposed to the odour of the three trout only. The post hoc Mann–Whitney U tests on those three groups revealed that minnows did not display statistically different intensity responses to the odour of lake trout and brook trout (shoaling index: U=162.5, p=0.063; line crosses: U=163.0, p=0.065), but minnows did display a higher response intensity to lake trout odour than rainbow trout odour (shoaling index: U=124.0, p=0.006; line crosses: U=114.0, p=0.003). When comparing the intensity of response to brook trout versus rainbow trout, minnows did not significantly differ in their shoaling index (U=179.0, p=0.296), but they decreased the activity significantly more when exposed to brook trout than rainbow trout (U=116.0, p=0.009).
Figure 1.
Mean change (±s.e.) in (a) shoaling index and (b) line crosses for minnows conditioned with lake trout odour paired with either water (black bars) or conspecific alarm cues (white bars), and tested for a response to either lake trout, brook trout, rainbow trout, pike or sucker.
4. Discussion
Our results suggest that fathead minnows conditioned to recognize the odour of lake trout generalized their recognition to closely related species, the brook trout and rainbow trout, but not to distantly related predatory (pike) or non-predatory (sucker) fish. The absence of response to the sucker odour indicates that minnows did not rely on diet cues to generalize their recognition. The absence of a response to pike odour indicates that the generalization is limited to trout only, but not all fish predators. As expected, minnows responded with the highest response intensity to the odour of lake trout, the species they were conditioned to recognize as a threat. The level of generalization was dependent to some extent on the degree of relatedness of the other potential predators to the reference predator. Minnows did not respond differently to lake trout and brook trout, but the p values for both behavioural measures (0.065, 0.067) indicate that we may have weak support to say that minnows chemically differentiated the two species. We also have evidence suggesting that minnows responded with less intensity to rainbow trout odour than brook trout odour, hence displaying a graded response to other trout odour, reflecting the taxonomic closeness of these trout species to the reference predator.
The proximate mechanism behind this response pattern may be a difference in the suite of molecules that form the trout odour. In this case, odour molecules among the trout species are probably similar as they are recognized by the minnows, but are not identical as the minnows clearly differentiate the odours. Alternatively, the graded responses could be explained by the existence of a concentration gradient of specific chemicals. Fathead minnows have been demonstrated to adjust the intensity of their antipredator response according to the concentration of predator odour they are exposed to (Ferrari et al. 2006). Here, minnows may have learned to recognize specific chemicals from lake trout that are present in high concentration and may have adjusted the intensity of their antipredator response during subsequent exposures to other trout odours according to the concentration of these particular chemicals.
(a) Plasticity of generalization of predator recognition
(i) Taxonomy of generalization
This paper presents evidence that fathead minnows are able to chemically generalize the antipredator responses from lake trout to closely related salmonid fish. Griffin et al. (2001) demonstrated that tammar wallabies have the ability to visually generalize their antipredator response from a red fox to a cat. Stankowich & Coss (2007) used felid predator models and showed that Columbian black-tailed deer (Odocoileus hemionus comlombianus) exhibited a strong antipredator response to a model puma, their current predator, an intermediate response to a novel tiger model, but did not differ in their responses to a model jaguar or a model mule deer. Whether the ability of black-tailed deer to generalize resulted from learning or whether it represents generalization from an innate recognition template deserves further consideration. Both fishes and mammals have the ability to generalize their recognition of predators to closely related novel predators, consequently it is not unreasonable to think that other vertebrates may also possess this ability. Given the considerable implications of these findings for prey risk assessment, we strongly encourage additional work by researchers studying both vertebrates and invertebrates.
(ii) When to generalize?
We hypothesize that the degree of flexibility in the generalization of predator recognition is dependent on the evolutionary history of predation experienced by each prey species. Species living in relative ‘isolation’ for long evolutionary periods might have a limited ability to learn and generalize predator recognition. For example, if a prey species is always exposed to the same species of predators over long periods of evolutionary time, then it is probable that animals evolving an innate recognition of those predators will be at a selective advantage, as they do not require the first ‘learning trial’ to identify the predator as a threat. However, prey species do not always possess innate recognition of predators, implying that there has not been enough time to genetically fix the response and/or there is a cost to genetically fixing such a response.
Two factors affecting generalization in a given environment may be the predictability of predation and predator diversity. We hypothesize that it would be beneficial for prey to have innate predator recognition in environments where predictability of attack from a given predator is high and predator diversity is relatively low. Conversely, it would be beneficial for prey to base their responses on learned predator recognition and have predator generalization abilities in environments where predation predictability is low and predator diversity is high. An unknown aspect of innate predator recognition is whether prey are cuing on specific or general characteristics of the predators they respond to, i.e. the extent to which they can generalize a response to a novel predator.
Imagine a prey animal living in an environment where the ratio of ‘predators to non-predators’ is high (e.g. a rodent exposed to 10 species of birds, 9 being predatory and 1 not). The rodent would probably benefit from generalizing its predator recognition to all birds, as it would do better if it was always scared of a bird, given the probability that failing to respond will result in death. Conversely, if the ratio of ‘predators to non-predators’ is low (e.g. a rodent exposed to 10 species of birds, 1 being predatory and 9 not), the prey may do better if it specifically learned to recognize the only predatory species and hence not be scared of the non-predatory ones. Keep in mind that responding to predators is costly as it takes time and effort away from fitness-related activities such as foraging or reproduction (Lima & Dill 1990). Hence, the ability to generalize predator recognition is likely to be directly related to the predation history experienced by prey species in a given habitat. Consequently, it is probable that prey species may have innate recognition of some predators and learned recognition and generalization of some others. Perhaps the best way to approach predator recognition is to think of it as a continuum from ‘innate predator recognition’ to ‘learned predator recognition without generalization’ and finally to ‘learned predator recognition with generalization’. We refer to this as the ‘Predator Recognition Continuum Hypothesis’.
Fathead minnows used in our study are common through most of central North America. They inhabit ponds, lakes and rivers and can easily move from one to another during floods. Hence, as a species, their small size and wide distribution will probably result in exposures to a wide variety of predators, particularly when considered over an evolutionary time-scale. Hence it might be adaptive for a species like fathead minnows to be able to have flexibility (or plasticity) in the recognition pattern of potential predators.
(iii) What to generalize?
Prey animals probably cue on some specific characteristics of the predators, such as shape, colour or odour. Stankowich & Coss (2007) showed that black-tailed deer do generalize their visual recognition of a puma to a tiger, but not to a jaguar. While all of these felids have the same general shape, they differ in their coat pattern. In this case, deer generalize from a felid with a uniform coat (a puma) to a felid with a striped-coat (a tiger), which implies that deer do not cue solely on coat colour to recognize predators. However, the spotted coat pattern of the jaguar seems to deceive the prey, as the deer are not able to recognize the jaguar as a predator.
In any theoretical consideration of the generalization of predator recognition, we need to consider what cues the prey should use to generalize the predators. A predator's diet has been demonstrated to be an important factor in predator labelling in many species. For example, many fishes have been demonstrated to label a novel fish as a predator when detecting conspecific alarm cues in the fish's diet (Chivers & Mirza 2001). Likewise, rodents cue in on the breakdown of sulphur products in the diet of their predators (Fendt 2006). Thus, one can make the argument that diet plays a role in the generalization of predator recognition. Here, we argue that diet is a labelling tool, which allows prey to label a novel species as predatory. This phenomenon does not require any true ‘recognition’ of the predator, but instead the recognition of cues indicating risk. In contrast, true predator generalization requires the ability of prey to use specific characteristics of already known predators to respond to somehow similar unknown species, and thus should be independent of diet effects.
Blumstein (2002) discussed the effect of relaxed predation pressure on predator recognition in tammar wallabies. He argues that while visual predator recognition could be retained for several thousands of years of predator relaxation, chemical and acoustic predator recognition needed to be learned. Similarly, it may be possible that prey may generalize predator recognition using one type of stimulus but not another. Further research needs to address the use of different stimulus types in generalization of predator recognition. Such work may reveal fascinating taxonomic predispositions towards particular sensory systems.
(iv) Generalization of non-predator recognition
A thorough consideration of generalization should include not only what predators prey can generalize to recognize as a threat but also what non-predators prey can generalize to recognize as not a threat. In one study, Griffin et al. (2002) tried to condition tammar wallabies to recognize a juvenile goat as a threat, but wallabies did not acquire a fear response to the goat. Three scenarios could explain these results. Firstly, it is possible that wallabies had previous experience with goats in their environment and had previously learned that goats were not a threat, as goat cues were never associated with risk. Thus, learning to recognize the predator failed due to latent inhibition (Acquistapace et al. 2003; Ferrari & Chivers 2006b). Secondly, it is possible that wallabies were previously exposed to a close relative of the goat and thus, as before, did not learn to associate the danger with the sight of the goat due to generalization. Thirdly, it is possible that wallabies innately recognize goats as a non-predator. It is not unrealistic to imagine that the costs associated with responding to non-predators may be high enough for prey to genetically fix the recognition of non-predator characteristics. Thus, there may be a generalization of non-predators. This is an exciting topic that deserves further consideration.
More studies on the topic of generalization would allow us to answer questions such as: how specific or general is predator recognition; what types of information prey are using to recognize predators; which factors affect the specificity of learned predator recognition; and how does the evolutionary history of predation drive these differences. Factors limiting generalization of predator recognition might be of prime importance for endangered species that are translocated in new habitats and exposed to new predator communities. Moreover, the propensity of some species to rapidly and adaptively respond to new communities of predators might help us predict the level of invasiveness of those species.
Acknowledgments
These ideas were conceived by M. C. O. Ferrari and D. P. Chivers. We thank Gary R. Bortolotti for his comments on the manuscript. The Natural Sciences and Engineering Research Council of Canada and the University of Saskatchewan provided financial support to D. P. Chivers and F. Messier. All work reported herein was in accordance with the Guidelines to the Care and Use of Experimental Animals published by the Canadian Council on Animal Care and was conducted under the University of Saskatchewan Committee of Animal Care and Supply protocol no. 20050136.
References
- Acquistapace P, Hazlett B.A, Gherardi F. Unsuccessful predation and learning of predator cues by crayfish. J. Crust. Biol. 2003;23:364–370. doi:10.1651/0278-0372(2003)023[0364:UPALOP]2.0.CO;2 [Google Scholar]
- Berejikian B.A, Tezaka E.P, LaRaeb A.L. Innate and enhanced predator recognition in hatchery-reared chinook salmon. Environ. Biol. Fishes. 2003;67:241–251. doi:10.1023/A:1025887015436 [Google Scholar]
- Bevelhimer M.S, Stein R.A, Carline R.F. Assessing significance of physiological differences among three esocids with a bioenergetics model. Can. J. Fish. Aquat. Sci. 1985;42:57–69. [Google Scholar]
- Blumstein D.T. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 2002;29:685–692. doi:10.1046/j.1365-2699.2002.00717.x [Google Scholar]
- Brown G.E, Chivers D.P, Smith R.J.F. Localized defecation of pike: a response to labelling by cyprinid alarm pheromone? Behav. Ecol. Sociobiol. 1995;36:105–110. doi:10.1007/s002650050130 [Google Scholar]
- Chivers D.P, Mirza R.S. Predator diet cues and the assessment of predation risk by aquatic vertebrates: a review and prospectus. In: Marchlewska-Koj D.A, Lepri J.J, Müller-Schwarze D, editors. Chemical signals in vertebrates. vol. 9. Plenum Press; New York, NY: 2001. [Google Scholar]
- Chivers D.P, Smith R.J.F. The role of olfaction in chemosensory-based predator recognition in the fathead minnow Pimephales promelas. J. Chem. Ecol. 1993;19:623–633. doi: 10.1007/BF00984997. doi:10.1007/BF00984997 [DOI] [PubMed] [Google Scholar]
- Chivers D.P, Smith R.J.F. Fathead minnows Pimephales promelas, acquire predator recognition when alarm substance is associated with the sight of unfamiliar fish. Anim. Behav. 1994;48:597–605. doi:10.1006/anbe.1994.1279 [Google Scholar]
- Chivers D.P, Smith R.J.F. Chemical alarm signaling in aquatic predator/prey interactions: a review and prospectus. Ecoscience. 1998;5:338–352. [Google Scholar]
- Curio E, Ernst U, Vieth W. Cultural transmission of enemy recognition: one function of mobbing. Science. 1978;202:899–901. doi: 10.1126/science.202.4370.899. doi:10.1126/science.202.4370.899 [DOI] [PubMed] [Google Scholar]
- Darwish T.L, Mirza R.S, Leduc A.O.H.C, Brown G.E. Acquired predator recognition of novel predator odour cocktails by juvenile glowlight tetras. Anim. Behav. 2005;70:83–89. doi:10.1016/j.anbehav.2004.09.017 [Google Scholar]
- Fendt M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J. Chem. Ecol. 2006;32:2617–2627. doi: 10.1007/s10886-006-9186-9. doi:10.1007/s10886-006-9186-9 [DOI] [PubMed] [Google Scholar]
- Ferrari M.C.O, Chivers D.P. The role of learning in the development of threat-sensitive predator avoidance: how do fathead minnows incorporate conflicting information? Anim. Behav. 2006a;71:19–26. doi:10.1016/j.anbehav.2005.02.016 [Google Scholar]
- Ferrari M.C.O, Chivers D.P. The role of latent inhibition in acquired predator recognition by fathead minnows. Can. J. Zool. 2006b;84:505–509. doi:10.1139/Z06-027 [Google Scholar]
- Ferrari M.C.O, Trowell J.J, Brown G.E, Chivers D.P. The role of leaning in the development of threat-sensitive predator avoidance in fathead minnows. Anim. Behav. 2005;70:777–784. doi:10.1016/j.anbehav.2005.01.009 [Google Scholar]
- Ferrari M.C.O, Kapitania-Kwok T, Chivers D.P. The role of learning in the development of threat-sensitive predator avoidance: the use of predator cue concentration by fathead minnows. Behav. Ecol. Sociobiol. 2006;60:522–527. doi:10.1007/s00265-006-0195-z [Google Scholar]
- Goth A. Innate predator-recognition in Australian brush-turkey (Alectura lathami, Megapodiidae) hatchlings. Behaviour. 2001;138:117–136. [Google Scholar]
- Griffin A.S, Evans C.S, Blumstein D.T. Learning specificity in acquired predator recognition. Anim. Behav. 2001;62:577–589. doi:10.1006/anbe.2001.1781 [Google Scholar]
- Griffin A.S, Evans C.S, Blumstein D.T. Selective learning in a marsupial. Ethology. 2002;108:1103–1114. doi:10.1046/j.1439-0310.2002.00840.x [Google Scholar]
- Higgins J.J. Brooks/Cole—Thomson Learning; Pacific Grove, CA: 2004. Introduction to modern non-parametric statistics. [Google Scholar]
- Lima S.L, Bednekoff P.A. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 1999;153:649–659. doi: 10.1086/303202. doi:10.1086/303202 [DOI] [PubMed] [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Mathis A, Smith R.J.F. Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike's diet. Anim. Behav. 1993;46:645–656. doi:10.1006/anbe.1993.1241 [Google Scholar]
- McLean I.G, Lundie-Jenkins G, Jarman P.J. Teaching an endangered mammal to recognise predators. Biol. Conserv. 1996;56:51–62. doi:10.1016/0006-3207(95)00038-0 [Google Scholar]
- Mirza R.S, Ferrari M.C.O, Kiesecker J.M, Chivers D.P. Responses of American toad tadpoles to predation cues: behavioural response thresholds, threat-sensitivity and acquired predation recognition. Behaviour. 2006;143:887–889. doi:10.1163/156853906778017926 [Google Scholar]
- Rochette R, Dill L.M, Himmelman J.H. A field test of threat sensitivity in a marine gastropod. Anim. Behav. 1997;54:1053–1062. doi: 10.1006/anbe.1997.0488. doi:10.1006/anbe.1997.0488 [DOI] [PubMed] [Google Scholar]
- Sih A, Ziemba R, Harding K.C. New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol. Evol. 2000;15:3–4. doi: 10.1016/s0169-5347(99)01766-8. doi:10.1016/S0169-5347(99)01766-8 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. Freeman and Company; New York, NY: 2003. Biometry: the principles and practice of statistics in biological research. [Google Scholar]
- Stankowich T, Coss R.G. The re-emergence of felid camouflage with the decay of predator recognition in deer under relaxed selection. Proc. R. Soc. B. 2007;274:175–182. doi: 10.1098/rspb.2006.3716. doi:10.1098/rspb.2006.3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe K.L. Innate and learned components of defence by flickers against a novel nest competitor, the European starling. Ethology. 2004;110:779–791. doi:10.1111/j.1439-0310.2004.01016.x [Google Scholar]
- Wisenden B.D. Chemically-mediated strategies to counter predation. In: Collin S.P, Marshall N.J, editors. Sensory processing in the aquatic environment. Springer; New York, NY: 2003. pp. 236–251. [Google Scholar]
- Woody D.R, Mathis A. Acquired recognition of chemical stimuli from an unfamiliar predator: associative learning by adult newts, Notophthalmus viridescens. Copeia. 1998;1998:1027–1031. doi:10.2307/1447352 [Google Scholar]
- Zar J.H. 4th edn. Prentice-Hall, Inc; New Jersey, NJ: 1999. Biostatistical analysis. [Google Scholar]