Abstract
Sexual conflict between males and females over mating is common. Females that copulate with extrapair mates outside the pair-bond may gain (i) direct benefits such as resources or increased paternal care, (ii) indirect genetic benefits for their offspring, or (iii) insurance against infertility in their own social mate. Few studies have been able to demonstrate the different contexts in which females receive varying types of benefits from extrapair mates. Here, I examined sexual conflict, female extrapair mate choice, and patterns of extrapair paternity in the cooperatively breeding superb starling Lamprotornis superbus using microsatellite markers. Although extrapair paternity was lower than many other avian cooperative breeders (14% of offspring and 25% of nests), females exhibited two distinct mating patterns: half of the extrapair fertilizations were with males from inside the group, whereas half were with males from outside the group. Females with few potential helpers copulated with extrapair mates from within their group and thereby gained direct benefits in the form of additional helpers at the nest, whereas females paired to mates that were relatively less heterozygous than themselves copulated with extrapair mates from outside the group and thereby gained indirect genetic benefits in the form of increased offspring heterozygosity. Females did not appear to gain fertility insurance from copulating with extrapair mates. This is the first study to show that individuals from the same population mate with extrapair males and gain both direct and indirect benefits, but that they do so in different contexts.
Keywords: cooperative breeding, extrapair paternity, extrapair fertilization, sexual conflict, genetic heterozygosity, fertility insurance
1. Introduction
Conflict between males and females over reproduction is common in many species of animals (Arnqvist & Rowe 2005). Sexual conflict over mating often occurs because males and females benefit differentially from mating with (i) multiple individuals, since males typically have a higher optimal mating frequency than females or (ii) specific individuals, since males are typically less choosy than females about mates (Wedell et al. 2006). Although males typically have more to gain from mating multiply than do females (Bateman 1948; but see Andersson & Simmons 2006), studying why females might engage in, or even initiate, mating with multiple males has generated substantial controversy (Westneat & Stewart 2003; Arnqvist & Kirkpatrick 2005, 2007; Albrecht et al. 2006; Charmantier & Sheldon 2006; Hadfield et al. 2006; Qvarnsrtom et al. 2006; Griffith 2007). Irrespective of which sex initiates extrapair matings, there is good evidence to suggest that females actively make extrapair mate-choice decisions (Jennions & Petrie 2000; Griffith et al. 2002; Westneat & Stewart 2003; Mays & Hill 2004; Neff & Pitcher 2005; Hoffman et al. 2007) and even dynamically allocate paternity (Safran et al. 2005). However, despite a great deal of empirical work on female extrapair mate choice, few studies have been able to demonstrate the different contexts in which females receive varying types of benefits from extrapair mates (Westneat & Stewart 2003).
Females may copulate with males outside the pair-bond to (i) guard against infertility in their own social mate (fertility insurance hypothesis; Wetton & Parkin 1991; Sheldon 1994) or gain two non-exclusive types of benefits (reviewed in Birkhead & Møller 1992; Griffith et al. 2002; Cockburn 2004). The potential benefits that females may receive from extrapair mates include: (ii) direct benefits such as resources or increased paternal care provided by helpers at the nest (direct benefits hypothesis; Wolf 1975; Burke et al. 1989; Colwell & Oring 1989) or (iii) indirect genetic reproductive benefits including obtaining ‘good genes’ for their offspring (genetic quality hypothesis; Møller 1988; Hamilton 1990; Westneat et al. 1990; Birkhead & Møller 1992; Gray 1997), maximizing genetic diversity among their offspring (genetic diversity hypothesis; Westneat et al. 1990) or maximizing genetic compatibility between themselves and the father of the offspring (genetic compatibility hypothesis; Zeh & Zeh 1996; Kempenaers et al. 1999; Tregenza & Wedell 2000). These hypotheses have not been tested simultaneously in any cooperative breeders (Cockburn 2004), despite the fact that cooperatively breeding species are tractable and powerful study systems in which to test these ideas since having helpers at the nest may reduce the potential costs of female extrapair mating decisions. In other words, if cuckoldry results in a reduction of paternal care and constrains females from seeking extrapair mates (Dixon et al. 1994; Albrecht et al. 2006), then alloparental care by helpers may compensate for a loss of paternal care and liberate females to mate more promiscuously outside of their pair-bond (Mulder et al. 1994).
Here, I examine sexual conflict, female extrapair mate choice and patterns of extrapair paternity in the cooperatively breeding superb starling, Lamprotornis superbus. Superb starlings are plural breeders that live in large social groups of up to 30 individuals in which up to six socially monogamous pairs per group build their own separate nests (Rubenstein 2006). They are endemic to East African savannas (Feare & Craig 1999; Fry et al. 2000), which are characterized by a highly unpredictable environment where dry season rainfall varies greatly among years (D. Rubenstein & I. Lovette 2007, unpublished data) and influences breeding roles (Rubenstein 2007a) and offspring sex allocation (Rubenstein 2007b) in superb starlings. Reproductive conflict, or conflict over reproductive opportunities and breeding roles among group members, as well as divorce (both within and among years) is high in superb starlings (Rubenstein 2007a,c) suggesting that sexual conflict over extrapair matings may also be intense. After estimating extrapair paternity rates using microsatellite markers, I use long-term demographic data, comparisons of genetic heterozygosity in parents and offspring and behavioural observations of helper nestling provisioning to test predictions about (i) from where extrapair males should come, (ii) when females should engage in extrapair fertilizations, (iii) which extrapair males should females copulate with, and (iv) what effect extrapair fertilizations will have on offspring genetic heterozygosity (electronic supplementary material, table 1). Here, I describe the circumstances under which females may make different types of extrapair mating decisions, thereby constructing informative predictions about which individuals are likely to engage in extrapair matings in avian species and why they might do so.
2. Material and methods
(a) Study system and species
Breeding activities of seven social groups of superb starlings were monitored from April 2001 to December 2005 at the Mpala Research Centre, Laikipia, Kenya (0°17′ N, 37°52′ E). One additional group was added in January 2002 and another was added in January 2003. Birds defended year-round territories and bred during both the long (March–May) and short rains (November). Superb starlings lived in groups of 10–35 (mean=21) individuals with up to six breeding pairs per group (mean=3.5) during the long rains and up to four breeding pairs per group (mean=1.7) during the short rains (Rubenstein 2006). Pairs frequently renested multiple times during the long rains—but not during the short rains—and it was common for pairs to lay three or four clutches of eggs in a season if early laid clutches failed or were depredated. Pairs occasionally fledged more than one brood in a single season. Helpers had a positive effect on parental fitness by increasing the number of offspring fledged (Rubenstein 2007b). More than 90% of nests had at least one helper (Rubenstein 2006), and although both sexes helped at the nest, males did a greater proportion of the provisioning (Rubenstein 2007b). Helpers included both offspring and other first-order relatives (e.g. parents, siblings), as well as other group members that were less related, or even unrelated, to the breeding pair (Rubenstein 2006). Non-group members were never allowed near the nest and were actively chased from the territory during the breeding season (D. Rubenstein 2001–2005, personal observation).
(b) Captures and sampling
Active nests were checked every 1–3 days during the incubation and nestling stages, and nestlings were banded and sampled between 5 and 8 days after hatching. Adults were captured annually during the dry season using baited wire traps. Each individual was given a unique set of colour leg bands and a numbered metal leg ring. Over the course of the study, 476 birds were captured (more than 97% of the birds in the study population), including 247 nestlings sampled from 100 nests from 10 breeding seasons, five during the long rains (204 offspring) and five during the short rains (43 offspring). During this period, 1134 eggs were laid in 352 nests (895 eggs in 276 nests during the long rains and 239 eggs in 76 nests during the short rains), but most did not survive long enough to be sampled owing to extremely high nest predation rates (nearly 75% of nests failed with more than 90% due to nest predation; Rubenstein 2006). Infertility rates were estimated as the proportion of nests in each year that contained at least one egg that did not hatch. Examination of a subset of these eggs found no evidence of embryonic development (D. Rubenstein 2001–2005, personal observation). Nests that were abandoned, or where no eggs hatched, were excluded from the analysis. This work was approved by the Cornell Institutional Animal Care and Use Committee (01-27).
(c) Behavioural observations
Observations of copulations were rare; only three were observed during the study (electronic supplementary material). Focal observations (1–2 h) at nests were conducted during the nest building and incubation stages to identify the social breeders. Social mothers were identified as the female at a nest that incubated eggs; social fathers were defined as those that remained near the incubating female while she was on the nest. Observers used spotting scopes and were hidden under a tree or behind a blind more than 30 m from the nest. After eggs hatched, focal observations (1–3 h) were used to identify helpers at the nest. Although most nests were observed multiple times after eggs hatched (range=1–9 observations; mean=3.3 observations), repeated observations were not always possible due to the high nest predation rates (Rubenstein 2006). The identity of all birds that came within approximately 30 m of the nest and whether each bird brought food was recorded. Breeders were defined as the social parents of the nest, whereas helpers were defined as all individuals other than the parents that brought food to a nest.
(d) Microsatellite genotyping, parentage and relatedness analyses
Seventeen microsatellite markers were chosen from a set of 31 polymorphic loci previously developed for superb starlings (Rubenstein 2005). Two of these markers showed evidence of null alleles and were excluded from the primary parentage analysis (electronic supplementary material, table 2). Blood from superb starlings was collected in Queens lysis buffer (Seutin et al. 1991) and genomic DNA was extracted using a DNeasy Tissue Kit (QIAGEN). The forward primer of each pair was labelled using the fluorescent dyes 6-FAM, NED, PET or VIC (Applied Biosystems) and polymerase chain reaction (PCR) was conducted using methods and conditions published previously (Rubenstein 2005). Genotyping was performed on a 3100 Genetic Analyzer (Applied Biosystems). All alleles were scored automatically and confirmed visually using Genemapper v. 3.0 software (Applied Biosystems). For each locus, 30–32 individuals were screened twice to estimate genotyping errors; no such errors were found.
Cervus v. 2.0 software (Marshall et al. 1998) was used to analyse parentage data (electronic supplementary material). Cervus uses a likelihood-based approach to infer parentage by simulating the critical difference in LOD (natural logarithm of the likelihood ratio) scores between candidate parents for assignment at chosen levels of confidence (95 and 80% in this study). Based upon the observed allele frequencies, Cervus calculates the probability of parental assignment when (i) neither parent is known and (ii) when one parent is known. Two simulations for parentage analysis were generated, one that accounted for relatedness among candidate fathers and another that did not (electronic supplementary material, table 3). Although the two simulations gave different success rates for identifying a suitable candidate father in the parentage analyses, they always identified the same candidates.
Kinship v. 1.3.1 software (Goodnight & Queller 1999) was used to calculate pairwise relatedness values between pair-bonded social mates in order to estimate genetic similarity (Tarvin et al. 2005; Freeman-Gallant et al. 2006). Kinship uses the population allele frequencies and the genotypes of the two individuals under consideration to calculate a pairwise relatedness value. Separate values were calculated for each year of the study because the coefficients are dependent upon the genotypes present in the population. Pairwise relatedness values are equivalent to coefficients of relatedness. A negative pairwise relatedness value means that the two individuals are less related to each other than are two individuals chosen at random from the population. Mean pairwise relatedness between social mates at nests containing extra-group extrapair offspring, nests containing within-group extrapair offspring and those without extrapair offspring was compared using GLMM. Nest type was included in the model as a fixed effect, and mother was included as a random effect to account for repeated measures from the same individual over successive broods or years.
(e) Genetic heterozygosity
Microsatellite genotypes were used to calculate two measures of genetic heterozygosity: (i) standardized observed heterozygosity (SH), a measure of individual heterozygosity that weights the heterozygosity at each locus by population allele frequencies (Coltman et al. 1999; Pemberton et al. 1999) and (ii) internal relatedness (IR) which measures similarity of parental half-genotypes within an individual, and is thus a measure of inbreeding (Amos et al. 2001). Since both measures of heterozygosity are dependent upon the genotypes present in the population, separate sets of values were calculated for each year of the study. Genetic heterozygosity of social mothers and fathers at nests with extra-group extrapair offspring, within-group extrapair offspring and no extrapair offspring was compared using GLMM. Nest type, parent sex and their interaction were included in the model as fixed effects, whereas mother was included as a random effect to account for repeated measures from the same individual over successive broods or years. The analysis included 32 mothers from 87 nests in which both parents were genotyped. Post hoc comparisons of each nest type were made using independent contrasts of least square means. Since many of the extrapair sires were unmarked extra-group males, it was not possible to directly determine if females were copulating with extra-group extrapair mates that were more genetically heterozygous than their social mates. However, it was possible to determine if females were copulating with more genetically heterozygous within-group extrapair mates by comparing the genetic heterozygosity of a female's within-group extrapair mate to that of her social mate using a paired t-test. Additionally, genetic heterozygosity of extrapair offspring and within-pair offspring from corresponding nests that contained either extra- or within-group extrapair offspring was compared using a model with nest type, offspring type and their interaction as fixed effects and mother as a random effect. The analysis included 17 mothers from 18 nests that included both within-pair and extrapair offspring.
(f) Patterns of extrapair paternity
An ANOVA that accounted for unequal variances was used to compare the number of surviving offspring from the previous 2 years (i.e. potential helpers) in nests containing extra-group extrapair offspring, nest containing within-group extrapair offspring and those without extrapair offspring. For nests with within-group extrapair offspring, cases in which the extrapair father was another breeder in the group (n=2) were excluded to constrain the analysis to females that copulated with subordinate extrapair males. Logistic regression was used to determine if the number of surviving offspring from the previous 2 years (i.e. potential helpers) predicted if female would obtain within-group extrapair fertilizations by comparing females at nests with within-pair extrapair offspring to females at all other nests.
3. Results and discussion
(a) Parentage analysis
Parentage analysis revealed that 34 of 247 (14%) offspring were the result of extrapair fertilizations and 25 of 100 (25%) nests contained at least one extrapair offspring. Superb starlings can breed twice annually and extrapair paternity rates were similar during the long (14%, 28 of 204) and short rains (14%, 6 of 43) breeding seasons. Extrapair paternity rates in superb starlings were slightly higher than those in European starlings, Sturnus vulgaris: 9–10% of offspring and 29–32% of nests (Pinxten et al. 1993; Smith & von Schantz 1993). However, extrapair paternity rates were lower than those observed in most other cooperative breeders in which microsatellites have been used to examine parentage (mean=43% of offspring; Double & Cockburn 2000, 2003; Li & Brown 2000; Richardson et al. 2001; Hatchwell et al. 2002; Hughes et al. 2003; Webster et al. 2004; Berg 2005), particularly in plural breeders (mean =61% of offspring; Double & Cockburn 2000, 2003; Li & Brown 2000; Hughes et al. 2003). There was no evidence of inbreeding between mothers and sons or between fathers and daughters, or of intraspecific brood parasitism. Overall, 16 of 34 (47%) extrapair fertilizations (7% of all offspring) were with males from outside the group (hereafter, extra-group extrapair fertilizations); 18 of 34 (53%) extrapair fertilizations (7% of all offspring) were with males from inside the group (hereafter, within-group extrapair fertilizations); 9 of 100 (9%) nests contained at least one extra-group extrapair offspring; and 16 of 100 (16%) nests contained at least one within-group extrapair offspring. Since half of all extrapair fertilizations were with extra-group males and half were with within-group males, these two groups are treated separately in the subsequent analysis of female extrapair mate choice.
(b) Within-group extrapair fertilizations
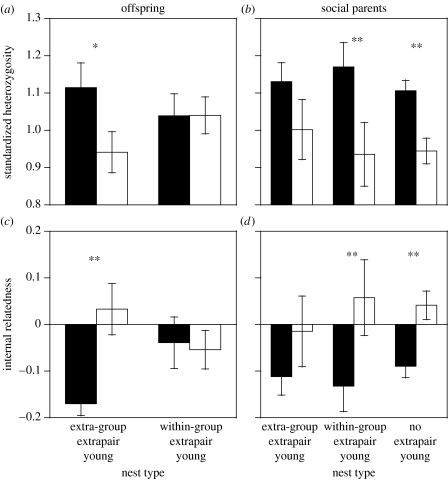
Female superb starlings would be expected to copulate with extrapair males from within the group either for direct benefits in the form of additional alloparental care, or for indirect genetic benefits in the form of increased offspring heterozygosity. There was no evidence that females gained indirect genetic benefits from copulating with extrapair males from within the group; within-group extrapair mates were not more genetically heterozygous than a female's social mate (paired t-test: SH: t=0.64, d.f. =9, p=0.54; IR: t=0.75, d.f. =9, p=0.47), and offspring produced by extrapair mates were not more genetically heterozygous than their nest mates who were fathered by the social mate (table 1; figure 1). In contrast, there was good evidence that females gained direct benefits from copulating with within-group extrapair males, as in some other species of plural breeders (Li & Brown 2000, 2002). Of the 15 nests that contained within-group extrapair offspring, the extrapair sire was a non-pair-bonded male (i.e. non-social breeder) who became a helper at nine nests while those nests contained the extrapair offspring, and another pair-bonded breeding male at two nests. Although focal observations were not conducted at the remaining four nests, in one case, the extrapair sire became a helper the following breeding season, and in another case, the extrapair sire became the social mate in the following breeding season. Thus, in at least 11 of 15 (73%) nests with within-group extrapair offspring, the extrapair sire was a subordinate male and an actual or probable helper, suggesting that within-group extrapair mates may have been chosen to provide additional alloparental care. Although it is possible that females could have obtained other indirect genetic benefits like good genes, this is unlikely since the majority of within-group extrapair sires were subordinate non-breeding group members.
Table 1.
Genetic heterozygosity of offspring and social parents at different types of nests. (Results are from post hoc independent contrasts of least square means from models comparing SH and IR between (i) extrapair and within-pair offspring from the same nest and (ii) social mothers and fathers at nests containing extra-group extrapair offspring, nests containing within-group extrapair offspring and nests without extrapair offspring.)
| nest type | number | standardized observed heterozygosity | internal relatedness | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| extrapair offspring versus within-pair offspring | |||||
| extra-group extrapair offspring | 6 | 3.50 | 0.079 | 8.22 | 0.011 |
| within-group extrapair offspring | 12 | 0.0001 | 0.99 | 0.093 | 0.76 |
| social mother versus social father | |||||
| extra-group extrapair offspring | 9 | 1.81 | 0.18 | 1.39 | 0.24 |
| within-group extrapair offspring | 10 | 6.68 | 0.011 | 5.93 | 0.016 |
| no extrapair offspring | 68 | 21.49 | <0.0001 | 19.20 | <0.0001 |
Figure 1.
Genetic heterozygosity of offspring and social parents at different types of nests. Genetic heterozygosity was calculated as both SH and IR. In (a) and (b), black bars indicate extra pair offspring and white bars indicate within-pair offspring. Offspring fathered by extra-group extrapair males were more heterozygous (i.e. high SH and low IR) than those fathered by social mates, whereas offspring fathered by within-group extrapair males had similar SH and IR to those fathered by social mates. In (c) and (d), black bars indicate social fathers and white bars indicate social mothers. Parents at nests with extra-group extrapair offspring did not differ in their SH or IR, but parents at nests with within-group extrapair offspring and at nests without extrapair offspring differed in both SH and IR. Mean±s.e. values are given. **, comparisons that were significantly different at p<0.05 and *, comparisons that were different at p<0.1. Samples sizes are indicated in table 1.
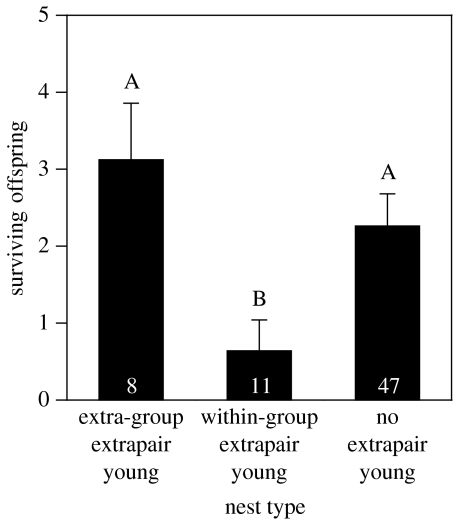
Females were more likely to copulate with extrapair males from inside the group when they had fewer of their own offspring from previous years available to help. The number of surviving offspring that a female had from the previous 2 years (i.e. potential helpers) differed among the nest types (Welch ANOVA for unequal variances: F=6.40, d.f. =2, p=0.0078; figure 2), and females were more likely to have offspring fathered by within-group extrapair mates if they had fewer surviving offspring (logistic regression: Χ2=6.04, d.f.=1, p=0.014). Thus, when females needed additional helpers at the nest, they were more likely to copulate with extrapair males who could, and did, help provision their offspring.
Figure 2.
The number of surviving offspring from the previous 2 years for females at different types of nests. Females who copulated with within-group extrapair mates had fewer surviving offspring (i.e. potential helpers) from the previous 2 years than did females who copulated with extra-group extrapair mates or those that did not have extrapair fertilizations. Mean±s.e. values are given. Different letters indicate means that were significantly different at p<0.05. Samples sizes are indicated at the bottom of each bar.
(c) Extra-group extrapair fertilizations
In contrast to within-group extrapair fertilizations, female superb starlings would be expected to copulate with extra-group extrapair mates for indirect genetic benefits, not for direct benefits (i.e. alloparental care) since only permanent group members provide alloparental care and help at the nest (Rubenstein 2006). In support of this prediction, I found that extra-group extrapair offspring were more heterozygous than the within-pair offspring (i.e. non-extrapair offspring) from the same nest (table 1; figure 1). Similar results have been shown in blue tits, Parus caruleus (Foerster et al. 2003), but not in reed buntings, Emberiza schoeniclus (Kleven & Lifjeld 2005). Although heterozygosity as measured with microsatellites does not always correlate with fitness (Coltman & Slate 2003; Balloux et al. 2004; Pemberton 2004; Slate et al. 2004) and other factors such as female preferences for rare male phenotypes could generate similar patterns in offspring heterozygosity, numerous studies have found a positive relationship between heterozygosity and various fitness components including body mass and survival (Coulson et al. 1998, 1999; Pujolar et al. 2005; Da Silva et al. 2006), disease susceptibility (Coltman et al. 1999; Cassinello et al. 2001; Acevedo-Whitehouse et al. 2003; Hawley et al. 2005) and reproductive success (Slate et al. 2000; Amos et al. 2001; Hoffman et al. 2004; Seddon et al. 2004; Charpentier et al. 2005).
Females were more likely to copulate with extra-group extrapair mates for indirect genetic benefits when they were paired to males with relatively lower heterozygosity than themselves. Fathers were significantly more heterozygous than mothers at nests without extrapair offspring and at nests with within-group extrapair offspring, but fathers were not more heterozygous than mothers at nests with extra-group extrapair offspring (table 1; figure 1). Although there was no difference in the heterozygosity of social fathers at nests containing extra-group extrapair offspring, within-group extrapair offspring and no extrapair offspring (GLMM: SH: F2,53=0.60, p=0.55; IR: F2,53=0.14, p=0.87), males that became pair-bonded breeders at least once during the study were significantly more heterozygous than those that did not (t-test: SH: t=3.10, d.f.=134, p=0.0024; IR: t=3.26, d.f. =134, p=0.0014). However, there were no differences in pair-wise relatedness (genetic similarity), between socially paired males and females at nests containing extra-group extrapair offspring, within-group extrapair offspring and no extrapair offspring (GLMM: F2,53=0.95, p=0.39).
(d) Infertility
Females would be expected to engage in extrapair fertilizations to guard against infertility in their own social mate if at least some of the eggs of the social mate tended to be infertile. Overall, 17% of nests that hatched young during the study contained at least one egg that failed to hatch (short rains: range=0–25%, mean=10%; long rains: range=8–39%, mean=21%). Nests containing within-group or extra-group extrapair offspring were not more likely to contain an egg that failed to hatch than those that did not contain extrapair offspring (chi-square test: Χ2=0.47, d.f.=2, p=0.79). Additionally, since the fertility insurance hypothesis predicts no differences in the genetic heterozygosity of extrapair and within-pair offspring from the same nest, the observed differences in offspring heterozygosity at nests containing extra-group extrapair offspring (table 1; figure 1) fail to support this idea.
4. Conclusions
To my knowledge, this is the first study to show that females from the same population copulate with extrapair mates and gain both direct and indirect benefits, but that they do so in different contexts. Females with few potential helpers copulated with extrapair mates from within their group and thereby gained direct benefits in the form of additional helpers at the nest, whereas females paired to mates that were relatively less heterozygous than themselves copulated with extrapair mates from outside the group and thereby gained indirect genetic benefits in the form of increased offspring heterozygosity. There was little evidence to suggest that females engaged in extrapair copulations to guard against infertility in their own social mate.
Although it is not clear if female superb starlings make active extrapair mating decisions (electronic supplementary material), the results of this study suggest that females could be making extra-group extrapair mating decisions with respect to the relative heterozygosity of their social mate and within-group extrapair mating decisions with respect to their need for additional help at the nest. However, since there were no differences in the relatedness of the social pairs at the different nest types, female superb starlings do not appear to be making extrapair mating decisions based on the genetic similarity to their social mate, as is the case in some (Eimes et al. 2004; Kleven et al. 2005; Tarvin et al. 2005; Freeman-Gallant et al. 2006), but not all avian species studied (Kleven & Lifjeld 2005; Bouwman et al. 2006; Edly-Wright et al. 2007). Although relatedness did not appear to directly influence female extrapair mate choice, it could indirectly influence female choice by impacting group social dynamics, which could affect female exposure to extra-group males (Rubenstein 2007c). Like superb starlings, female fur seals, Arctocephalus gazella, appear to actively choose mates based more on levels of heterozygosity than on relatedness (Hoffman et al. 2007).
Ultimately, these results are most consistent with predictions from the genetic compatibility hypothesis and suggest that females may have copulated with extra-group extrapair males, which were more genetically compatible than their social mates so that their offspring would have higher heterozygosity. It is also possible that extra-group extrapair males could have been of higher quality than a female's social mate, which would support the genetic quality hypothesis. However, since the genetic diversity hypothesis predicts no differences in the genetic heterozygosity of extrapair and within-pair offspring from the same nest, the observed differences in offspring heterozygosity at nests containing extra-group extrapair offspring fail to support this idea. Since it is not yet known in birds if, and how, females actively assess the heterozygosity (or genetic similarity) of males or if some post-copulatory process such as sperm competition, cryptic female choice, interactions between sperm and ova or differential embryo survival, is acting to increase the fitness of more heterozygous males (Jennions & Petrie 2000; Marshall et al. 2003; Tarvin et al. 2005), additional work is needed to understand the mechanisms underlying female extrapair mate choice for indirect genetic benefits. Future studies examining patterns of extrapair paternity will also benefit from focusing on sexual conflict and the evolutionary interactions between male and females over mating (Westneat & Stewart 2003; Griffith 2007), as well as the potential interactions between selection for direct and indirect benefits (Oneal et al. 2007).
Acknowledgments
I thank the Kenyan Ministry of Education, Science and Technology, the National Museums of Kenya Ornithology Department, the Kenya Wildlife Service and the Mpala Research Centre for enabling this work. I also thank J. Ekiru, F. Lomojo, G. Rana, J. Ronjore, W. Watetu, S. Bogdanowicz, S. McRae and L. Stenzler for their help in the field and laboratory, and E. Adkins-Regan, S. Emlen, I. Lovette, S. McRae, D.I. Rubenstein, P. Sherman, A. Townsend and M. Wikelski who provided their comments on the manuscript. This work was supported by fellowships from the Howard Hughes Medical Institute, the Smithsonian Institution, the Cornell University College of Agriculture and Life Sciences and the Miller Institute for Basic Research at the University of California at Berkeley, as well as by grants from the National Science Foundation (IBN-407713), the American Museum of Natural History Chapman Fund, the American Ornithologists’ Union, the Wilson Ornithological Society, the Society for Integrative and Comparative Biology, the Animal Behaviour Society, the Harvard Travellers Club, the Society of Sigma Xi, Cornell University, the Cornell Laboratory of Ornithology Benning Fund and Cornell Sigma Xi.
Supplementary Material
The supplementary material contains a table of the predictions tested in this study (table 1), as well as some data on copulation behaviour in superb starlings. It also contains a more detailed explanation of the parentage analysis, as well as tables showing microsatellite allele frequencies (table 2) and simulation parameters (table 3)
References
- Acevedo-Whitehouse K, Gulland F, Greig D, Amos W. Disease susceptibility in California sea lions. Nature. 2003;422:35. doi: 10.1038/422035a. doi:10.1038/422035a [DOI] [PubMed] [Google Scholar]
- Albrecht T, Kreisinger J, Pialek J. The strength of direct selection against female promiscuity is associated with rates of extrapair fertilizations in socially monogamous songbirds. Am. Nat. 2006;167:739–744. doi: 10.1086/502633. doi:10.1086/502633 [DOI] [PubMed] [Google Scholar]
- Amos W, Wilmer J.W, Fullard K, Burg T.M, Croxall J.P, Bloch D, Coulson T.N. The influence of parental relatedness on reproductive success. Proc. R. Soc. B. 2001;268:2021–2027. doi: 10.1098/rspb.2001.1751. doi:10.1098/rspb.2001.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Simmons L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. doi:10.1016/j.tree.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 2005;165:S26–S37. doi: 10.1086/429350. doi:10.1086/429350 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines revisited: a reply to Griffith. Am. Nat. 2007;169:282–283. doi:10.1086/510606 [Google Scholar]
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Balloux F, Amos W, Coulson T. Does heterozygosity estimate inbreeding in real populations? Mol. Ecol. 2004;13:3021–3031. doi: 10.1111/j.1365-294X.2004.02318.x. doi:10.1111/j.1365-294X.2004.02318.x [DOI] [PubMed] [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Berg E.C. Parentage and reproductive success in the white-throated magpie-jay, Calocitta formosa, a cooperative breeder with female helpers. Anim. Behav. 2005;70:375–385. doi:10.1016/j.anbehav.2004.11.008 [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; London, UK: 1992. Sperm competition in birds. [Google Scholar]
- Bouwman K.M, Burke T, Komdeur J. How female reed buntings benefit from extra-pair mating behaviour: testing hypotheses through patterns of paternity in sequential broods. Mol. Ecol. 2006;15:2589–2600. doi: 10.1111/j.1365-294X.2006.02955.x. doi:10.1111/j.1365-294X.2006.02955.x [DOI] [PubMed] [Google Scholar]
- Burke T, Davies N.B, Bruford M.W, Hatchwell B.J. Parental care and mating behaviour of polyandrous dunnocks Prunella modularis related to paternity by DNA fingerprinting. Nature. 1989;338:249–251. doi:10.1038/338249a0 [Google Scholar]
- Cassinello J, Gomendio M, Roldan E.R.S. Relationship between coefficient of inbreeding and parasite burden in endangered gazelles. Conserv. Biol. 2001;15:1171–1174. doi:10.1046/j.1523-1739.2001.0150041171.x [Google Scholar]
- Charmantier A, Sheldon B.C. Testing genetic models of mate choice evolution in the wild. Trends Ecol. Evol. 2006;21:417–419. doi: 10.1016/j.tree.2006.06.001. doi:10.1016/j.tree.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Charpentier M, Setchell J.M, Prugnolle F, Knapp L.A, Wickings E.J, Peignot P, Hossaert-McKey M. Genetic diversity and reproductive success in mandrills (Mandrillus sphinx) Proc. Natl Acad. Sci. USA. 2005;102:16 723–16 728. doi: 10.1073/pnas.0507205102. doi:10.1073/pnas.0507205102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn A. Mating systems and sexual conflict. In: Koenig W.D, Dickinson J, editors. Cooperative breeding in birds: recent research and new theory. Cambridge University Press; Cambridge, UK: 2004. pp. 81–101. [Google Scholar]
- Coltman D.W, Slate J. Microsatellite measures of inbreeding: a meta-analysis. Evolution. 2003;57:971–983. doi: 10.1111/j.0014-3820.2003.tb00309.x. doi:10.1554/0014-3820(2003)057[0971:MMOIAM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Coltman D.W, Pilkington J.G, Smith J.A, Pemberton J.M. Parasite-mediated selection against inbred Soay sheep in a free-living island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. doi:10.2307/2640828 [DOI] [PubMed] [Google Scholar]
- Colwell M.A, Oring L.W. Extra-pair paternity in the spotted sandpiper: a female mate acquisition technique. Anim. Behav. 1989;38:675–684. doi:10.1016/S0003-3472(89)80013-2 [Google Scholar]
- Coulson T.N, Pemberton J.M, Albon S.D, Beaumont M, Marshall T.C, Slate J, Guiness F.E, Clutton-Brock T.H. Microsatellites reveal heterosis in red deer. Proc. R. Soc. B. 1998;265:489–495. doi: 10.1098/rspb.1998.0321. doi:10.1098/rspb.1998.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson T, Albon S, Slate J, Pemberton J. Microsatellite loci reveal sex-dependent responses to inbreeding and outbreeding in red deer calves. Evolution. 1999;53:1951–1960. doi: 10.1111/j.1558-5646.1999.tb04575.x. doi:10.2307/2640453 [DOI] [PubMed] [Google Scholar]
- Da Silva A, Luikart G, Yoccoz N.G, Cohas A, Allaine D. Genetic diversity–fitness correlation revealed by microsatellite analyses in European alpine marmots (Marmota marmota) Conserv. Genet. 2006;7:371–382. doi:10.1007/s10592-005-9048-y [Google Scholar]
- Dixon A, Ross D, O'Malley S.L.C, Burke T. Paternal investment inversely related to degree of extra-pair paternity in the reed bunting. Nature. 1994;371:698–700. doi:10.1038/371698a0 [Google Scholar]
- Double M, Cockburn A. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. B. 2000;267:465–470. doi: 10.1098/rspb.2000.1023. doi:10.1098/rspb.2000.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double M, Cockburn A. Subordinate superb fairy-wrens (Malurus cyaneus) parasitize the reproductive success of attractive dominant males. Proc. R. Soc. B. 2003;270:379–384. doi: 10.1098/rspb.2002.2261. doi:10.1098/rspb.2002.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edly-Wright C, Schwagmeyer P.L, Parker P.G, Mock D.W. Genetic similarity of mates, offspring health and extrapair fertilization in house sparrows. Anim. Behav. 2007;73:367–378. doi:10.1016/j.anbehav.2006.08.008 [Google Scholar]
- Eimes J.A, Parker P.G, Brown J.L, Brown E.R. Extrapair fertilization and genetic similarity of social mates in the Mexican jay. Behav. Ecol. 2004;16:456–460. doi:10.1093/beheco/ari010 [Google Scholar]
- Feare C, Craig A. Princeton University Press; Princeton, NJ: 1999. Starlings and mynas. [Google Scholar]
- Foerster K, Delhey K, Johnson A, Lifjeld J.T, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. doi:10.1038/nature01969 [DOI] [PubMed] [Google Scholar]
- Freeman-Gallant C.R, Wheelright N.T, Meiklejohn K.E, Sollecito S.V. Genetic similarity, extrapair paternity, and offspring quality in Savanna sparrows (Passerculus sandwichensis) Behav. Ecol. 2006;17:952–958. doi:10.1093/beheco/arl031 [Google Scholar]
- Fry C.H, Keith S, Urban E.K. Academic Press; San Diego, CA: 2000. The birds of Africa. [Google Scholar]
- Goodnight K.F, Queller D.C. Computer software for performing likelihood rests of pedigree relationships using genetic markers. Mol. Ecol. 1999;8:1231–1234. doi: 10.1046/j.1365-294x.1999.00664.x. doi:10.1046/j.1365-294x.1999.00664.x [DOI] [PubMed] [Google Scholar]
- Gray E.M. Do female red-winged blackbirds benefit genetically from seeking extra-pair copulations? Anim. Behav. 1997;53:605–623. doi:10.1006/anbe.1996.0337 [Google Scholar]
- Griffith S.C. The evolution of infidelity in socially monogamous passerines: neglected components of direct and indirect selection. Am. Nat. 2007;169:274–281. doi: 10.1086/510601. doi:10.1086/510601 [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Hadfield J.D, Burgess M.D, Lord A, Phillimore A.B, Clegg S.M, Owens I.P.F. Direct versus indirect sexual selection: genetic basis of colour, size and recruitment in wild birds. Proc. R. Soc. B. 2006;273:1347–1353. doi: 10.1098/rspb.2005.3459. doi:10.1098/rspb.2005.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D. Mate choice near and far. Am. Zool. 1990;30:341–352. [Google Scholar]
- Hatchwell B.J, Ross D.J, Chaline N, Fowlie M.K, Burke T. Parentage in the cooperative breeding system of long-tailed tits, Aegithalos caudatus. Anim. Behav. 2002;64:55–63. doi:10.1006/anbe.2002.3033 [Google Scholar]
- Hawley D.M, Sydenstricker K.V, Kollias G.V, Dhondt A.A. Genetic diversity predicts pathogen resistance and cell-mediated immunocompetence in house finches. Biol. Lett. 2005;1:326–329. doi: 10.1098/rsbl.2005.0303. doi:10.1098/rsbl.2005.0303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J.I, Boyd I.L, Amos W. Exploring the relationship between parental relatedness and male reproductive success in the Antarctic fur seal Arctocephalus gazella. Evolution. 2004;58:2087–2099. doi: 10.1111/j.0014-3820.2004.tb00492.x. doi:10.1554/04-099 [DOI] [PubMed] [Google Scholar]
- Hoffman J.I, Forcada J, Trathan P.N, Amos W. Female fur seals show active choice for males that are heterozygous and unrelated. Nature. 2007;445:912–914. doi: 10.1038/nature05558. doi:10.1038/nature05558 [DOI] [PubMed] [Google Scholar]
- Hughes J.M, Mather P.B, Toon A, Ma J, Rowley I, Russell E. High levels of extra-group paternity in a population of Australian magpies Gymnorhina tibicen: evidence from microsatellite analysis. Mol. Ecol. 2003;12:3441–3450. doi: 10.1046/j.1365-294x.2003.01997.x. doi:10.1046/j.1365-294X.2003.01997.x [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Kempenaers B, Congdon B, Boag P.T, Robertson R.J. Extrapair paternity and egg hatchability in tree swallows: evidence for the genetic compatibility hypothesis? Behav. Ecol. 1999;10:304–311. doi:10.1093/beheco/10.3.304 [Google Scholar]
- Kleven O, Lifjeld J.T. No evidence for increased offspring heterozygosity from extrapair mating in the reed bunting (Emeriza schoeniclus) Behav. Ecol. 2005;16:561–565. doi:10.1093/beheco/ari027 [Google Scholar]
- Kleven O, Jacobsen F, Robertson R.J, Lifjeld J.T. Extrapair mating between relatives in the barn swallow: a role for kin selection? Biol. Lett. 2005;1:389–392. doi: 10.1098/rsbl.2005.0376. doi:10.1098/rsbl.2005.0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-H, Brown J.L. High frequency of extrapair fertilization in a plural breeding bird, the Mexican jay, revealed by DNA microsatellites. Anim. Behav. 2000;60:867–877. doi: 10.1006/anbe.2000.1554. doi:10.1006/anbe.2000.1554 [DOI] [PubMed] [Google Scholar]
- Li S.-H, Brown J.L. Reduction of maternal care: a new benefit of multiple matings. Behav. Ecol. 2002;13:87–93. doi:10.1093/beheco/13.1.87 [Google Scholar]
- Marshall T.C, Slate J, Kruuk L.E.B, Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. doi:10.1046/j.1365-294x.1998.00374.x [DOI] [PubMed] [Google Scholar]
- Marshall R.C, Buchanan K.L, Catchpole C.K. Sexual selection and individual genetic diversity in a songbird. Proc. R. Soc. B. 2003;270:S248–S250. doi: 10.1098/rsbl.2003.0081. doi:10.1098/rsbl.2003.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays H.L, Hill G.E. Choosing mates: good genes versus genes that are a good fit. Trends Ecol. Evol. 2004;19:554–559. doi: 10.1016/j.tree.2004.07.018. doi:10.1016/j.tree.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Møller A.P. Paternity and paternal care in the swallow. Anim. Behav. 1988;36:996–1005. doi:10.1016/S0003-3472(88)80059-9 [Google Scholar]
- Mulder R.A, Dunn P.O, Cockburn A, Lazenbycohen K.A, Howell M.J. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. B. 1994;255:223–229. doi:10.1098/rspb.1994.0032 [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Oneal E, Connallon T, Knowles L.L. Conflict between direct and indirect benefits of female choice in desert Drosophila. Biol. Lett. 2007;3:29–32. doi: 10.1098/rsbl.2006.0565. doi:10.1098/rsbl.2006.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. Measuring inbreeding depression in the wild: the old ways are best. Trends Ecol. Evol. 2004;19:613–615. doi: 10.1016/j.tree.2004.09.010. doi:10.1016/j.tree.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Pemberton J.M, Coltman D.W, Coulson T.N, Slate J. Using microsatellites to measure the fitness consequences of inbreeding and outbreeding. In: Goldstein D.B, Schlotterer C, editors. Microsatellites: evolution and application. Oxford University Press; Oxford, UK: 1999. pp. 151–164. [Google Scholar]
- Pinxten R, Hanotte O, Eens M, Verheyen R.F, Dhondt A.A, Burke T. Extra-pair paternity and intraspecific brood parasitism in the European starling, Sturnus vulgaris: evidence from DNA fingerprinting. Anim. Behav. 1993;45:795–809. doi:10.1006/anbe.1993.1093 [Google Scholar]
- Pujolar J.M, Maes G.E, Vancoillie C, Volckaert F.A.M. Growth rate correlates to individual heterozygosity in the European eel, Anguilla anguilaa L. Evolution. 2005;59:189–199. doi:10.1554/04-377 [PubMed] [Google Scholar]
- Qvarnsrtom A, Brommer J.E, Gustafsson L. Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nature. 2006;441:84–86. doi: 10.1038/nature04564. doi:10.1038/nature04564 [DOI] [PubMed] [Google Scholar]
- Richardson D.S, Jury F.L, Blaakmeer K, Komdeur J, Burke T. Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis) Mol. Ecol. 2001;10:2263–2273. doi: 10.1046/j.0962-1083.2001.01355.x. doi:10.1046/j.0962-1083.2001.01355.x [DOI] [PubMed] [Google Scholar]
- Rubenstein D.R. Isolation and characterization of polymorphic microsatellite loci in the plural cooperatively breeding superb starling, Lamprotornis superbus. Mol. Ecol. Notes. 2005;5:739–744. doi:10.1111/j.1471-8286.2005.01049.x [Google Scholar]
- Rubenstein, D. R. 2006 The evolution of the social and mating systems of the plural cooperatively breeding superb starling, Lamprotornis superbus PhD dissertation, Cornell University, Ithaca, NY.
- Rubenstein D.R. Stress hormones and sociality: integrating social and environmental stressors. Proc. R. Soc. B. 2007a;274:967–975. doi: 10.1098/rspb.2006.0051. doi:10.1098/rspb.2006.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein, D. R. 2007b Temporal but not spatial environmental variation drives adaptive offspring sex allocation in a plural cooperative breeder. Am. Nat 170 (doi:10.1086/518671) [DOI] [PubMed]
- Rubenstein, D. R. 2007c Territory quality drives intraspecific patterns of extrapair paternity. Behav. Ecol.
- Safran R.J, Neuman C.R, McGraw K.J, Lovette I.J. Dynamic paternity allocation as a function of male plumage color in barn swallows. Science. 2005;309:2210–2212. doi: 10.1126/science.1115090. doi:10.1126/science.1115090 [DOI] [PubMed] [Google Scholar]
- Seddon N, Amos W, Mulder R.A, Tobias J.A. Mate heterozygosity predicts territory size, song structure and reproductive success in a cooperatively breeding bird. Proc. R. Soc. B. 2004;271:1823–1829. doi: 10.1098/rspb.2004.2805. doi:10.1098/rspb.2004.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seutin G, White B.N, Boag P.T. Preservation of avian blood and tissue samples for DNA analysis. Can. J. Zool. 1991;69:82–90. [Google Scholar]
- Sheldon B.C. Male phenotype, fertility, and the pursuit of extra-pair copulations by females. Proc. R. Soc. B. 1994;257:25–30. doi:10.1098/rspb.1994.0089 [Google Scholar]
- Slate J, Kruuk L.E.B, Marshall T.C, Pemberton J.M, Clutton-Brock T.H. Inbreeding depression influences lifetime breeding success in a wild population of red deer (Cervus elaphus) Proc. R. Soc. B. 2000;267:1657–1662. doi: 10.1098/rspb.2000.1192. doi:10.1098/rspb.2000.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slate J, David P, Dodds K.G, Veenvliet B.A, Glass B.C, Broad T.E, McEwan J.C. Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity. 2004;93:255–265. doi: 10.1038/sj.hdy.6800485. doi:10.1038/sj.hdy.6800485 [DOI] [PubMed] [Google Scholar]
- Smith H.G, von Schantz T. Extra-pair paternity in the European starling: the effect of polygyny. Condor. 1993;95:1006–1015. doi:10.2307/1369436 [Google Scholar]
- Tarvin K.A, Webster M.S, Tuttle E.M, Pruett-Jones S. Genetic similarity of social mates predicts levels of extrapair paternity in splendid fairy-wrens. Anim. Behav. 2005;70:945–955. doi:10.1016/j.anbehav.2005.01.012 [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: an invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Webster M.S, Tarvin K.A, Tuttle E.M, Pruett-Jones S. Reproductive promiscuity in the splendid fairy-wren: effects of group size and auxiliary reproduction. Behav. Ecol. 2004;15:907–915. doi:10.1093/beheco/arh093 [Google Scholar]
- Wedell N, Kvarnemo C, Lessells C.M, Tregenza T. Sexual conflict and life histories. Anim. Behav. 2006;71:999–1011. doi:10.1016/j.anbehav.2005.06.023 [Google Scholar]
- Westneat D.F, Stewart I.R.K. Extra-pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Syst. 2003;34:365–396. doi:10.1146/annurev.ecolsys.34.011802.132439 [Google Scholar]
- Westneat D.F, Sherman P.W, Morton M.L. The ecology and evolution of extra-pair copulations in birds. In: Power D.M, editor. Current ornithology. Plenum Press; New York, NY: 1990. pp. 331–369. [Google Scholar]
- Wetton J.H, Parkin D.T. An association between fertility and cuckoldry in the house sparrow Passer domesticus. Proc. R. Soc. B. 1991;245:227–233. doi:10.1098/rspb.1991.0114 [Google Scholar]
- Wolf L.L. ‘Prostitution’ behaviour in a tropical hummingbird. Condor. 1975;77:140–144. doi:10.2307/1365783 [Google Scholar]
- Zeh J.A, Zeh D.W. The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc. R. Soc. B. 1996;263:1711–1717. doi:10.1098/rspb.1996.0250 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material contains a table of the predictions tested in this study (table 1), as well as some data on copulation behaviour in superb starlings. It also contains a more detailed explanation of the parentage analysis, as well as tables showing microsatellite allele frequencies (table 2) and simulation parameters (table 3)