Abstract
Constraints on form should determine how organisms diversify. Owing to competition for the limited space within the body, investment in adjacent structures may frequently represent an evolutionary compromise. For example, evolutionary trade-offs between eye size and jaw muscles in cichlid fish of the African great lakes are thought to represent a constructional constraint that influenced the diversification of these assemblages. To test the evolutionary independence of these structures in Lake Malawi cichlid fish, we measured the mass of the three major adductor mandibulae (AM) muscles and determined the eye volume in 41 species. Using both traditional and novel methodologies to control for resolved and unresolved phylogenetic relationships, we tested the evolutionary independence of these four structures. We found that evolutionary change in the AM muscles was positively correlated, suggesting that competition for space in the head has not influenced diversification among these jaw muscles. Furthermore, there was no negative relationship between change in total AM muscle mass and eye volume, indicating that there has been little effect of the evolution of eye size on AM evolution in Lake Malawi cichlids. The comparative approach used here should provide a robust method to test whether constructional constraints frequently limit phenotypic change in adaptive radiations.
Keywords: evolutionary constraint, comparative method, feeding, trade-off, vision
1. Introduction
For many morphological phenotypes, allocation to one structure may compromise investment in other structures. These compromises, or trade-offs, can operate at many levels of biological organization and among diverse components of organismal design (Garamszegi et al. 2002; Striedter & Northcutt 2006). From a developmental perspective, if extensive somatic investment is made in one structure, then it could limit the amount of soma that can be dedicated to the formation of another (Emlen 2001; Moczek & Nijhout 2004). It is also possible that constructional trade-offs constrain investment in phenotypes because the structural space in organisms is limiting (Barel 1984). If one structure is enlarged, then it could limit the size of other structures, especially those in adjacent areas. For instance, debate continues regarding the modular versus compensatory evolution of mammalian brains (Finlay & Darlington 1995; Barton & Harvey 2000; de Winter & Oxnard 2001). Similarly, the craniofacial morphology of cichlid fish is one of the most extensively studied organismal phenotypes used to bolster the idea that constructional constraints are evolutionarily important. To examine a long postulated constructional constraint, we examined evolutionary trade-offs in eye size and adductor mandibulae (AM) muscle masses among Lake Malawi cichlid fish.
Nowhere has the rate and extent of trophic diversification been as extreme as in the monophyletic assemblage of approximately 1000 species of Lake Malawi cichlids. In rapidly evolving groups like these fish, modularity, or the independent evolution of phenotypes, has long been implicated as critical in the evolution of the feeding apparatus (Liem 1973, 1979; Albertson et al. 2003, 2005; Hulsey 2006; Hulsey et al. 2006). The identification of constraints (genetic, developmental, functional and/or constructional) in the cichlid trophic apparatus would therefore allow one to determine which aspects of the trophic apparatus are more evolvable and which elements generally change in an integrated manner. For instance, the three AM muscles may evolve with respect to one another in several ways. The AMs in most teleosts probably differentiate from a single muscle mass present early in ontogeny (Hernandez et al. 2005), suggesting that the adult adductors may be strongly positively correlated. Likewise, functional studies might lead one to infer that change in one muscle may be positively correlated with change in another to coordinate the forces exerted when the jaws are used during feeding (Anker 1978; Wainwright et al. 2004). However, it is also possible that the AM subdivisions may have evolved largely independently of one another as has been postulated for other aspects of the cichlid trophic apparatus (Liem 1973). While AM1 and AM2 function primarily during feeding, AM3 may be most important in respiration (Osse 1969; von Herbing et al. 1996a), indicating that AM3 might be functionally modular. Alternatively, because the AM muscles are confined to the cheek region of the jaw, they may compete extensively for space in this relatively constrained region of the head. In tetraodontiform fish, where there has been extensive duplication of AM muscles, total AM mass does not increase as the number of muscles increases. Therefore, the overall volume available for divergence in the AMs may be constrained (Friel & Wainwright 1997). Since the mass of a muscle is a good predictor of its volume and the mechanical properties of vertebrate muscle can generally be estimated accurately if the muscle mass and pinnation angle are known (Calow 1973), muscle mass predicts both the spatial and functional properties of the AMs. If constructional competition for space were to influence the AM mass and function in cichlids, we might expect a negative correlation among the masses of adductor muscles during evolution.
Vision appears to be critically important to the diversification of Malawi cichlids (Knight & Turner 1999, Carleton et al. 2000; Parry et al. 2005), and several visual abilities may be tied to the size of the cichlid eye (Otten 1981; Meer et al. 1984). In vertebrates, larger eyes can achieve a greater pupil diameter as well as focal length and may permit greater visual sensitivity and resolution (Kiltie 2000; Humphries & Ruxton 2002; Thomas et al. 2006). In cichlids and other vertebrates, eye size may also influence the AM muscle mass through constructional constraints (Barel 1983; Gosline 1989). Some of the best evidence for these putative negative correlations in fish has been found in the Lake Victoria species flock (Barel 1984; Strauss 1984) that contains many cichlid species that are either extinct or endangered, but are closely related to the species found in Lake Malawi (Kocher et al. 1995). Notably, developmental studies also suggest that a trade-off exists between ocular and mandibular arch musculature (von Scheven et al. 2006). If eye size were to commonly limit AM size during trophic diversification, we would expect change in these muscles and also in eye size to have a negative correlation. If eye size increases and constructional constraints are important, this change should result in relatively smaller jaw-closing muscles. Alternatively, if there were a positive or lack of correlation between eye volume and the masses of the muscles, this would suggest that there is little constraint imposed by eye size on AM size in Malawi cichlids.
When assessing the correlated evolution of characters, past examinations of constructional constraints have generally not adequately accounted for the effects of body size in comparisons (Strauss 1984). Phenotypes generally change extensively as organisms grow. Constructional studies have also rarely accounted for the potential influence of shared evolutionary history on the associations among characters. Species are not evolutionarily independent data points and incorporating phylogenetic hypotheses into the analysis of association among characters provides a means to more robustly determine how characters coevolved (Felsenstein 1985). One problem with analysing phenotypic correlations among rapidly radiating groups like the cichlids in Lake Malawi is the general difficulty found when attempting to recover bifurcating relationships among species (Kornfield & Smith 2000; Won et al. 2006). To determine the constructional constraints among eye size and the size of the adductors, it seems critical to evaluate the influence of both body size and the numerous possible influences of phylogenetic history on the correlations recovered among these structures.
We first documented variation in AM and eye size within a diverse subset of the Malawi species flock. A hypothesis for the phylogenetic relationships among the species examined was then reconstructed using the ND2 gene to provide an evolutionary framework for comparisons among these species. Since this gene failed to adequately resolve bifurcating relationships among numerous groups in the phylogeny, we augmented the resolved backbone of this topology with randomly generated phylogenetic relationships. Finally, both independent contrast analyses and Mantel tests were used to examine the relationships among the AMs and eye size in the Malawi cichlid species flock.
2. Material and methods
(a) Morphology
Forty-one cichlid species were collected in several locations (table 1) from the southern end of Lake Malawi, Africa in July 2005. Fish were preserved in formalin and then transferred to 70% ethanol for long-term storage. On one to three specimens of each species, the length of the head between the posterior edge of the preopercle and the tip of the upper jaw was measured to estimate head length (HL). Standard length (SL) of the fish was also measured.
Table 1.
The species and their collection sites within Lake Malawi are shown: OP, Otter Point; OS, Otter Sand; TW, Thumbi West; MB, Mbenji Island; and ML, Maleri Islands. Species are arranged from those with the smallest to those with the largest total AM residuals. Standard length, SL; head length, HL; mass of the three adductors, AM1, AM2 and AM3; eye volume, EV and diet categories are given. The diet categories are abbreviated: P, piscivore; Pk, planktivore; A, algivore; B, planktivore/algivore; I, insectivore; M, molluscivore; E, egg stealer; C, parasite cleaner; F, fin biter; and G, plant gleaner.
| genus | species | site | n | SL (mm) | HL (mm) | AM1 (mg) | AM2 (mg) | AM3 (mg) | EV (mm3) | diet |
|---|---|---|---|---|---|---|---|---|---|---|
| Fossorochromis | rostratus | OP | 2 | 70.3 | 19.3 | 8.2 | 7.8 | 1.8 | 156.2 | P |
| Protomelas | fenestratus | TW | 3 | 68.5 | 19.2 | 8.7 | 7.9 | 2.6 | 188.6 | I |
| Tropheops | orange chest | TW | 2 | 81.5 | 18.5 | 9.5 | 10.4 | 2.3 | 193.2 | A |
| Copadichromis | eucinostomus | OS | 2 | 96.8 | 22.1 | 15.6 | 13.1 | 3.5 | 232.1 | Pk |
| Ctenopharynx | pictus | TW | 3 | 82.2 | 22.8 | 16.0 | 13.6 | 2.8 | 242.2 | Pk |
| Tropheops | gracilior | OP | 1 | 73.6 | 17.4 | 11.8 | 5.5 | 1.4 | 170.0 | A |
| Tropheops | red cheek | TW | 2 | 76.1 | 18.1 | 12.5 | 7.6 | 2.3 | 173.0 | A |
| Pseudotropheus | crabro | M | 1 | 72.6 | 18.1 | 13.9 | 10.6 | 2.1 | 138.9 | C |
| Copadichromis | mbenjii | MB | 3 | 87.8 | 18.2 | 15.0 | 12.8 | 2.5 | 319.3 | Pk |
| Tropheops | broad mouth | OP | 1 | 76.1 | 18.7 | 14.8 | 10.0 | 2.2 | 159.7 | A |
| Cyrtocara | moorii | OS | 2 | 99.3 | 24.5 | 25.5 | 23.0 | 4.1 | 333.7 | I |
| Taeniolethrinops | praeorbitalis | OS | 2 | 140.0 | 43.8 | 77.4 | 55.5 | 22.0 | 800.2 | I |
| Chilotilapia | euchilus | TW | 1 | 120.4 | 36.3 | 60.1 | 38.8 | 12.2 | 651.6 | I |
| Labeotropheus | trewavasae | TW | 1 | 95.5 | 22.5 | 27.1 | 27.6 | 6.7 | 227.1 | A |
| Rhamphochromis | esox | OS | 1 | 119.7 | 31.5 | 49.4 | 40.1 | 4.8 | 255.8 | P |
| Hemitilapia | oxyrhynchus | OS | 2 | 91.1 | 16.6 | 18.1 | 9.8 | 2.5 | 438.2 | G |
| Maravichromis | mola | OS | 2 | 103.6 | 30.3 | 50.3 | 24.3 | 8.8 | 449.5 | M |
| Nimbochromis | linni | TW | 1 | 88.9 | 26.2 | 39.1 | 22.3 | 3.4 | 220.2 | P |
| Tropheops | microstoma | OP | 2 | 73.2 | 18.9 | 21.3 | 14.8 | 2.0 | 177.3 | A |
| Labidochromis | gigas | TW | 1 | 70.8 | 16.8 | 18.5 | 10.6 | 1.9 | 124.0 | A |
| Placidochromis | spilopterus | ML | 1 | 106.6 | 30.3 | 54.4 | 30.2 | 11.4 | 627.2 | E |
| Metriaclima | callainos | TW | 1 | 84.4 | 20.0 | 28.8 | 19.1 | 4.8 | 154.7 | B |
| Cynotilapia | afra | TW | 3 | 72.5 | 16.8 | 22.4 | 17.3 | 2.4 | 132.0 | Pk |
| Labeotropheus | fuelleborni | TW | 3 | 98.9 | 23.1 | 38.6 | 37.3 | 6.2 | 376.8 | A |
| Placidochromis | johnstoni | OS | 1 | 146.5 | 40.4 | 101.4 | 67.9 | 17.9 | 506.7 | I |
| Melanochromis | vermivorus | TW | 1 | 69.1 | 16.3 | 22.1 | 17.0 | 3.6 | 95.1 | I |
| Dimidiochromis | compressiceps | OS | 1 | 144.9 | 43.4 | 145.9 | 78.3 | 15.3 | 422.7 | P |
| Placidochromis | milomo | ML | 1 | 136.8 | 42.0 | 135.6 | 74.2 | 26.4 | 957.3 | I |
| Nimbochromis | polystigma | OS | 3 | 92.0 | 25.0 | 58.5 | 37.3 | 7.4 | 220.6 | P |
| Pseudotropheus | elongatus | TW | 2 | 75.7 | 17.6 | 32.9 | 19.3 | 2.4 | 112.6 | A |
| Trematocranus | placodon | OS | 3 | 144.5 | 37.5 | 127.3 | 73.0 | 25.7 | 1100.9 | M |
| Otopharynx | heterodon | TW | 1 | 132.7 | 34.2 | 120.7 | 70.0 | 23.4 | 736.3 | Pk |
| Melanochromis | auratus | TW | 2 | 77.9 | 16.9 | 43.8 | 36.4 | 5.2 | 101.4 | I |
| Tyrannochromis | nigriventer | OP | 2 | 130.4 | 41.7 | 163.5 | 102.3 | 31.9 | 459.3 | P |
| Aristochromis | christyi | OP | 2 | 108.5 | 31.0 | 111.1 | 61.1 | 10.3 | 384.9 | P |
| Metriaclima | aurora | TW | 3 | 78.8 | 15.1 | 26.8 | 23.7 | 3.3 | 210.1 | B |
| Docimodus | evelynae | ML | 1 | 72.3 | 7.8 | 11.7 | 12.4 | 2.1 | 152.3 | C |
| Genyochromis | mento | TW | 2 | 78.7 | 20.1 | 69.2 | 64.7 | 7.3 | 108.3 | F |
| Tyrannochromis | macrostoma | OP | 3 | 132.9 | 37.2 | 213.5 | 115.5 | 24.3 | 393.6 | P |
| Taeniolethrinops | furcicauda | OS | 1 | 140.5 | 35.6 | 223.8 | 115.4 | 26.0 | 578.7 | I |
| Metriaclima | livingstonii | TW | 1 | 67.0 | 6.7 | 13.1 | 9.7 | 1.5 | 117.0 | B |
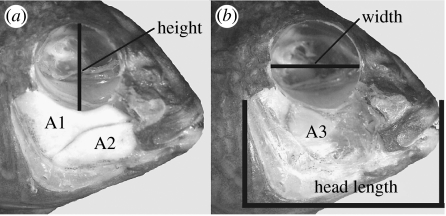
Prior to eye measurements and muscle dissection, the eye was removed from the orbit. Three measurements were made on the orbit with calipers to estimate the volume of the eye (figure 1). Both the width and height of the orbit were measured as the orbit can deviate from a perfect circle (Barel 1984). In cichlids, the interior components of the two eyes meet in the centre of the head. Therefore, the distance spanning the width of the head between the two most dorsal points of the eyes was measured. To estimate the volume of the eye, this distance was halved to represent the width of a single eye. Using these three measurements and the formula for the volume of a cylinder, we calculated the volume of the eye.
Figure 1.
Craniofacial structural measurements. The three adductor mandibulae were dissected from the cichlid head. Then, (a) the height and (b) width of the orbit were measured. In cichlids, the interior components of the two eyes meet in the centre of the head. The third dimension of the cylindrically shaped eye was measured as the width of the head at the dorsalmost point where orbit height was measured. This third measurement was then halved to estimate the length of one cylindrically modelled orbit and combined with the other two measurements to estimate the eye volume.
For all individuals, mass of each of the three AMs was measured to estimate the volume of each muscle. The AM1, AM2 and AM3 were dissected wholly from the head (figure 1) and placed into 70% ethanol. Prior to measurement, each muscle was removed from its vial, patted twice on paper towel and immediately weighed. The wet mass of each AM muscle was thereby determined to the nearest 0.1 mg using a digital balance.
To estimate the relationship between the muscle masses and eye volume, these measurements were adjusted by head size. Since mass generally scales with the third power of length, the cube root of the masses of the muscle and eye sizes was first found. Then, the mass of AM1, AM2, AM3 and their combined mass as well as the volume of the eye were log transformed to account for increased variance as measurements increase with body size. Subsequently, the residuals of these five measurements were obtained from linear regressions on log HL to limit the influence of HL measurement error on residual estimates of the muscle and eye phenotypes. Once the residuals of the morphological measurements were obtained, the variation of these values around the mean measurement made was estimated. This allowed us to determine the range of size-standardized residuals for each character. The correlations among the three AMs were then examined. The correlations among the residuals from HL were also examined to test the null hypothesis that eye size had no relationship with total AM mass.
(b) DNA isolation and sequencing
To provide a phylogenetic hypothesis of the relationships of the species examined, the ND2 gene of 34 Malawi species (GenBank accession numbers: EF585251–EF585283) were combined with a few Malawi cichlids that were previously sequenced (Appendix A). The five ND2 sequences of the species Heterochromis multidens, Boulengerochromis microlepis, Oreochromis niloticus, Astatotilapia nubila and Astatotilapia burtoni were included as outgroups. All species we sequenced were collected from the wild in Lake Malawi from the locations in table 1. For sequencing, total genomic DNA was isolated from fin clips at the Hubbard Centre for Genome Studies, University of New Hampshire. A 1 μl (100 ng of DNA) aliquot of this solution was used to provide a DNA template for the polymerase chain reaction (PCR). The entire ND2 gene was PCR amplified using primers from Kocher et al. (1995). Amplifications were carried out in a MT Research Peltier DNA thermocycler. The PCR volume was 25 μl (18 μl H2O, 2.75 μl 10×MgCl2 PCR buffer, 1.25 μl MgCl2, 2.0 μl dNTPs (10 mM), 1.25 μl of each primer (10 μM), 0.25 μl Taq and 0.5 μl DNA; approx. 15–20 ng). Thermal cycling conditions consisted of an initial denaturation step of 94°C (2.0 min), 54°C (1.0 min) and 72°C (1.5 min). A final incubation of 72°C for 4 min was added to ensure complete extension of amplified products. Subsequently, the 1.1 kb PCR products were separated from unincorporated primers and dNTPs using electrophoresis in agarose gels run in Tris–acetate buffer (pH 7.8). Ethidium bromide (1.5 mg μl−1) was added to the gels for visualization. Positively amplified DNA was then purified using an enzymatic combination of 1 μl exonuclease I (10.0 U μl−1) and 1 μl shrimp alkaline phosphatase (2.0 U μl−1) per 10 μl of PCR product. Treated PCR products were sequenced by the High Throughput DNA Sequencing Facility at the University of Washington. Complete gene sequences were assembled from individual reactions using the program Sequencher v. 4.6 (Gene Codes, Ann Arbor, MI). Sequences were aligned using Clustal X (Thompson et al. 1999) and codon positions were defined using MacClade v. 4.0 (Maddison & Maddison 2000).
(c) Phylogenetic analysis
In our analysis, we included a total of 41 recognized species of Lake Malawi cichlids. ModelTest v. 3.06 (Posada & Crandall 1998) was used to identify the best model of molecular evolution for each codon site. With the ND2 gene partitioned into its codon sites, Bayesian analyses were executed to find approximations of the maximum likelihood tree using MrBayes v. 3.0 (Ronquist & Huelsenbeck 2003). The analyses treated the transition–transversion matrices, number of invariant sites and γ-shaped parameters as unlinked or independent for each codon site. Flat prior probability distributions for all parameters were assumed before analysis. We ran five separate Bayesian analyses for 1 000 000 generations with four Markov chains in each run. We sampled trees from the Markov Chain Monte Carlo search algorithm every 100 generations. After each analysis, the log-likelihood scores were plotted against generation to identify the point at which likelihood values reached equilibrium. In all five, the equilibrium was reached at approximately 50 000 generations, and sample points prior to generation 100 000 in each run were discarded as ‘burn-in’ samples. The remaining samples from all runs combined were used to produce a majority rule consensus tree in PAUP* v. 4.0b10 (Swofford 2002). The percentage of all trees that recovered a particular clade (the clade's posterior probability) was depicted on the best likelihood topology found during the Bayesian analyses.
(d) Comparative analyses
To assess the putative correlations among muscle masses as well as between total AM mass and eye volume, we first examined the correlation between Malawi species values. However, since species are not evolutionarily independent (Felsenstein 1985), we also performed several independent contrast analyses once these measurements were corrected for size. The specimens used in these analyses were roughly the same size regardless of the maximum or average body sizes these species achieve in the wild. Therefore, we corrected our cranial measurements for HL before the independent contrast analyses because specimen sizes examined were independent of evolutionary history. HL correction incorporated through multiple regression within a phylogenetic framework also produced highly similar results suggesting corrections of HL prior to evolutionary analyses, provided robust inferences (results not shown).
For the phylogenetic backbone of these analyses, we used the above ND2 phylogeny. We first performed an independent contrast analysis with the single best likelihood topology recovered from the Bayesian analysis. However, many of the species relationships were recovered as virtual polytomies due to a lack of base-pair changes among these putatively recently diverged species (Kocher et al. 1995). For an independent contrast analysis, it is necessary to have a strictly bifurcating topology. Therefore, we also used a modification of the method proposed in Losos (1994). We used the 50% majority rule consensus ND2 tree as a backbone topology for relationships and then augmented this with 100 randomly constructed topologies that rendered the polytomies into bifurcating relationships. To generate these topologies, the 50% majority rule consensus tree recovered from the Bayesian analysis was imported into Tree Edit v. 1.0 (Rambaut & Charleston 2002) and the numerous polytomies in the tree were randomly resolved 100 times. For both the best tree and the majority rule tree, the outgroup species used in the phylogeny reconstruction were pruned from the topologies. Finally, the best topology and the 100 semi-random topologies for the species remaining in the tree were exported into CAIC (Purvis & Rambaut 1995). Phylogenetic independent contrast analyses were then performed. We used the recovered Bayesian branch lengths for the single best likelihood topology and treated all branches as equal length in the randomized topologies to assess the independent contrast correlations among the residuals of the transformed values. Although the assumption of equal branch lengths is not likely to be realistic, we used this assumption for the randomized topologies because it limited the influence of the randomly determined branch lengths on the contrast analysis. For this randomized analysis, the ‘crunch’ algorithm was used in CAIC as it treats all variables as continuous.
Although the ND2 gene provides a putative phylogeny of Malawi cichlids, the mitochondrial gene tree may poorly reflect the species phylogeny of this diverse assemblage. Like any type of molecular marker used to reconstruct the relationships among such recently diverged species (Won et al. 2006), the mitochondrial gene tree derived from ND2 sequences may be misleading because of hybridization (Streelman et al. 2004) and retention of ancestral polymorphism (Parker & Kornfield 1997). To use a complementary method that avoids the problem of non-independence for parametric tests but that assesses the correlations among traits, we employed Mantel tests implemented in Genepop v. 3.4 (http://wbiomed.curtin.edu.au/genepop/; Raymond & Rousset 1995). The Mantel tests computed the Spearman correlation coefficients among empirical pairwise trait matrices (e.g., AM1 mass versus AM2 mass) and calculated test statistics based on permuted distance matrices (in this case, 1000 permutations).
3. Results
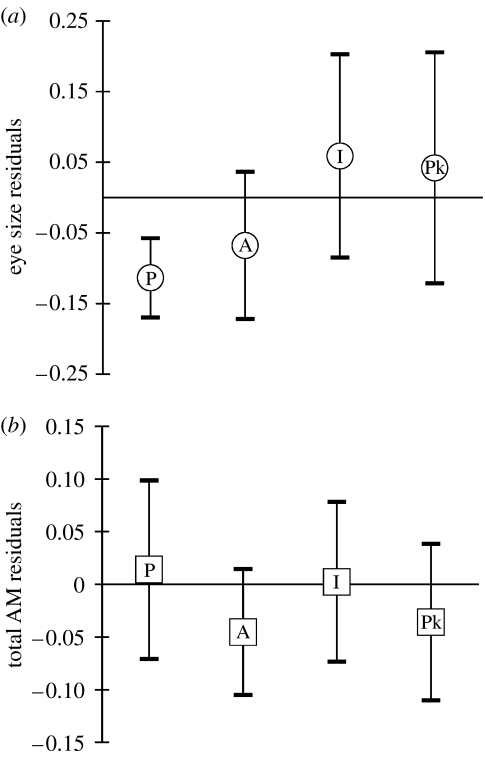
Generally, the AM1 was the largest muscle of the three AMs in all the cichlids examined (table 1). The AM2 was the next largest and the AM3 was consistently the smallest of the three muscles. The AM1 and AM2 mass residuals were highly positively correlated (r=0.93; p<0.001) among species. Likewise, the AM3 was also positively correlated with both AM1 (r=0.81; p<0.001) and AM2 (r=0.84; p<0.001). The smallest residuals of total AM mass were found in the species of Tropheops and the piscivorous Fossorochromis rostratus. The largest total residuals were found in the fin biter Genyochromis mento and in the piscivorous Tyrannochromis macrostoma. When species values were standardized by average HL, the average expected total mass of the AMs was 76.1 mg. After controlling for HL, total AM muscle mass in the Malawi cichlids ranged 7.1-fold. The volume of the eye when adjusted by HL showed a limited association with trophic guild (table 1). Although piscivores such as F. rostratus, Rhamphochromis esox and Nimbochromis spp. had relatively small eye volume residuals (figure 2), the confidence interval for residual values of these species and others grouped by trophic guild generally overlapped with mean species values. However, planktivores such as Copadichromis mbenjii did exhibit consistently larger eye volume residuals. In the members of the Malawi flock examined here, average eye volume, when adjusted for HL, was 292 mm3 and varied 4.7-fold among species.
Figure 2.
Residuals of (a) eye volume and (b) total AM mass on HL are shown for different trophic categories. The four categories displayed for each trait are P, piscivores; A, algivores; I, insectivores; and Pk, planktivores. Each category included here had more than five species and species names are given in table 1. Piscivores tended to have small eyes and larger AMs when compared with planktivores that exhibit larger eyes and somewhat smaller AMs although the variation makes comparative conclusions difficult. The variation in each diet category is substantial and no statistics are done on the data shown because there is no enough power to test these patterns with phylogenetically controlled tests.
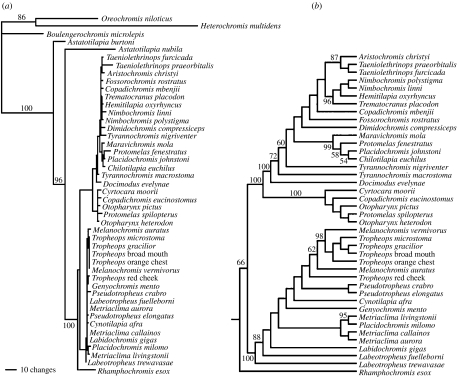
The results from our phylogenetic analysis of the ND2 gene (figure 3) are largely concordant with previous analyses (Kocher et al. 1995). Rhamphochromis esox was found to be the sister group to all other species examined. There appear to be at least two major clades recovered that comprise most of the species flock. The ‘mbuna’ genera such as Labeotropheus, Metriaclima, Tropheops and Melanochromis form one clade that has virtually no resolution due to a complete absence of phylogenetically informative sites among species or even genera. The extreme example of this is that Melanochromis vermivorus, Tropheops broad mouth, Tropheops gracilior, Tropheops orange chest and Tropheops microstoma had identical ND2 haplotypes. Metriaclima aurora, Metriaclima callainos, Pseudotropheus crabro and G. mento likewise had identical ND2 haplotypes. The second large clade contains many diverse groups that include sand-dwelling and pelagic species such as Trematocranus placodon and Copadichromis spp. Only 40% of nodes were supported with greater than 50% bootstrap support. Numerous relationships among many species had limited support due to few shared substitutions in the ND2 gene.
Figure 3.
Phylogeny of the Malawi cichlids. (a) The amount of sequence divergence and basic topology of the ND2 phylogeny recovered was similar in its broad relationships to those recovered in previous analyses (Kocher et al. 1995). Numerous species had little to no shared sequence divergence among them and therefore failed to provide the bifurcations necessary for a comparative contrast analysis. The posterior probability support for the outgroups is shown on the phylogram of the topology showing the best likelihood (a). (b) Owing to the short branch lengths, the posterior probabilities for Malawi cichlid relationships are depicted on one potential cladogram of relationships for these species. For the phylogenetically controlled comparative analyses, the 50% majority rule ND2 topology was augmented with 100 arbitrarily chosen sets of randomly chosen bifurcating relationships ((b) is one example). All nodes exhibiting less than 50% posterior probability support were randomly resolved to create a completely bifurcating topology, and all branch lengths were treated as equivalent in length to calculate the independent contrasts.
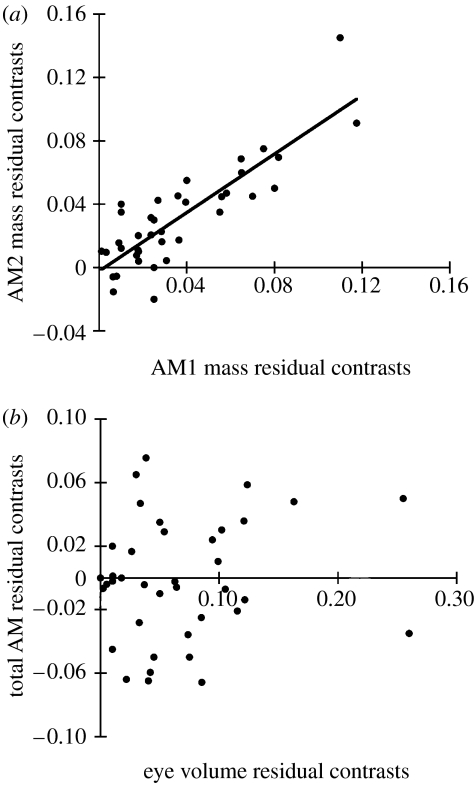
The comparative analyses performed with both the ND2 topologies and the Mantel tests provide consistent evolutionary scenarios regarding the evolution of the craniofacial structures examined. The masses of the AMs were always recovered as evolving in concert. The correlation between the mass of AM1 and AM2 (figure 4a) was especially strong and was always highly significant (p<0.0001) for the best topology (r=0.84), all 100 randomized topologies (r=0.91±0.01 s.e.) and for the Mantel tests (Spearman's r=0.83, p<0.0001). When examining the independent contrasts on the ND2 topologies, the relationships between AM1 and AM3 (best: r=0.76 and randomized: r=0.74±0.02 s.e.) and AM2 and AM3 (best: r=0.67 and randomized: r=0.81±0.02 s.e.) were likewise always highly significant (p<0.0001). Similar results were obtained from the Mantel tests of these variables (A1 versus A3 r=0.59; p<0.0001 and A2 versus A3 r=0.64; p<0.0001). However, when the relationship between the total AM residuals and residuals of eye volume were examined with the Mantel tests, there was a slightly positive relationship (r=0.06; p=0.04), but there was never a significant evolutionary correlation when accounting for the best topology (p=0.90) or any of the randomized topologies (range: p=0.98–0.23).
Figure 4.
(a) A representative example of the relationship among the residual AM masses displaying the strongly positive relationship recovered even when phylogeny was accounted for using the 100 randomly resolved ND2 topologies. (b) However, the relationship between eye volume residuals and total AM mass residuals was never significant once phylogeny was accounted for suggesting that the eye and adductors have not changed in concert during the evolution of the Malawi species flock.
4. Discussion
We recovered evidence of modularity among phenotypes in the Malawi cichlid traits examined, but found no evidence for constructional constraints. Every method used to test for an evolutionary association among the AMs demonstrated that all three are positively correlated with one another. The correlation among the AM1 and AM2 muscles is especially strong, which might have been expected given their shared function during feeding (Osse 1969; von Herbing et al. 1996b) and their common developmental origin (Hernandez et al. 2005). These positive correlations indicate that the cichlid AM probably do not compete for space in the cichlid head, and suggest that the muscles are not evolutionarily modular. Although modularity has widely been implicated as important in the evolution of the cichlid trophic apparatus (Liem 1973, 1979; Albertson et al. 2003; Hulsey et al. 2006), the strong correlations among the AMs suggest that they have not changed independently during the diversification of Malawi cichlids.
A greater appreciation of the function of the adductors during feeding (Alfaro et al. 2001; Korff & Wainwright 2004) and how they develop during ontogeny (Hernandez et al. 2005) could provide further insight into the causes of the correlated change among the adductors. Parrotfish species that bite and excavate coral tend to have larger muscles than parrotfish that scrape algae and dead coral (Bellwood & Choat 1990). Groups such as triggerfish that feed by biting chunks of coral and other hard-shelled prey also commonly have large adductor muscles (Turingan & Wainwright 1993). Although we did not have the quantitative diet information to adequately test the evolutionary relationships among masses of the AMs and feeding guilds in Malawi cichlids, cichlid genera that scrape or bite algae from the substrate like Tropheops or Labeotropheus did not have significantly larger AM masses. This is all the more interesting given that species such as Labeotropheus possess lower jaws designed for exceedingly high mechanical advantage (Albertson et al. 2005) when the jaws are closed via the AMs. There was very little clear differentiation in the adductor muscle masses among trophic guilds as they all appeared to overlap the mean value of the muscle masses. Nevertheless, the sevenfold range of mass variation in the adductors when adjusted for size suggests that there has been substantial diversification in the AM of Malawi cichlids. These patterns highlight the extensive gaps that remain in our general functional understanding of the link between the mass of the AMs and trophic abilities in teleost fish.
Eye volume and the mass of the adductors are not negatively correlated in Malawi cichlids. Although eye volume may influence adductor size in some cichlids, this does not appear to be true in the diverse set of Malawi cichlids examined here. In place of the negative correlation in size expected if eyes frequently compete for space with AMs, eye size had at best a positive but weak correlation with adductor size. This weak relationship suggests that the size of the eye is probably able to evolve independently of AM mass. This lack of correlated change among functionally important characters may be common in highly diverse systems such as the Malawi cichlids (Wainwright et al. 2004). Also, it is unclear if constructional constraints between eye size and jaw muscles may operate in other groups of fish, especially since some groups may have much larger eyes on average than the Lake Malawi cichlids (Pankhurst 1989; Huber et al. 1997). Since Lake Malawi may have exceptional water clarity compared with other water bodies inhabited by cichlids, it seems possible that visual abilities which are heavily influenced by eye volume and necessary in low-light environments could be relaxed in Lake Malawi. Comparisons of the relationships of eye size and AMs in other groups of cichlids would help to resolve whether eye size is actually less constraining in Lake Malawi.
Although the link between eye volume and many characteristics of vision are probably loose, the lack of an apparent structural trade-off between eye size and jaw muscle mass is tantalizing in Lake Malawi. In this group of cichlids, both sexual selection on male nuptial coloration and natural selection on the trophic apparatus have probably been critical in driving the rapid evolution of the approximately 1000 species present (Danley & Kocher 2001; Streelman et al. 2003). Our results suggest that there may be little interaction between the two adjacent components of the head we examined. Although there are a few deepwater species of Malawi cichlids that have greatly enlarged eyes, we did not examine those species here. Analyses of these characters in deepwater species may find greater compromises between eye volume and adductor mass. However, since they represent only approximately 1% of the species diversity in the lake (Turner et al. 2004), our results are probably representative of change throughout the diversification of the Lake Malawi flock. Analyses similar to those presented here using these species may demonstrate the exception in the lake that proves the apparent rule that eye volume has very little influence on adductor mass in this species flock.
Species flocks such as the Lake Malawi cichlids are so intensively studied precisely because of the putatively adaptive divergence that has occurred in these groups in such a short period of evolutionary time (Kocher et al. 1995; Danley & Kocher 2001). Owing to the increasing availability of phylogenetic trees, comparative analyses incorporating phylogeny provide some of the most powerful methods available to test hypotheses of constraint and adaptation, but use of this method is often intractable in adaptive radiations. In groups where data is plentiful but phylogenetic relationships remain unclear because numerous topologies could explain the data equally well, the use of alternative topologies that differ only slightly in their likelihood provides a viable option to robustly evaluate evolutionary correlations (Huelsenbeck & Rannala 2003). However, the rate at which some species flocks diverge often precludes the ability of even rapidly evolving molecular markers like the ND2 mitochondrial gene to recover the strictly bifurcating topologies necessary to reconstruct contrast correlations. One option could be to examine the correlation among species values and discount what little evolutionary history is recoverable or to produce completely random phylogenies (Losos 1994). In contrast, one could use only the single best topology recovered from a Bayesian analysis despite the limited robustness of the topology. The simple alternative methodology we used to examine evolutionary correlations accounted for those phylogenetic relationships consistently recovered while randomly resolving rapidly evolving or otherwise difficult to recover nodes. This randomization has the disadvantage of basing comparative analyses on phylogenetic hypothesis that contains inferred star-like bursts of diversification. However, for exceedingly rapidly diversifying clades, this may provide a close reflection of actual diversification. This hybrid of previously proposed methods probably provides the best option available for the comparative evaluation of hypothesized adaptations and constraints (Losos 1994) in the rapidly diversifying Malawi radiation.
The volume of the eye and masses of the AMs cannot become infinitely large, and therefore at some level, constructional constraints could operate. However, it seems probable that other constraints on these structures may generally operate in Malawi cichlids before constructional constraints become critical. It is also possible that because of the functional importance of eye size and AM mass, these structures may be imposing constructional constraints on other craniofacial features that were not examined. Identifying what promotes and places limits on the diversification of phenotypic evolution in radiations such as the Malawi cichlids remains fundamental to understanding the rates and pathways underlying how these groups have diversified. Ultimately, to understand the causes of correlated and independent change among the AMs and other components of the cichlid trophic apparatus, investigations should continue to examine the evolution of functional, developmental and genetic determinants of cichlid craniofacial form.
Acknowledgements
Specimens were collected and sacrificed in accordance with the nation of Malawi and the Georgia Institute of Technology IACUC A0521.
We thank T. D. Kocher, P. D. Danley, A. E. Howe, R. Roberts and R. Zatha for help in collecting fish; Stuart Grant for hospitality and boat time; A. J. Ambali, the University of Malawi, Malawi Parks Service and the Malawi government for logistics and permissions; the NSF (IOB 0546423) and an Alfred P. Sloan Research Fellowship in Computational and Evolutionary Molecular Biology (BR 4499) for funding support (to J.T.S.).
Appendix A.
Species and GenBank Numbers: Aristochromis christyi (EF585282); Chilotilapia euchilus (EF585280); Copadichromis eucinostomus (EF585268); Copadichromis mbenjii (EF585255); Ctenopharynx pictus (EF585254); Cynotilapia afra (EF585264); Cyrtocara moorii (AY930089); Dimidiochromis compressiceps (EF585267); Docimodus evelynae (EF585252); Fossorochromis rostratus (EF585281); Genyochromis mento (AF305297); Hemitilapia oxyrhynchus (EF585277); Labeotropheus trewavasae (EF585283); Labeotropheus fuelleborni (EF585259); Labidochromis gigas (EF585276); Maravichromis mola (EF585274); Melanochromis vermivorus (EF585270); Melanochromis auratus (AY930069); Metriaclima callainos (EF585271); Metriaclima aurora (EF585266); Metriaclima livingstonii (EF585273); Nimbochromis linni (EF585279); Nimbochromis polystigma (EF585262); Otopharynx heterodon (EF585278); Placidochromis spilopterus (EF585253); Placidochromis johnstoni (EF585269); Placidochromis milomo (EF585251); Protomelas fenestratus (AF305301); Pseudotropheus crabro (EF585256); Pseudotropheus elongatus (EF585272); Rhamphochromis esox (AF305252); Taeniolethrinops praeorbitalis (AF305318); Taeniolethrinops furcicauda (EF585263); Trematocranus placodon (EF585261); Tropheops gracilior (EF585260); Tropheops orange chest (EF585275); Tropheops red cheek (EF585265); Tropheops broad mouth (EF559101); Tropheops microstoma (EF585258); Tyrannochromis nigriventer (AF305307); Tyrannochromis macrostoma (EF585257); Boulengerochromis microlepis (AF317229); Oreochromis niloticus (AF317237); Heterochromis multidens (AF317269); Astatotilapia burtoni (AF305245); Astatotilapia nubila (AF305242).
References
- Albertson R.C, Streelman J.T, Kocher T.D. Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc. Natl Acad. Sci. USA. 2003;100:5252–5257. doi: 10.1073/pnas.0930235100. doi:10.1073/pnas.0930235100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson R.C, Streelman J.T, Kocher T.D, Yelick P.C. Integration and evolution of the cichlid mandible: the molecular basis of alternative feeding strategies. Proc. Natl Acad. Sci. USA. 2005;102:16 287–16 292. doi: 10.1073/pnas.0506649102. doi:10.1073/pnas.0506649102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro M.E, Janovetz J, Westneat M.W. Motor control across trophic strategies: muscle activity of biting and suction feeding fishes. Am. Zool. 2001;41:1266–1279. doi:10.1668/0003-1569(2001)041[1266:MCATSM]2.0.CO;2 [Google Scholar]
- Anker G.C. The morphology of the head muscles of a generalized Haplochromis species: H. elegans. Trewavas 1993 (Pisces, Cichlidae) Neth. J. Zool. 1978;28:234–271. [Google Scholar]
- Barel C.D.N. Towards a constructional morphology of the cichlid fishes (Teleostei, Perciformes) Neth. J. Zool. 1983;33:357–424. [Google Scholar]
- Barel C.D.N. Form-relations in the context of constructional morphology: the eye and suspensorium of lacustrine Cichlidae (Pisces, Teleostei): with a discussion on the implications for phylogenetic and allometric form-interactions. Neth. J. Zool. 1984;34:439–502. [Google Scholar]
- Barton R.A, Harvey P.H. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. doi:10.1038/35016580 [DOI] [PubMed] [Google Scholar]
- Bellwood D.R, Choat J.H. A functional analysis of grazing in parrotfishes (Family Scaridae)—the ecological implications. Environ. Biol. Fish. 1990;28:189–214. doi:10.1007/BF00751035 [Google Scholar]
- Calow L.J. Mechanical analysis of a hind leg of a frog (Rana temporaria) J. Zool. 1973;171:293–321. [Google Scholar]
- Carleton K.L, Harosi F.I, Kocher T.D. Visual pigments of African cichlid fishes: evidence for ultraviolet vision from microspectrophotometry and DNA sequences. Vision Res. 2000;40:879–890. doi: 10.1016/s0042-6989(99)00238-2. doi:10.1016/S0042-6989(99)00238-2 [DOI] [PubMed] [Google Scholar]
- Danley P.D, Kocher T.D. Speciation in rapidly diverging systems: lessons from Lake Malawi. Mol. Ecol. 2001;10:1075–1086. doi: 10.1046/j.1365-294x.2001.01283.x. doi:10.1046/j.1365-294X.2001.01283.x [DOI] [PubMed] [Google Scholar]
- de Winter W, Oxnard C.E. Evolutionary radiations and convergences in the structural organization of mammalian brains. Nature. 2001;409:710–714. doi: 10.1038/35055547. doi:10.1038/35055547 [DOI] [PubMed] [Google Scholar]
- Emlen D.J. Costs and the diversification of exaggerated animal structures. Science. 2001;291:1534–1536. doi: 10.1126/science.1056607. doi:10.1126/science.1056607 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. doi:10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Finlay B.L, Darlington R.B. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. doi:10.1126/science.7777856 [DOI] [PubMed] [Google Scholar]
- Friel J.P, Wainwright P.C. A model system of structural duplication: homologies of adductor mandibulae muscles in tetraodontiform fishes. Syst. Biol. 1997;46:441–463. doi:10.2307/2413691 [Google Scholar]
- Garamszegi L.Z, Moller A.P, Erritzoe J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. B. 2002;269:961–967. doi: 10.1098/rspb.2002.1967. doi:10.1098/rspb.2002.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline W.A. Two patterns of differentiation in the jaw musculature of teleostean fishes. J. Zool. Lond. 1989;218:649–662. [Google Scholar]
- Hernandez L.P, Patterson S.E, Devoto S.H. The development of muscle fiber type identity in zebrafish cranial muscles. Anat. Embryol. 2005;209:323–334. doi: 10.1007/s00429-004-0448-4. doi:10.1007/s00429-004-0448-4 [DOI] [PubMed] [Google Scholar]
- Huber R, Van Staaden M.J, Kaufman L.S, Liem K.F. Microhabitat use, trophic patterns, and the evolution of brain structure in African cichlids. Brain Behav. Evol. 1997;50:167–182. doi: 10.1159/000113330. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P, Rannala B. Detecting the correlation between characters in a comparative analysis with uncertain phylogeny. Evolution. 2003;57:1237–1247. doi: 10.1111/j.0014-3820.2003.tb00332.x. doi:10.1554/01-012 [DOI] [PubMed] [Google Scholar]
- Hulsey C.D. Function of a key morphological innovation: fusion of the cichlid pharyngeal jaw. Proc. R. Soc. B. 2006;273:669–675. doi: 10.1098/rspb.2005.3375. doi:10.1098/rspb.2005.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey C.D, García de León F.J, Rodiles-Hernández R. Micro- and macroevolutionary decoupling of cichlid jaws: a test of Liem's key innovation hypothesis. Evolution. 2006;60:2096–2109. doi:10.1554/05-587.1 [PubMed] [Google Scholar]
- Humphries S, Ruxton G.D. Why did some ichthyosaurs have such large eyes? J. Exp. Biol. 2002;205:439–441. doi: 10.1242/jeb.205.4.439. [DOI] [PubMed] [Google Scholar]
- Kiltie R.A. Scaling of visual acuity with body size in mammals and birds. Funct. Ecol. 2000;14:226–234. doi:10.1046/j.1365-2435.2000.00404.x [Google Scholar]
- Knight M.E, Turner G.F. Reproductive isolation among closely related Lake Malawi cichlids: can males recognize conspecific females by visual cues? Anim. Behav. 1999;58:761–768. doi: 10.1006/anbe.1999.1206. doi:10.1006/anbe.1999.1206 [DOI] [PubMed] [Google Scholar]
- Kocher T.D, Conroy J.A, Mckaye K.R, Stauffer J.R, Lockwood S.F. Evolution of NADH dehydrogenase subunit 2 in East African cichlid fish. Mol. Phylogenet. Evol. 1995;4:420–432. doi: 10.1006/mpev.1995.1039. doi:10.1006/mpev.1995.1039 [DOI] [PubMed] [Google Scholar]
- Korff W.L, Wainwright P.C. Motor pattern control for increasing crushing force in the striped burrfish (Chilomycterus schoepfi) Zoology. 2004;107:335–346. doi: 10.1016/j.zool.2004.09.001. doi:10.1016/j.zool.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Kornfield I, Smith P.F. African cichlid fishes: model systems for evolutionary biology. Annu. Rev. Ecol. Syst. 2000;31:163–196. doi:10.1146/annurev.ecolsys.31.1.163 [Google Scholar]
- Liem K.F. Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst. Zool. 1973;22:425–441. doi:10.2307/2412950 [Google Scholar]
- Liem K.F. Modulatory multiplicity in the feeding mechanism in cichlid fishes, as exemplified by the invertebrate pickers of Lake Tanganyika. J. Zool. Lond. 1979;189:93–125. [Google Scholar]
- Losos J.B. An approach to the analysis of comparative data when a phylogeny is unavailable or incomplete. Syst. Biol. 1994;43:117–123. doi:10.2307/2413584 [Google Scholar]
- Maddison D.R, Maddison W.P. Sinauer Associates; Sunderland, MA: 2000. MacClade 4.0. [Google Scholar]
- Meer H, Van Der J, Anker G.C. Retinal resolving power and sensitivity of the photopic system in seven haplochromine species (Pisces, Teleostei) Neth. J. Zool. 1984;34:197–209. [Google Scholar]
- Moczek A.P, Nijhout H.F. Tradeoffs during the development of primary and secondary sexual traits in a horned beetle. Am. Nat. 2004;163:184–191. doi: 10.1086/381741. doi:10.1086/381741 [DOI] [PubMed] [Google Scholar]
- Osse J.W.M. Functional morphology of the head of the perch (Perca fluviatilis): an electromyographical study. Neth. J. Zool. 1969;19:289–392. [Google Scholar]
- Otten E. Vision during growth of a generalized Haplochromis species Haplochromis elegans, Pisces: Cichlidae. Neth. J. Zool. 1981;31:650–700. [Google Scholar]
- Pankhurst N.W. The relationship of ocular morphology to feeding modes and activity periods in shallow marine teleosts from New Zealand. Environ. Biol. Fish. 1989;26:201–212. doi:10.1007/BF00004816 [Google Scholar]
- Parker A, Kornfield I. Evolution of the mitochondrial DNA control region in the mbuna (Cichlidae) species flock of Lake Malawi, east Africa. J. Mol. Evol. 1997;45:70–83. doi: 10.1007/pl00006204. doi:10.1007/PL00006204 [DOI] [PubMed] [Google Scholar]
- Parry J.W.L, Carleton K.L, Spady T, Carboo A, Hunt D.M, Bowmaker J.K. Mix and match color vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Curr. Biol. 2005;15:1734–1739. doi: 10.1016/j.cub.2005.08.010. doi:10.1016/j.cub.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall K.A. Model Test: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. doi:10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC): an apple macintosh application for analyzing comparative data. Bioinformatics. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. doi:10.1093/bioinformatics/11.3.247 [DOI] [PubMed] [Google Scholar]
- Rambaut, A. & Charleston, M. 2002 TreeEdit: phylogenetic tree editor v. 1.0 alpha 10. See http://evolve.zoo.ox.ac.uk/software/TreeEdit/main.html
- Raymond M, Rousset F. Genepop (version 1.2)—population genetics software for exact tests and ecumenicism. J. Hered. 1995;86:248–249. [Google Scholar]
- Ronquist F, Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. doi:10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Strauss R.E. Allometry and functional feeding morphology in haplochromine cichlids. In: Echelle A.A, Kornfield I.L, editors. Evolution of fish species flocks. University of Maine Press; Orono, ME: 1984. pp. 217–229. [Google Scholar]
- Streelman J.T, Albertson R.C, Kocher T.D. Genome mapping of the orange blotch colour patterns in cichlid fishes. Mol. Ecol. 2003;12:2465–2471. doi: 10.1046/j.1365-294x.2003.01920.x. doi:10.1046/j.1365-294X.2003.01920.x [DOI] [PubMed] [Google Scholar]
- Streelman J.T, Gmyrek S.L, Kidd M.R, Kidd C, Robinson R.L, Hert E, Ambali A.J, Kocher T.D. Hybridization and contemporary evolution in an introduced cichlid fish from Lake Malawi National Park. Mol. Ecol. 2004;13:2471–2479. doi: 10.1111/j.1365-294X.2004.02240.x. doi:10.1111/j.1365-294X.2004.02240.x [DOI] [PubMed] [Google Scholar]
- Striedter G.F, Northcutt R.G. Head size constrains forebrain development and evolution in ray-finned fishes. Evol. Dev. 2006;8:215–222. doi: 10.1111/j.1525-142X.2006.00091.x. doi:10.1111/j.1525-142X.2006.00091.x [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer; Sunderland, MA: 2002. PAUP*: phylogenetic analyses using parsimony (* and other methods) beta version 4.0. [Google Scholar]
- Thomas R.J, Szekely T, Powell R.F, Cuthill I.C. Eye size, foraging methods and the timing of foraging in shorebirds. Funct. Ecol. 2006;20:157–165. doi:10.1111/j.1365-2435.2006.01073.x [Google Scholar]
- Thompson J.D, Plewniak F, Poch O. A comprehensive comparison of multiple sequence alignment programs. Nucleic Acid Res. 1999;27:2682–2690. doi: 10.1093/nar/27.13.2682. doi:10.1093/nar/27.13.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turingan R.G, Wainwright P.C. Morphological and functional bases of durophagy in the queen triggerfish, Balistes vetula (Pisces, Tetraodontiformes) J. Morphol. 1993;215:101–118. doi: 10.1002/jmor.1052150202. doi:10.1002/jmor.1052150202 [DOI] [PubMed] [Google Scholar]
- Turner G.F, Robinson R.L, Shaw P.W, Carvalho G.R. Identification and biology of Diplotaxodon, Rhamphochromis and Pallidochromis. In: Snoeks J, editor. The cichlid diversity of Lake Malawi/Nyasa: identification, distribution and taxonomy. Cichlid Press; El Paso, TX: 2004. pp. 198–251. [Google Scholar]
- von Herbing H.I, Miyake T, Hall B.K, Boutilier R.G. The ontogeny of feeding and respiration in larval Atlantic cod (Gadus morhua), Teleostei, Gadiformes: (I) morphology. J. Morphol. 1996a;227:15–36. doi: 10.1002/(SICI)1097-4687(199601)227:1<15::AID-JMOR2>3.0.CO;2-O. doi:10.1002/(SICI)1097-4687(199601)227:1<15::AID-JMOR2>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- von Herbing H.I, Miyake T, Hall B.K. The ontogeny of feeding and respiration in larval Atlantic cod (Gadus morhua): (II) function. J. Morphol. 1996b;227:37–50. doi: 10.1002/(SICI)1097-4687(199601)227:1<37::AID-JMOR3>3.0.CO;2-M. doi:10.1002/(SICI)1097-4687(199601)227:1<37::AID-JMOR3>3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- von Scheven G, Alvares L.E, Mootoosamy R.C, Dietrich S. Neural tube derived signals and FGF8 act antagonistically to specify eye versus mandibular arch muscles. Development. 2006;133:2731–2745. doi: 10.1242/dev.02426. doi:10.1242/dev.02426 [DOI] [PubMed] [Google Scholar]
- Wainwright P.C, Bellwood D.R, Westneat M.W, Grubich J.R, Hoey A.S. A functional morphospace for the skull of labrid fishes: patterns of diversity in a complex biomechanical system. Biol. J. Linn. Soc. 2004;82:1–25. doi:10.1111/j.1095-8312.2004.00313.x [Google Scholar]
- Won Y.J, Sivasundar A, Wang Y, Raincrow J, Hey J. Nuclear gene variation and the molecular dating of the cichlid species flock of Lake Malawi. Mol. Biol. Evol. 2006;23:828–837. doi: 10.1093/molbev/msj101. doi:10.1093/molbev/msj101 [DOI] [PubMed] [Google Scholar]