Abstract
Interval timing—sensitivity to elapsing durations—has recently been found to occur in an invertebrate pollinator, the bumble-bee (Bombus impatiens). Here, bumble-bees were required to time the interval between the start of foraging in a patch of low-quality artificial flowers providing 25% sucrose and the availability of a high-quality flower (HQF) that provided 50% sucrose after a fixed delay. The delay changed after every 20 foraging bouts in the order 30–150–30 s. Bees visited the HQF sooner when the delay was 30 s than when it was 150 s, and visits to the HQF peaked near the end of both delays. When the delay changed to 150 s, bees appeared to time both the previous 30 s delay and the new delay. To examine whether bees also learned what kind of reward was provided at the HQF, its usual reward was replaced with 25% sucrose in a final foraging bout. Bumble-bees rejected the HQF on the reward-replacement test. These results show that bumble-bees remembered both when reward was produced by the HQF and what type of reward was produced. These findings indicate that bumble-bees can learn both the timing and content of reward production.
Keywords: interval timing, bumble-bee, Bombus impatiens, pollinator, foraging
1. Introduction
Interval timing—sensitivity to elapsing durations—is thought to function in many different contexts, including communication (von Frisch 1967; Seeley & Tovey 1994), navigation (Srinivasan et al. 2000) and foraging (Stephens & Krebs 1986; Gill 1988; Gallistel 1990; Seeley & Tovey 1994; Clayton & Dickinson 1998; Ohashi 2002; Henderson et al. 2006). Interval timing ability is known to occur in several vertebrates, including humans (Richelle & Lejeune 1980; Lejeune & Wearden 1991), and was recently discovered in bumble-bees, Bombus impatiens (Boisvert & Sherry 2006). Ecological considerations suggest that pollinators might possess keen interval timing abilities; both insect and avian pollinators make a variety of decisions that would appear to require the ability to estimate elapsed durations. Many pollinators show resource fidelity—the repeated use of renewing nectar sources (Thomson et al. 1982; Waser 1986; Chittka et al. 1999; Osborne et al. 1999)—and are therefore faced with the problem of scheduling revisits to resources that vary according to temporal schedules. Traplining—the use of spatially distinct resources in repeatable circuits—is one form of resource fidelity shown by avian and insect pollinators (Heinrich 1979; Gill 1988). Among avian pollinators, traplining hermit hummingbirds (Phaethornis superciliosus) and territorial rufous hummingbirds (Selasphorns rufus) have shown the ability to estimate elapsed durations associated with nectar replenishment under free-living conditions (Gill 1988; Henderson et al. 2006). In addition, invertebrate and vertebrate pollinators may make foraging decisions involving vastly different time-scales, ranging from a few seconds (for intra-patch choices) to several hours (inter-foraging bout choices; Menzel 1999).
Most empirical demonstrations of interval timing rely on operant procedures. The most commonly used method is the fixed-interval (FI) procedure (Ferster & Skinner 1957; Richelle & Lejeune 1980; Lejeune & Wearden 1991; Boisvert & Sherry 2006). In an FI procedure, an animal receives a reward on the first response that occurs after a FI of time has elapsed since presentation of a time marker, often a light cue or food delivery. Two or more FIs can be incorporated within a session to analyse the timing of multiple intervals concurrently, in which case the procedure is described as a mixed-FI schedule. Vertebrates trained on FI procedures withhold responses for one-third to two-thirds of the interval duration, and the maximum rate of responding usually occurs at the end of the interval (Lejeune & Wearden 1991). Under mixed-FI conditions, vertebrates often show two bursts of responding, one at each of the FI values in the mixture (Catania & Reynolds 1968; Leak & Gibbon 1995; Whitaker et al. 2003).
Recently, we tested bumble-bees under conditions in which proboscis extension was reinforced after a fixed duration had elapsed, or after either of two fixed durations had elapsed (Boisvert & Sherry 2006). Across a range of FI values between 6 and 36 s, bees withheld responding for one-third to one-half of the interval durations, and the maximum rates of responding occurred at the end of the intervals. When bees were exposed to multiple FI values during a single session, they often showed two bursts of responding, each located near the specific FI values in the mixture, although on other trials, bees timed only a single FI value.
Although often conceived of as foraging problems, operant procedures may fail to capture the complexity of interval timing in natural contexts. Timing behaviour may be more precise when measured in more naturalistic contexts (Gallistel 1990). In addition, animals may reveal the ability to remember temporal information with other features of the environment in more complex contexts.
In a preliminary experiment, we asked whether bumble-bees would learn to time a delay between the onset of foraging in one patch and the production of reward in another location in a large screened enclosure connected to a bumble-bee colony. Each of 12 artificial flowers in a low-quality patch contained 2.5 μl of 25% sucrose at the start of each foraging bout. A 13th flower—hereafter called the high-quality flower (HQF)—had a distinctive corolla and was located 36 cm from the low-quality patch. This flower was initially empty but produced 40 μl of 50% sucrose after a fixed time-interval that began on the first visit made to any flower in the low-quality patch. Five bees completed an experiment in which the delay was set at 90 s for an initial 20 foraging bouts and then changed to 60 s for a further 20 foraging bouts. Mean wait times—the time until the first visit to the HQF during each foraging bout—were significantly longer when the delay was 90 s (M=80.49 s, s.e.m.=5.18) than when it was 60 s (M=63.00 s, s.e.m. =2.75), t(4)=3.58, p=0.023. Visits to the HQF peaked during the last 15 s of the delay in both delay conditions. These findings suggest that bumble-bees quickly learned to time the delays. However, because the change of delays was confounded with training experience, it is possible that the change in bees' behaviour reflected a process other than timing.
We extended our preliminary findings in a reversal design that included a broader range of delays. We also asked whether bumble-bees could learn about the quality of reward provided by the HQF. As before, a HQF and a patch of 12 low-quality flowers were available at the start of each foraging bout. Following either 30 or 150 s, the HQF produced 40 μl of 50% sucrose. The delay changed after every 20 foraging bouts according to an ABA design, with the A stages characterized by the 30 s delay and the B stage characterized by the 150 s delay. On a 61st foraging bout, the reward provided by the HQF was replaced (unbeknownst to the bee) with 25% sucrose.
2. Material and methods
(a) Study animals and housing
Female worker bumble-bees (B. impatiens) lived in commercially prepared hives (Biobest Canada Ltd, Leamington, Ontario), which were housed in the laboratory and provided with pollen daily. Individual workers were uniquely identified with coloured nail polish applied to the thorax. Data were collected on five bees in this experiment. None of the bees had previous foraging experience.
(b) Apparatus
The experiment was conducted in the laboratory in a wire-mesh screened enclosure adapted from Gegear & Laverty (1998) connected to the colony. The enclosure measured 122.5 cm×91.5 cm (floor dimensions)×93.5 cm. Bees entered the chamber through a Plexiglas tunnel. The floor of the enclosure was Styrofoam, covered with green construction paper. Flowers were constructed from blue, 1.5 ml centrifuge tubes (Gordon Technologies, Inc., Mississauga, Ontario) with caps removed. A corolla was fitted to the open end of each low-quality flower using a round piece of blue acetate (3 cm in diameter). The corolla used for the HQF was blue with black hatching. Eppendorf micropipettes (Brinkmann Instruments Ltd, Mississauga, Ontario) were used to dispense 2.5 μl of 25% sucrose solution in each of the low-quality flowers. A syringe fitted with 100 cm of tubing (Clay Adams, PE-90) was used to deliver 40 μl of 50% sucrose solution to the HQF from outside the screened enclosure. The experimenter remained in a fixed position in the testing room during the experiment, and delivery of reward was not signalled by any acoustic, olfactory or movement cues. Flowers were placed in holes in the Styrofoam floor of the enclosure, with 12 cm between the adjacent flowers in the low-quality patch and 36 cm between the HQF and the nearest flower in the low-quality patch.
(c) Procedure
(i) Pre-training phase
To ensure bees had equivalent foraging experience, each bee received five trials with 30 low-quality flowers available in the enclosure, followed by five trials with only the HQF, containing 40 μl of 50% sucrose, available. ‘Trial’ and ‘foraging bout’ are used here synonymously and refer to the period between a bee's entrance and exit from the enclosure.
(ii) Training phase
Twelve baited flowers in the low-quality patch and a single unbaited HQF were available at the start of each foraging bout. A bee was permitted to enter the screened enclosure and forage freely. The experimenter started a timer on the first reward taken in the low-quality patch. Drained flowers in the low-quality patch were not refilled during a foraging bout. Immediately after the fixed delay elapsed, the experimenter filled the HQF with 40 μl of 50% sucrose solution using the syringe located outside the enclosure. The foraging bout ended when the bee exited the enclosure. Following each foraging bout, all flowers and corollas were washed with unscented dish soap and new flowers were used for the next trial. Each bee completed 61 foraging bouts. The fixed delay changed after every 20 foraging bouts according to an ABA reversal design, with a 30 s delay used for foraging bouts 1–20 and 41–60, and a 150 s delay used for foraging bouts 21–40. The 61st foraging bout was a reward-replacement test in which the HQF was filled after a 30 s delay with 40 μl of 25% rather than the usual 50% sucrose. All trials were videotaped for later analysis. We recorded all flowers visited, the time of each visit and the time spent taking sucrose solution from the HQF.
(d) Data analysis
The number of low-quality flowers drained and wait times—the time elapsed to the first visit to the HQF—were taken from the last five foraging bouts during each delay phase in the experiment and analysed by repeated measures ANOVA. Tukey's HSD tests were used for post hoc comparisons. Inter-flower movements were analysed from videotape. Visits to the HQF were collected in 15 s time bins throughout each delay and compiled from all foraging bouts. Alpha was set at 0.05 for all analyses.
3. Results and discussion
(a) Behaviour in the low-quality patch
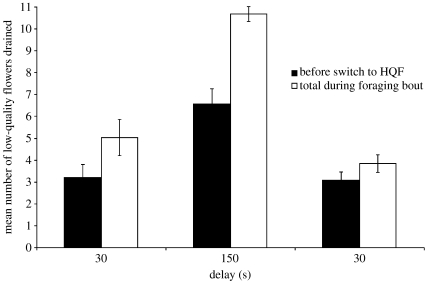
The number of flowers drained before switching to the HQF (figure 1, filled bars) differed across the three phases of the experiment, F(2,8)=26.90, p<0.001. Bumble-bees drained more flowers during the 150 s delay phase than during both 30 s delay phases (p<0.01). The number of flowers drained did not differ between the 30 s delay phases. The total number of low-quality flowers drained (figure 1, open bars) also differed across the phases of the experiment, F(2,8)=32.97, p<0.001. The total number of flowers drained during the 150 s delay phase exceeded the number drained in each of the 30 s delay phases (p<0.01), while the numbers drained in the two 30 s phases did not differ statistically. A comparison between the number of low-quality flowers drained before switching to the HQF and the total number drained for each delay phase showed significant differences for the first 30 s delay and the 150 s delay phases (p<0.05).
Figure 1.
Mean number of low-quality flowers drained during 30 and 150 s delay conditions. The mean number of low-quality flowers drained over the last five foraging bouts is shown for each condition. Filled bars show the number of low-quality flowers drained before switching to the HQF and open bars show the total number of low-quality flowers drained during each foraging bout. Error bars are standard errors of the mean.
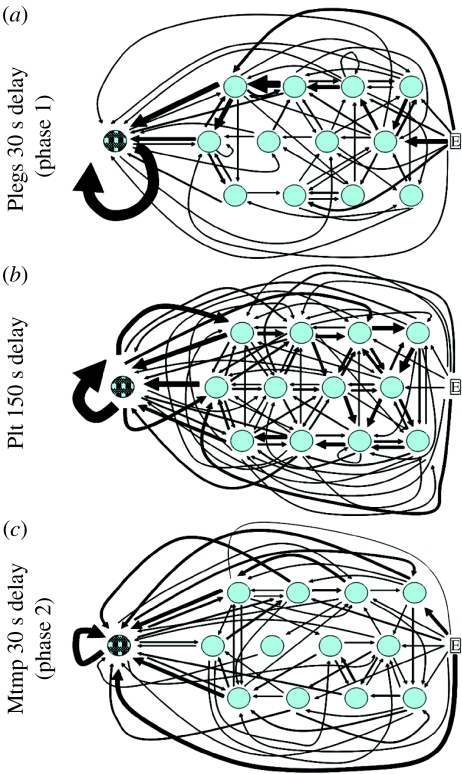
To examine whether bumble-bees adopted systematic routes of travel among flowers in the low-quality patch (i.e. traplining), we examined movements between all possible flower pairs. For each foraging bout, we recorded the first flower visited upon entering the enclosure and all visits thereafter. We then counted the number of visits between all combinations of flower pairs. Frequency counts were used to construct schematics for each delay phase for each bee. Representative diagrams for three bees are shown in figure 2. The frequency of movements between two flowers is shown in the diagrams by the thickness of the arrows; the thicker the arrow, the more frequent the visits. In figure 2, the thickest arrow corresponds to 20 movements in the direction indicated, while the thinnest arrows correspond to a single movement. There was no evidence for systematic traplining by any of the bees; a bee arriving at a flower did not always depart in the same direction. Some inter-flower movements, however, were common. These included movements from the three flowers nearest the HQF to the HQF and movements from the HQF back to the HQF (figure 2). Upon entering the enclosure, some bees often visited the HQF before visiting a low-quality flower (figure 2c).
Figure 2.
Inter-flower movements. Frequency of movements by three bees between all possible flower pairs for all foraging bouts. Thicker arrows indicate more frequent movements. Blue circles show low-quality flowers and the blue/black hatched circle represents the HQF. ‘E’ is the entrance to the enclosure from the colony. The abbreviations (a) Plegs, (b) Plt and (c) Mtmp identify individual bees.
(b) Behaviour at the HQF
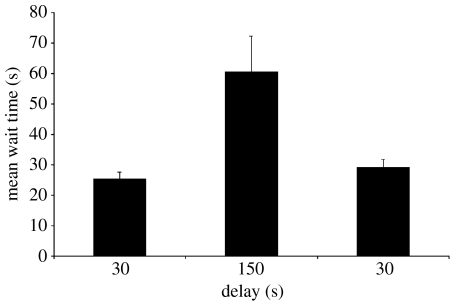
Wait times (figure 3) differed across the three phases of the experiment, F(2,8)=19.38, p=0.001. Bumble-bees waited longer to visit the HQF when the delay was 150 s than when it was 30 s, whether the 30 s delay preceded (p<0.01) or followed (p<0.01) the longer delay. Wait times did not differ between the first and second 30 s delay phases.
Figure 3.
Mean wait times for 30 and 150 s delay conditions. Wait times (time elapsed to the first visit to the HQF) were averaged over the last five foraging bouts in each condition. Error bars are standard errors of the mean.
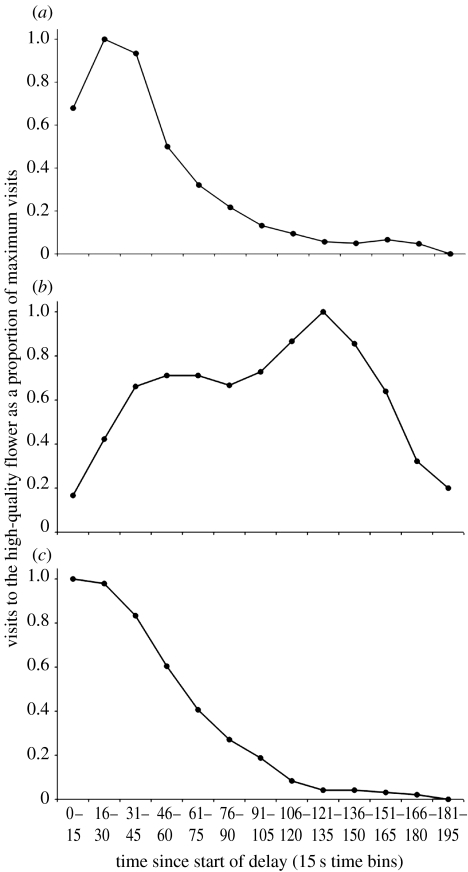
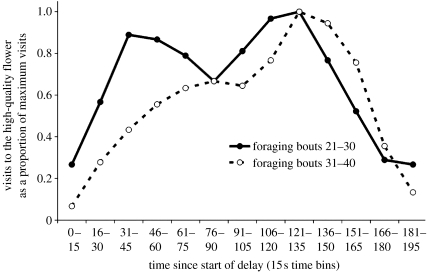
Visits to the HQF also varied as a function of time elapsed during the delay (figure 4). Visits to the HQF peaked before the 30 s delay elapsed in both the first and second 30 s delay phases. When all foraging bouts during the 150 s phase are considered, visits peak between 121 and 135 s. Visits during the first and second block of 10 foraging bouts during this phase are shown in figure 5. During the first block, the function is clearly bimodal, with an early peak at 31–45 s and a later peak at 136–150 s. This suggests that bees continued to time the previously experienced 30 s delay while they learned to time the 150 s delay. During the second block of 10 foraging bouts, the function became unimodal, with a single peak at 121–135 s, suggesting that bees had adjusted to the longer delay. Bumble-bees therefore quickly changed the timing of their visits to the HQF within 10–20 foraging bouts in anticipation of reward production and may have timed both the short and long durations concurrently soon after the switch to the 150 s delay.
Figure 4.
Visits to the HQF during 30 and 150 s delay conditions. Visits are shown as a proportion of maximum visits, recorded in 15 s time bins. Visits were aggregated across foraging bouts and then total visits in each time bin were divided by the maximum value (see Boisvert & Sherry 2006 for details). Data from (a) the first 30 s delay phase, (b) the 150 s delay phase and (c) the second 30 s delay phase.
Figure 5.
Visits to the HQF during the 150 s delay condition. Visits are shown as a proportion of maximum visits, recorded in 15 s time bins. The solid line shows data from the first half of the 150 s delay phase (foraging bouts 21–30), while the dashed line shows data from the second half of the 150 s delay phase (foraging bouts 31–40).
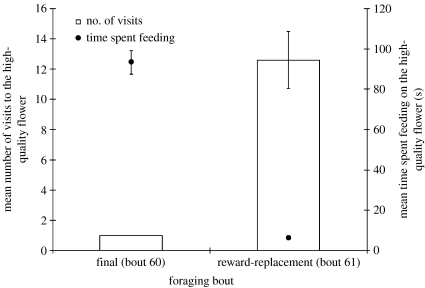
Bumble-bees clearly learned when reward was produced by the HQF. Learning to time the delay to reward production, however, did not require that they have any expectation about the quality of the reward. At least three properties of the HQF made it superior to the low-quality flowers: it had a greater sugar concentration and a greater volume, and involved lower flight costs once it produced reward (because there was no further need to switch flowers). The most salient of these properties may have been its sucrose concentration. To assess whether bees formed expectations about the sucrose concentration—in addition to learning when it would occur—bees completed an additional 61st foraging bout in which the reward produced by the HQF was replaced with 25% sucrose. The question of interest was: how would bees behave upon discovering the 25% sucrose in the HQF? On reward-replacement tests, we recorded the number of visits to the HQF that occurred after the delay had elapsed and the time spent feeding during the first of these visits. These data were compared to the final foraging bout during the second 30 s delay phase (the 60th foraging bout). All bees made a single visit to the HQF following the delay on the 60th foraging bout and fed on the 50% sucrose for approximately 90 s. In contrast, bees spent an average of 6 s on the HQF on the first visit after the delay and rejected it repeatedly before returning to the colony. Significantly more visits were made to the HQF following delay on the reward-replacement test than on the 60th foraging bout, t(4)=6.15, p=0.004. Less time was spent on the flower during the first visit following the delay on the reward-replacement test than on the previous foraging bout, t(4)=14.49, p<0.001. These data are shown in figure 6. Bumble-bees rejected 25% sucrose when it was offered by the HQF, despite having just accepted 25% sucrose rewards from low-quality flowers during the same foraging bout. It was striking that bees often returned to the low-quality patch and consumed 25% sucrose from low-quality flowers instead of from the HQF during the reward-replacement test. The consumption of 25% sucrose cannot therefore account for the bees' behaviour on the reward-replacement test. It was, instead, the unexpected occurrence of 25% sucrose at the HQF that caused bees to reject the HQF on these trials. This indicates that bumble-bees learned not only when the high-quality reward would be available, but also what kind of reward it provided.
Figure 6.
Number of visits and time spent feeding on the HQF. Bars show the number of visits to the HQF after the delay elapsed during the final foraging bout and the reward-replacement test. Circles show the time spent feeding on the HQF on the first visit after the delay elapsed during the final foraging bout and the reward-replacement test. The time-interval equalled 30 s on these foraging bouts. Error bars are standard errors of the mean.
4. General discussion
The behaviour of bumble-bees in the foraging problem described here suggests that bees are able to adjust their behaviour to the temporal structure of a complex foraging environment while remembering non-temporal information about the concentration of floral nectar rewards available. Their ability to do so developed rapidly, in as few as 20 foraging bouts. The task used here differs in a number of ways from the procedures usually used to examine interval timing. In most operant FI studies, only a narrow range of behaviour is possible in the conditioning chamber, few stimuli compete for a subject's attention with those associated with the duration to be timed and the interval itself is signalled by a single stimulus event, such as the onset and offset of a key light. Here, bumble-bees could perform a broad range of behaviour in the screened enclosure, many stimuli could compete for the bee's attention and the event that marked the onset of the interval to be timed was the bee's own behaviour at one of many similar stimuli, the low-quality flowers. In addition, during any given foraging bout, bumble-bees may have been monitoring which low-quality flowers had already been depleted.
Natural foraging behaviour of insect and avian pollinators often seems to imply the ability to track elapsing time. Some bumble-bees and hummingbirds are known to visit specific resources in repeatable sequences (Heinrich 1979; Thomson et al. 1982, 1997). Faithful use of resources whose nectar availability varies with time raises the possibility that such fidelity reflects knowledge of the temporal properties of reward availability. Gill (1988) showed that free-living traplining hermit hummingbirds were able to adjust revisitation intervals to feeders to closely match experimentally imposed refill intervals. Similarly, Henderson et al. (2006) showed that territorial rufous hummingbirds learned the timing of reward availability for artificial flowers across the delays several minutes long. It is unknown whether bumble-bees can learn to adjust their behaviour under similar conditions. Under the current conditions, bumble-bees did not show systematic traplining within the low-quality patch, although movements between some pairs of flowers within the patch were more frequent than others. On the other hand, bees were required to visit the low-quality patch in order to obtain reward in the HQF, i.e. they had to trapline from the low-quality patch to the HQF. Here, traplining at the level of the patch was mediated by timing the delay associated with reward production by the HQF. Our findings therefore indicate that interval timing can mediate some (albeit simple) forms of trapline foraging.
Bees could have timed the delays in different ways. For example, bees may have relied on internal timing mechanisms to guide behaviour during the delay. Alternatively, they may have used their own behaviour to indicate when to visit the HQF. The notion that behaviour mediates timing performance by animals is formalized in the behavioural theory of timing (Killeen & Fetterman 1988). Key evidence for the latter would include systematic behaviour changes during the delay. As noted above, although bees' inter-flower movements were often biased in favour of certain pairs, bumble-bees did not move through the low-quality patch on traplines. Moreover, there was no indication that bees displayed distinct categories of behaviour (such as grooming, resting, etc.), which could be correlated with time elapsed during the delay. Bees rarely did anything but forage until they had depleted the HQF. In another series of experiments examining interval timing by bumble-bees (M. J. Boisvert 2006, Unpublished doctoral dissertation), bees' performance was inconsistent with the behavioural theory of timing; no systematic changes in behaviour occurred during FI trials in which bees timed short delays in an automated chamber.
Bees drained twice as many low-quality flowers before switching to the HQF on the 150 s delay as they did on the 30 s. Given that average wait times were twice as long on the 150 s delay as they were on the 30 s delay, this might indicate that bees used one rule like ‘visit the HQF after draining n low-quality flowers’ during the 30 s delay and another rule like ‘visit the HQF after draining 2n low-quality flowers’ during the 150 s delay. One observation noted during training is instructive here. It was not unusual upon entering the enclosure for bees to visit the HQF before visiting any low-quality flower. However, this rarely occurred during the 150 s delay phase. This suggests that bees were not using a counting rule (because the count would be zero at the start of a trial), nor were they using a ‘check HQF first’ rule (because they only did this on 30 s delay trials). The pattern of behaviour shown by bees in these experiments is best explained by an internal timing mechanism, rather than a counting strategy.
One aspect of the present procedure has implications for theories of timing. Unlike traditional FI procedures, multiple rewards were collected while the delay interval elapsed. Most theories of animal interval timing have been advanced to explain the performance of animals trained in operant procedures such as the FI schedule in which a subject times a duration between successive food rewards. Scalar expectancy theory (Gibbon 1977, 1991) proposes that animals compare an estimate of current elapsing time with a value of remembered time sampled from memory. The occurrence of a biologically meaningful event, such as food, is proposed to trigger encoding of a time estimate in memory. Tuned-trace theory (Staddon et al. 2002) proposes that each occurrence of reward leaves a memory trace for the time marker, whereupon the trace decays according to a negatively accelerating function. Tuned-trace theory proposes that animals remember the level of a trace associated with the previous reward and respond when a particular trace value is reached. Here, bees were required to time the interval between the first of several low-quality rewards and the high-quality reward. Both scalar expectancy theory and tuned-trace theory would seem to have difficulty explaining bumble-bees' timing behaviour in the present experiment because multiple low-quality rewards were typically collected during the interval to be timed.
The results of this experiment show that bumble-bees can accurately time the duration of intervals up to 150 s in a foraging context, which captures some features of natural foraging for floral nectar in the wild. Bumble-bees showed wait times and peaks in responding comparable with those described for vertebrates in studies of timing. The results further show that bumble-bees can learn the duration of time-intervals and respond to changes in interval durations relatively quickly, in this study within 20 foraging trials. In addition to learning when reward was produced, bumble-bees also learned what nectar concentration was produced.
Acknowledgments
We wish to thank W. A. Roberts for helpful comments, Alexa Tullett for help with observations and J. Ladich for constructing the screened enclosure. This research was supported by a grant from the Natural Science and Engineering Research Council of Canada to D.F.S. We declare no financial conflicts of interest.
References
- Boisvert M.J, Sherry D.F. Interval timing by an invertebrate, the bumble bee Bombus impatiens. Curr. Biol. 2006;16:1636–1640. doi: 10.1016/j.cub.2006.06.064. doi:10.1016/j.cub.2006.06.064 [DOI] [PubMed] [Google Scholar]
- Catania A.C, Reynolds G.S. A quantitative analysis of responding maintained by interval schedules of reinforcement. J. Exp. Anal. Behav. 1968;11:327–383. doi: 10.1901/jeab.1968.11-s327. doi:10.1901/jeab.1968.11-s327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L, Thomson J.D, Waser N.M. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften. 1999;86:361–377. doi:10.1007/s001140050636 [Google Scholar]
- Clayton N.S, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. doi:10.1038/26216 [DOI] [PubMed] [Google Scholar]
- Ferster C.B, Skinner B.F. Appleton-Century-Crofts; New York, NY: 1957. Schedules of reinforcement. [Google Scholar]
- Gallistel C.R. MIT Press; Cambridge, MA: 1990. The organization of learning. [Google Scholar]
- Gegear R.J, Laverty T.M. How many flower types can bumble bees work at the same time? Can. J. Zool. 1998;76:1358–1365. doi:10.1139/cjz-76-7-1358 [Google Scholar]
- Gibbon J. Scalar expectancy theory and weber's law in animal timing. Psychol. Rev. 1977;84:279–325. doi:10.1037/0033-295X.84.3.279 [Google Scholar]
- Gibbon J. Origins of scalar timing. Learn. Motiv. 1991;22:3–38. doi:10.1016/0023-9690(91)90015-Z [Google Scholar]
- Gill F.B. Trapline foraging by hermit hummingbirds: competition for an undefended, renewable resource. Ecology. 1988;69:1933–1942. doi:10.2307/1941170 [Google Scholar]
- Heinrich B. Harvard University Press; Cambridge, MA: 1979. Bumblebee economics. [Google Scholar]
- Henderson J, Hurly T.A, Bateson M, Healy S.D. Timing in free-living rufous hummingbirds Selasphorus rufus. Curr. Biol. 2006;16:512–515. doi: 10.1016/j.cub.2006.01.054. doi:10.1016/j.cub.2006.01.054 [DOI] [PubMed] [Google Scholar]
- Killeen P.R, Fetterman J.G. A behavioral theory of timing. Pychol. Rev. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. doi:10.1037/0033-295X.95.2.274 [DOI] [PubMed] [Google Scholar]
- Leak T.M, Gibbon J. Simultaneous timing of multiple intervals: implications of the scalar property. J. Exp. Psychol. Anim. Behav. Process. 1995;21:3–19. doi:10.1037/0097-7403.21.1.3 [PubMed] [Google Scholar]
- Lejeune H, Wearden J.H. The comparative psychology of fixed-interval responding: some quantitative analyses. Learn. Motiv. 1991;22:84–111. doi:10.1016/0023-9690(91)90018-4 [Google Scholar]
- Menzel R. Memory dynamics in the honeybee. J. Comp. Physiol. A. 1999;185:323–340. doi:10.1007/s003590050392 [Google Scholar]
- Ohashi K. Consequences of floral complexity for bumblebee-mediated geitonogamous self-pollination in Salvia nipponica Miq (Labiatae) Evoltion. 2002;56:2414–2423. doi: 10.1111/j.0014-3820.2002.tb00167.x. doi:10.1554/0014-3820(2002)056[2414:COFCFB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Osborne J.L, Clark S.J, Morris R.J, Williams I.H, Riley J.R, Smith A.D, Reynolds D.R, Edwards A.S. A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J. Appl. Ecol. 1999;36:519–533. doi:10.1046/j.1365-2664.1999.00428.x [Google Scholar]
- Richelle M, Lejeune H. Pergamon Press; New York, NY: 1980. Time in animal behaviour. [Google Scholar]
- Seeley T.D, Tovey C.A. Why search time to find a food-storer bee accurately indicates the relative rates of nectar collecting and nectar processing in honey bee colonies. Anim. Behav. 1994;47:311–316. doi:10.1006/anbe.1994.1044 [Google Scholar]
- Srinivasan M.V, Zhang S.W, Altwein M, Tautz J. Honeybee navigation: nature and calibration of the “odometer”. Science. 2000;287:851–853. doi: 10.1126/science.287.5454.851. doi:10.1126/science.287.5454.851 [DOI] [PubMed] [Google Scholar]
- Staddon J.E.R, Chelaru I.M, Higa J.J. A tuned-trace theory of interval-timing dynamics. J. Exp. Anal. Behav. 2002;77:105–124. doi: 10.1901/jeab.2002.77-105. doi:10.1901/jeab.2002.77-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D.W, Krebs J.R. Princeton University Press; Princeton, NJ: 1986. Foraging theory. [Google Scholar]
- Thomson J.D, Maddison W.P, Plowright R.C. Behavior of bumble bee pollinators of Aralia hispida Vent. (Araliaceae) Oecologia. 1982;54:326–336. doi: 10.1007/BF00380001. doi:10.1007/BF00380001 [DOI] [PubMed] [Google Scholar]
- Thomson J.D, Slatkin M, Thomson B.A. Trapline foraging by bumble bees: II. Definition and detection from sequence data. Behav. Ecol. 1997;8:199–210. doi:10.1093/beheco/8.2.199 [Google Scholar]
- von Frisch K. Harvard University Press; Cambridge, MA: 1967. The dance language and orientation of bees. [Google Scholar]
- Waser N.M. Flower constancy: definition, cause and measurement. Am. Nat. 1986;127:593–603. doi:10.1086/284507 [Google Scholar]
- Whitaker S, Lowe C.F, Wearden J.H. Multiple-interval timing in rats: performance on two-valued mixed fixed-interval schedules. J. Exp. Psychol. Anim. Behav. Process. 2003;29:277–291. doi: 10.1037/0097-7403.29.4.277. doi:10.1037/0097-7403.29.4.277 [DOI] [PubMed] [Google Scholar]