Abstract
The palaeoecology of basal turtles from the Late Triassic was classically viewed as being semi-aquatic, similar to the lifestyle of modern snapping turtles. Lately, this view was questioned based on limb bone proportions, and a terrestrial palaeoecology was suggested for the turtle stem. Here, we present independent shell bone microstructural evidence for a terrestrial habitat of the oldest and basal most well-known turtles, i.e. the Upper Triassic Proterochersis robusta and Proganochelys quenstedti. Comparison of their shell bone histology with that of extant turtles preferring either aquatic habitats or terrestrial habitats clearly reveals congruence with terrestrial turtle taxa. Similarities in the shell bones of these turtles are a diploe structure with well-developed external and internal cortices, weak vascularization of the compact bone layers and a dense nature of the interior cancellous bone with overall short trabeculae. On the other hand, ‘aquatic’ turtles tend to reduce cortical bone layers, while increasing overall vascularization of the bone tissue. In contrast to the study of limb bone proportions, the present study is independent from the uncommon preservation of appendicular skeletal elements in fossil turtles, enabling the palaeoecological study of a much broader range of incompletely known turtle taxa in the fossil record.
Keywords: bone histology, palaeoecology, Testudinata, Proganochelys quenstedti, Proterochersis robusta
1. Introduction
The phylogenetic position of turtles within the Amniota remains one of the most enigmatic and controversial problems in vertebrate systematics (see Zardoya & Meyer 2001 for an overview). The peculiar bauplan of the turtles with a body encased in a rigid shell composed of a dorsal carapace and a ventral plastron makes morphological and osteological comparison with other vertebrate groups difficult. Therefore, it is very important to broaden our knowledge of potentially informative fields other than the osteological description of specimens to help in evaluating the competing hypotheses of turtle origins. The palaeoecology and taphonomic situation of fossil turtles is one such field. In the case of well-known stem turtles, i.e. the Upper Triassic Proganochelys quenstedti and Proterochersis robusta, their palaeoecology was classically interpreted to be semi-aquatic (Gaffney 1990). On the other hand, Upper Triassic Palaeochersis talampayensis from Argentina, South America and Lower Jurassic Australochelys africanus from South Africa were proposed to have a more terrestrial ecology than the purportedly semi-aquatic forms (Gaffney & Kitching 1994; Rougier et al. 1995). In the monographic compendium on the osteology of Proganochelys, Gaffney (1990, p. 25) noted that this basal turtle was probably roughly similar to the American snapper Macroclemys temminckii in ‘size, possible habitat and some morphologic features’, and the resulting life restoration (Gaffney 1990; figure 1) included a swimming P. quenstedti, underscoring a purportedly semi-aquatic habitat preference.
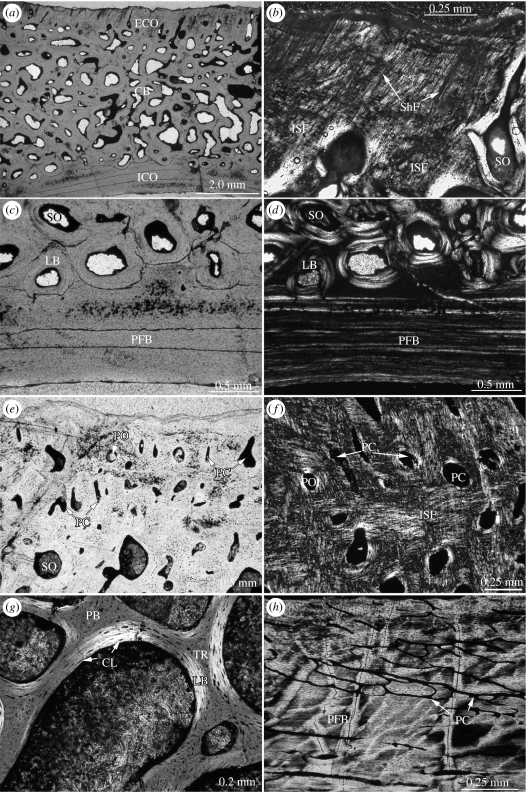
Figure 1.
Shell bone histology of extant turtles with (a–d) terrestrial and (e–h) aquatic ecologies: (a) complete thin section of right hyoplastron of C. picturata. The diploe structure of the bone has a robust appearance with well-developed thick and compact external and internal cortices, as is usually found in terrestrial turtles. Note typical cross-polarization of secondary osteons at transition of external cortex and interior cancellous bone; (b) close-up of interwoven structural fibre bundles (ISFs) of external cortex of right hypoplastron of G. pardalis. The bone tissue is only weakly vascularized by scattered thin primary vascular canals; (c) close-up of external cortex of costal of Terrapene carolina triunguis that, similarly to (a) and (b), consists of the dense ISFs and low vascularization typical for terrestrial turtles; (d) close-up of mainly avascular parallel-fibred bone of internal cortex of hyoplastron of Terrapene carolina triunguis. Vascularization of internal cortices is generally low in the terrestrial turtle taxa; (e) thin section of complete costal of C. fimbriatus. Note irregular ISF of external cortex and reduced thickness of internal cortex. The interior cancellous bone is dominated by slender bone trabeculae and irregular large vascular cavities, giving the bone a less compact appearance; (f) close-up of scalloped external cortex of neural3 of Chelydra serpentina, highly vascularized by primary vascular canals. Many canals end in small foramina at the external bone surface; (g) close-up of external cortex of left costal2 of Caretta caretta, extremely vascularized by circular primary vascular canals, primary osteons and secondary osteons; (h) close-up of internal cortex of left costal2 of Caretta caretta, highly vascularized by circular primary vascular canals and secondary osteons. Although not as strongly reduced in thickness, the compact nature of the internal cortex is lost based on the high vascularization; AT, adipose tissue; CB, cancellous bone; CL, bone cell lacunae; ECO, external cortex; GM, growth mark; ICO, internal cortex; ISF, interwoven collagenous structural fibre bundles; KS, keratinous shield; PC, primary vascular canal; PFB, parallel-fibred bone; PO, primary osteon; SO, secondary osteon.
Recently, however, Joyce & Gauthier (2004) forcefully argued for terrestrial habitats of P. quenstedti and P. talampayensis. The material of P. robusta could not be included in the study of Joyce & Gauthier (2004) since it lacks forelimbs that could be adequately measured. Based on forelimb proportion measurements, the authors were able to show that these basal turtles plotted in an ecological field which is defined by extant turtles living in terrestrial habitats.
The aim of this study is to test the ‘semi-aquatic habitat’ hypothesis against this new ‘terrestrial habitat’ hypothesis for basal turtles by analysing turtle shell bone microstructures. Our basic assumption is that, comparable with modern turtle taxa, the shell bones of basal turtle taxa are influenced to similar degrees by ecology, phylogeny and lifestyle. Thus, to elucidate the palaeoecology of the two stem Testudinata P. quenstedti and P. robusta, the shell bone histologies of extant turtles that prefer terrestrial habitats in comparison with those that primarily live in aquatic environments were analysed. A previous study has already shown that shell bone microstructures can be influenced by both phylogenetic and functional constraints to various degrees (Scheyer & Sánchez-Villagra 2007). However, especially in vertebrate groups that are secondarily adapted to an aquatic lifestyle, bone growth and microstructure, e.g. long bones and vertebrae, are predominantly influenced by functional adaptation to the buoyancy-giving habitat (Buffrénil & Schoevaert 1988; Ricqlès 1989; Buffrénil & Mazin 1990; Ricqlès & Buffrénil 2001; Scheyer & Sánchez-Villagra 2007).
In the first step, we will describe shell bone microstructures of a representative selection of extant turtles that dwell in either aquatic or terrestrial environments (figure 1). These taxa are part of a larger survey of turtle shell bone histology covering 36 extant and 66 fossil taxa from most suprageneric turtle taxa (Scheyer in press) and were chosen because they cover a large part of the phylogenetic tree of turtles, ensuring that functional influences on bone histology can easily be separated from phylogenetic ones. By choosing six extant turtle taxa to be exemplarily described, we further attempted to cover a representative size range, including small, medium and large species. In the second step, we will focus on the shell bone histology of P. quenstedti and P. robusta in detail, because this is paramount to future turtle shell bone descriptions (figure 2).
Figure 2.
Shell bone histology of Upper Triassic basal turtles (a–d) P. quenstedti and (e–h) P. robusta: (a) section of costal of P. quenstedti showing the compact diploe structure of the shell bone in normal transmitted light. As in terrestrial turtles (figure 1a), the diploe is well developed with thick external and internal compact cortices; (b) close-up of external cortex of former specimen in polarized light. Note the differences between Sharpey's fibres and ISFs. The bone tissue is weakly vascularized (compare to figure 1c); close-up of transition from interior cancellous bone to compact internal cortex of costal in (c) normal transmitted and in (d) polarized light. The transition is dominated by secondary osteons, the internal cortex by avascular parallel-fibred bone (compare to Figure 1d); (e) close-up of external cortex of peripheral of P. robusta in normal transmitted light; (f) close-up of the external cortex of peripheral of P. robusta in polarized light. The bone tissue is vascularized by scattered primary osteons and primary vascular canals; (g) close-up of bone trabeculae of interior cancellous bone of former specimen in polarized light and lambda compensator. Bone trabeculae are primary but lined with secondary lamellar bone; (h) close-up of internal cortex of plastron fragment (hyo- or hypoplastron) of P. robusta in polarized light, showing parallel-fibred bone tissue vascularized by a loose network of thin primary vascular canals (with pyrite infill). Note that the internal cortex thus shows a slightly raised vascularization compared with P. quenstedti. However, the level of vascularization comprises only a fraction of the vascularization and remodelling observed in the aquatic species (compare to figure 1e,h); CB, cancellous bone; CL, bone cell lacunae; ECO, external cortex; ICO, internal cortex; ISF, interwoven collagenous structural fibre bundles; LB, lamellar bone; PB, primary bone; PC, primary vascular canal; PFB, parallel-fibred bone; PO, primary osteon; ShF, Sharpey's fibres; SO, secondary osteon; TR, bone trabeculae.
In addition to representing a novel approach to the palaeoecology of fossil turtles, this study greatly increases the palaeoecological sample size because it is independent from the necessity of adequate limb preservation in fossil turtles that otherwise would be needed for proportion measurements sensu Joyce & Gauthier (2004).
2. Material and methods
The majority of fossil and extant turtles have a more or less aquatic lifestyle, and among living turtles, the most obvious terrestrial taxa belong to Testudinoidea (Bataguridae, Emydidae and Testudinidae). Although a broad taxonomic range of fossil and extant terrestrial and aquatic turtles was analysed by Scheyer (in press), and a categorization of the ecological adaptation of turtles based on histological gradations of the shell bones was attempted in that study, it goes beyond the scope of the current study to incorporate all the results of Scheyer (in press). Therefore, only a simplified categorization is given herein by concentrating on a few representative taxa and providing lists of taxa that share similar results, respectively, as electronic supplementary material. Although fossils are included in the electronic supplementary material, we focus on the well-known extant taxa in §4 because habitat preference is usually well studied and much more reliable in extant taxa. To represent the bone histology of terrestrial turtles, three extant testudinoid taxa were chosen. Taxa compiled in electronic supplementary material were found to share the terrestrial configuration in their shell bone microstructures, thus indicating that a phylogenetic instead of an adaptive signal in the shell bones can be ruled out.
(a) Extant terrestrial turtles
The African leopard tortoise Geochelone pardalis (Bell 1828; Testudinidae), the common box turtle Terrapene carolina triunguis (Agassiz 1857; Emydidae) and the Indochinese box turtle Cuora picturata (Stuart & Parham 2004), formerly known as C. galbinifrons picturata (Lehr et al. 1998; Bataguridae), were chosen to be presented in detail in §3. Although Cuora spp. constitute a group of semi-aquatic turtles, C. picturata was included in the study assuming that it shares habitat preferences with C. galbinifrons sensu lato which, according to Ernst & Barbour (1989, p. 149), is ‘probably the least aquatic species of Cuora’. Habitat selection among species similarly occurs in the genus Terrapene. For the sampled T. c. triunguis, Reagan (1974) noted multiple habitat changes from grassland to woodlands in spring, summer and autumn depending on temperature and humidity. The specimen of G. pardalis, a large species that occurs in savannah, bush and grassland habitats (Ernst & Barbour 1989), has a straight plastron length (SPL) of 245 mm (possibly subadult specimen), and the sampled specimen of C. picturata has an SPL of 151 mm. Although the carapace of the specimen of T. c. triunguis was fairly crushed prior to sampling, the plastron was still whole, having an SPL of 126 mm.
(b) Extant aquatic turtles
Three turtles, the common snapping turtle Chelydra serpentina (Linnaeus 1758; Chelydridae), the South American matamata Chelus fimbriatus (Schneider 1783; Chelidae) and the marine loggerhead turtle Caretta caretta (Linnaeus 1758; Cheloniidae), were chosen to represent a broad taxonomic sample of aquatic turtles. Chelydra serpentina and C. fimbriatus are freshwater turtles that prefer slow-moving water bodies, but C. serpentina occasionally enters brackish waters (Kinneary 1993). Both the turtles are bottom walkers; however, more active swimmers are also listed in the electronic supplementary material. The complete plastron length of C. serpentina was not measurable, because only the right hyo- and hypoplastron were obtained for histologic sectioning. However, as the medial anteroposterior extension of hyo- and hypoplastron spans 170 mm, the reconstructed plastron length is approximately 280 mm. The sampled C. fimbriatus (FMNH 269459) has an SPL of approximately 338 mm. Turtles that share histological details (Scheyer in press) with C. serpentina and C. fimbriatus are compiled in the electronic supplementary material. Hatchlings of C. caretta live in pelagic environments (Bowen et al. 1995), while the older individuals generally prefer near-shore habitats. The sampled individual of C. caretta had an SPL of approximately 425 mm (possibly subadult specimen). Taxa that more closely resemble C. caretta in their histology are compiled in the electronic supplementary material (after Scheyer in press).
(c) Fossil basal turtles
The well-preserved Late Triassic taxa P. quenstedti (Baur 1887) and P. robusta (Fraas 1913) from southern Germany are chosen to represent the basal Testudinata. Although Priscochelys hegnabrunnensis, a single shell fragment recently described from the Middle Triassic of southwestern Germany by Joyce & Karl (2006) may be older and may occupy the basal-most position in Testudinata to date, P. quenstedti is still the most basal well-preserved turtle known and the slightly more derived P. robusta from the lower Löwenstein Formation (Early Norian, Late Triassic) remains the oldest well-preserved turtle. Proterochersis robusta was and still is thought to be a stem pleurodire by some authors (Gaffney & Meylan 1988; Gaffney et al. 2006). All studies that score P. robusta as an independent terminal taxon, however, recover this taxon as a basal turtle instead (Rougier et al. 1995; Sukhanov 2006; Joyce 2007). According to Sukhanov (2006), P. talampayensis, recognized as a member of the Australochelyidae by Rougier et al. (1995), occupies an intermediate position between P. quenstedti and P. robusta. The sample of P. quenstedti included a fragment of crushed shell with bits of a plastron fragment and a peripheral (SMNS 17203; Gaffney 1990, pp. 150–151, figs 99 and 100), as well as the smallest of three fused posterior peripherals of SMNS 17203 (Gaffney 1990, p. 154, fig. 103). All fragments are from an individual found in the famous Plateosaurus quarry of Trossingen, southwestern Germany (Gaffney 1990, p. 15; Sander 1992) which is situated in the upper Löwenstein Formation (Norian, Late Triassic). Additionally, a costal fragment of a fragmentary shell (MB.R. 3449.2) from the Plateosaurus quarry at Halberstadt, eastern Germany, was sectioned (for details on this locality, see Sander 1992). The sample of P. robusta included a fragmentary peripheral and a small plastron fragment (hyo- or hypoplastron) of specimen SMNS 16442. Both the fragments were found in the lower Löwenstein Formation (Early Norian, Late Triassic) at Murrhardt, southwestern Germany. Shell bone material of P. talampayensis, the taxon that was included in the morphometric analysis of Joyce & Gauthier (2004), could not be obtained for sectioning. An overview of all used turtle shell material is compiled in table 1.
Table 1.
Material sectioned for the study including taxa names, accession numbers, element descriptions and general remarks.
| sampled taxa | specimen no. | sectioned shell elements | general remarks |
|---|---|---|---|
| Proganochelys quenstedti | SMNS 17203 | peripheral and plastron fragment | whole element was sampled; figured in Gaffney (1990: figs 99, 101) |
| SMNS 17203 | posterior peripheral | whole element was sampled; figured in Gaffney (1990: fig. 103) | |
| MB.R. 3449.2 | costal | whole element was sampled; labelled as ‘Triassochelys’ | |
| Proterochersis robusta | SMNS 16442 | peripheral | whole element was sampled |
| SMNS 16442 | hyo- or hypoplastron | whole element was sampled | |
| Geochelone pardalis | SMNS 12605 | neural2 | subsampled as drilled bone core (diameter 12 mm) |
| SMNS 12605 | costal2 (right) | subsampled as drilled bone core (diameter 12 mm) | |
| SMNS 12605 | hypoplastron (right) | subsampled as drilled bone core (diameter 12 mm) | |
| Terrapene carolina triunguis | FMNH 211806 | neural and costal (right) | articulated elements were sampled |
| FMNH 211806 | peripheral | whole element was sampled | |
| FMNH 211806 | hyo- and hypoplastron (left) | subsampled as drilled bone core (diameter 12 mm) | |
| Cuora picturata | YPM 13877 | neural6 | whole element was sampled |
| YPM 13877 | costal6 (left) | whole element was sampled | |
| YPM 13877 | peripheral8 | whole element was sampled | |
| YPM 13877 | hyoplastron (right) | whole element was sampled | |
| Caretta caretta | FMNH 98963 | costal2 (left) | subsampled as drilled bone core (diameter 22 mm) |
| FMNH 98963 | hyoplastron (left) | subsampled as drilled bone core (diameter 22 mm) | |
| Chelydra serpentina | YPM 10857 | neural3 | whole element was sampled |
| YPM 10857 | costal3 | whole element was sampled | |
| YPM 10857 | peripheral3 | whole element was sampled | |
| YPM 10857 | hyo- and hypoplastron | whole element was sampled | |
| Chelus fimbriatus | FMNH 269459 | costal | subsampled as drilled bone core (diameter 22 mm); bone stained green |
| FMNH 269459 | peripheral | subsampled as drilled bone core (diameter 22 mm); bone stained green | |
| FMNH 269459 | hyoplastron (right) | subsampled as drilled bone core (diameter 22 mm); bone stained green |
(d) Fossil and extant bone histology
For studying turtle shell bone histology, standard petrographic thin sections were prepared of the bone samples. All thin sections were produced at the Institute of Palaeontology, University of Bonn, Germany. In the case of the fossil taxa, i.e. the basal turtles, fragmentary shell bone elements from the carapace and plastron were sampled. In the case of extant turtle taxa, either whole shell bone elements were sampled or the shell bones were subsampled by core drilling (table 1). A diamond-studded coring bit mounted in a slow-moving power drill attached to a drill press was used to extract the bone core and the keratin shields, where applicable, from the turtle shell (Sander 2000). The description of the turtle shell elements follows Zangerl (1969), and the histological descriptions are based on Francillon-Vieillot et al. (1990) and Scheyer et al. (in press). Note that the orientation of the bio-apatite phase in bone is strongly connected to the original arrangement of the collagen phase. Therefore, both arrangements are intricately linked to each other, even if the bone gets diagenetically altered during fossilization, making the comparison of fossil and extant bone material possible.
(e) Acronyms used in main text
FMNH, The Field Museum, Chicago, IL, USA; MB, Naturhistorisches Forschungsinstitut und Museum für Naturkunde, Zentralinstitut der Humboldt-Universität zu Berlin, Berlin, Germany; SMNS, Staatliches Museum für Naturkunde Stuttgart, Stuttgart, Germany; YPM, Peabody Museum of Natural History at Yale University, New Haven, CT, USA.
3. Results
(a) Bone histology of extant terrestrial turtles
All three sampled terrestrial species show a diploe structure in the shell bones. Although overall shell bone thickness differs among the taxa, the external and internal cortices are generally well developed.
(i) External cortex
The external cortex consists of interwoven structural collagen fibres (ISFs), i.e. metaplastically ossified layers of the integument (Haines & Mohuiddin 1968). Fibre bundles of similar length and diameter are oriented perpendicular, parallel and diagonal to the external bone surface. Vascularization of the bone tissue is generally low with scattered small primary vascular canals and few scattered primary osteons. Remodelling of the cortical bone occurs at a transition zone to the interior cancellous bone with larger scattered secondary osteons. Growth marks that extend subparallel to the external bone surface are present in the external cortex in all three taxa.
(ii) Cancellous bone
The cancellous bone mostly consists of short and thick bone trabeculae and small- to medium-sized vascular spaces. The bone trabeculae are longer and more slender in the peripheral of C. picturata. Interstitial primary bone is found within the trabeculae and trabecular intersections, while the trabecular walls consist of lamellar bone. Bone cells and their lacunae are round and numerous in the primary bone. In the secondary lamellar bone, bone cells and lacunae are flattened and elongated in shape and more sparsely distributed.
(iii) Internal cortex
The internal cortices of the three sampled taxa consist of parallel-fibred bone. The bone tissue is generally weakly vascularized with few scattered primary vascular canals. The vascularization of the internal cortices is lowest in T. c. triunguis (most bone layers are avascular) and highest in C. picturata. Subsequent remodelling of the compact bone tissue occurs adjacent to the interior cancellous bone by secondary erosion cavities and scattered secondary osteons. Bone cell lacunae are flattened and elongated.
(b) Bone histology of extant aquatic and semi-aquatic turtles
Despite their widely differing phylogenetic affinities, all the three semi-aquatic to aquatic turtles show a rather uniform shell histology. While the shell bones of all three taxa also have a diploe structure, the amount and ratio of compact bone layers differs. In C. serpentina and C. fimbriatus, the thickness of the internal cortex is significantly reduced in comparison with the external cortex. In C. caretta, the internal cortex is not as strongly reduced as in the other two species.
(i) External cortex
In all three taxa, the bone tissue of the external cortex consists of metaplastic ISF, and the external bone surfaces are rough and porous. This rough surface texture is linked to vascular canals opening up to the surface of the bone. In C. fimbriatus, the dorsal external bone surfaces of the costal and the peripheral also show a strongly humped topography. The connective tissue between bone and keratinous shield either fills in topographical differences or it amplifies existing undulations in the bone surface topography leading to small humps in the shield cover. The larger surficial humps of the costal and the peripheral are based on variations in thickness of the external cortex. Within the ISF, the fibre bundles are of similar length and thickness, leading to a uniform spatial arrangement where fibre bundles equally trend perpendicular, subparallel and diagonal to the surface of the bone. In C. serpentina, however, the arrangement of the ISF can be locally dominated by fibre bundles that extend predominantly diagonally from the external bone surface towards the interior cancellous bone. This peculiar arrangement of fibre bundles is visible in most samples of C. serpentina. Vascularization of the bone tissue is high in all three taxa with the maximum level of vascularization occurring in C. caretta. In this taxon, the cortical bone starts losing its compact character due to the very high amount of primary osteons and primary vascular canals (often occurring in successive layers). In C. fimbriatus and C. serpentina, the amount of primary vascular canals and primary osteons is lower and the bone tissue appears still more compact. In these two taxa, the primary vascular canals often anastomose and branch, thereby forming reticular vascularization patterns.
(ii) Cancellous bone
The bone trabeculae of the cancellous bone are still largely primary, although, especially in the interior most part of the cancellous bone, secondary remodelling into long and slender secondary trabeculae proceeds. The larger cavities between the trabeculae show a lining with secondary lamellar bone. In C. fimbriatus, the transition between the external and internal cortical layers and the cancellous interior in-between is rather distinct instead of having interlaced intermediate zones as seen in C. caretta and C. serpentina. In C. caretta, areas where small vascular cavities predominate, alternate and interlace with areas where scattered, large vascular spaces dominate the cancellous bone.
(iii) Internal cortex
The internal cortices of all three taxa are made up of parallel-fibred bone. The arrangement of the fibres is roughly subparallel to the internal bone surface. Vascularization is moderate to high in C. serpentina and C. fimbriatus, mainly achieved by scattered primary vascular canals and scattered primary osteons. The primary osteons are similar in diameter and evenly spaced throughout the successive parallel-fibred layers of the internal cortex. The internal cortex of the samples of C. caretta is strongly vascularized. Similar to the external cortex, the compact nature of the bone is loosened up by primary vascular canals and numerous primary osteons.
(c) Bone histology of basal turtles
The shell bones of P. quenstedti and P. robusta have a very similar microstructure, both showing a diploe structure, in which well-developed external and internal cortices frame interior cancellous bone. The shell bones appear quite massive in thin section with internal and external cortices being of similar thickness.
(i) External cortex
The bone tissue consists of ISF. The interwoven structure is strongly dominated by fibre bundles that extend perpendicular or at high angles to the surface of the bone. Sharpey's fibres inserting at high angles into the cortical bone are also present and, due to their stronger mineralization, separable from the surrounding interwoven structural fibres of the bone matrix. The external cortex is vascularized with few scattered primary osteons and primary vascular canals. Cyclical growth marks are very poorly developed in the cortex to be countable. A few secondary osteons appear at the transition between external cortex and interior cancellous bone.
(ii) Cancellous bone
The cancellous bone in P. quenstedti is characterized by short secondarily remodelled trabeculae and moderate vascularization with small marrow cavities. In P. robusta, the bone trabeculae are generally longer and more slender. Primary interstitial bone is still present within the trabeculae and trabecular intersections. The lining of the trabeculae consists of secondary lamellar bone.
(iii) Internal cortex
The internal cortices in both basal turtles consist of parallel-fibred bone that can locally reach the organization grade of lamellar bone. Fibre bundles in the parallel-fibred bone are rather fine and of similar length and thickness. Coarser fibre bundles that may represent Sharpey's fibres are only found directly lateral to the incorporated rib of the costal plate of P. quenstedti and the distal bulge of the peripheral fragment of P. robusta. The distal bulge is recognized by an internal swelling of the cancellous bone and a convex curvature of the internal cortex. While the internal cortex in P. quenstedti is mainly avascular, a fine reticular vascularization pattern of thin primary vascular canals is locally developed in the plastral fragment of P. robusta.
4. Discussion
Histologic adaptation of turtles to an aquatic lifestyle includes overall reduction of shell bone tissue through reduction of cortical bone, increase of vascularization of cortical bone and the homogenization of cancellous and compact bone. However, it is apparent that none of these characteristics is encountered in basal turtles (see table 2 for comparison). On the other hand, the shells of P. quenstedti and P. robusta exhibit a bone microstructure generally consistent with that of the sampled turtles from terrestrial environments (electronic supplementary material). Both the basal turtles and the modern terrestrial turtles show a robust diploe structure with well-developed external and internal cortical bone layers. The metaplastic bone tissue is weakly vascularized in the external cortex, the vascularization of the parallel-fibred bone tissue of the internal cortex is low to absent, and the bone trabeculae are rather short and thick in diameter in the cancellous bone.
Table 2.
Comparison and synopsis of shell bone histology of basal turtles with that of modern terrestrial and aquatic turtles; ISF, interwoven collagenous structural fibre bundles; LB, lamellar bone; PFB, parallel-fibred bone.
| fossil basal turtles P. quenstedti & P. robusta | modern terrestrial turtles C. picturata, T. c. triunguis and G. pardalis | modern aquatic turtles C. caretta, C. serpentina and C. fimbriatus | |
|---|---|---|---|
| compact diploe-structure of shell bone | yes | yes | no |
| homogenization of cortical and cancellous bone | no | no | low (C. serpentina and C. fimbriatus) to high (C. caretta) |
| bone tissue of external cortex | ISF | ISF | ISF |
| vascularization of external cortex | low | low | moderate to strong |
| bone trabeculae of cancellous bone | generally shorter and thicker | generally shorter and thicker | generally longer and slender |
| reduction of internal cortex | no | no | moderate to strong |
| vascularization of internal cortex | low to absent | low to absent | moderate to strong |
| bone tissue of internal cortex | PFB locally grading into LB | PFB | PFB |
An exception to this general trend in terrestrial turtles was recently described for Hesperotestudo crassiscutata, a giant tortoise from the Pleistocene of Florida, USA, in which slightly increased cortical vascularization and more slender bone trabeculae were proposed as a light-weight construction of the shell bone directly linked to the immense size of the animals (Scheyer & Sánchez-Villagra 2007). Among extant tortoises, on the other hand, the increased levels of vascularization of Kinixys homeana and Geochelone carbonaria (electronic supplementary material) are not easily explained, and it may be speculated that bone microstructures in both taxa are linked to their respective peculiar shell morphologies (strongest sampled non-pathological humped carapace in G. carbonaria, unique carapacial hinge system in K. homeana).
Instead of an increased vascularization typical of semi-aquatic to mainly aquatic taxa, Mauremys cf. Mauremys mutica and Pangshura tentoria, two taxa inhabiting mainly swamps, marshes, ponds and creeks or rivers (Ernst & Barbour 1989) were found to express tendencies to terrestrial bone microstructures similar to C. picturata that elude a clear explanation with the current data available. Among marine turtles, dermochelyid armour plates retain a thick external cortex and lack overall homogenization into cancellous bone while the internal cortex becomes reduced. This leads to the conclusion that Dermochelys coriacea and Psephophorus sp. (electronic supplementary material) show quite a strong but not extreme adaptation to the aquatic milieu, in spite of the fact that their armour is secondary in nature. Although some gradation between the categories thus obviously occurs among groups, we are confident that the majority of taxa allow a well-founded categorization of ecological adaptation.
It is further inferred that overall composition of the integument and the specific locus of bone development in the integument are comparable in basal turtles and modern terrestrial turtles. Comparison of basal turtles with extant aquatic and terrestrial turtles (table 2) thus independently corroborates the hypothesis of Joyce & Gauthier (2004) that basal turtles lived in the terrestrial environment. However, while morphometric analysis must rely on the rare preservation of associated or articulated limb material, the analysis of shell bone microstructures allows palaeoecological study of a much wider range of taxa due to the much more frequent preservation of shell material.
Our results underscore that the evolutionary history of turtles began in the terrestrial environment and that the turtle ancestor was most probably a terrestrial animal. Still early in their history, turtles then adopted a semi-aquatic and eventually even a fully aquatic lifestyle, with diverse turtle lineages evolving ever more terrestrial forms. These are rare instances of secondary adaptation to the terrestrial environment in a vertebrate lineage, resulting in a convergent evolution of the shell bone microstructure of the basal turtles.
Acknowledgments
We would like to express our thanks to all institutions, museums, collections managers and their respective colleagues (a complete list is included in the electronic supplementary material), who provided us with the turtle shell material for histological thin section preparation. O. Dülfer is acknowledged for his technical support in preparing the sections. W. Joyce and one anonymous reviewer are thanked for greatly improving the manuscript. This project was funded by DFG grant no. SA-469/15.
Supplementary Material
Detailed information on the ecological/palaeoecological categorisation of turtle taxa, their authorship and specimen numbers, collections acronyms, as well as acknowledgements and additional references are given
References
- Agassiz L. Contributions to the natural history of the United States of America. vol. 1. Little, Brown; Boston, USA: 1857. [Google Scholar]
- Baur G. Ueber den Ursprung der Extremitäten der Ichthyopterygia. Berichte über die Versammlungen des Oberrheinischen Geologischen Vereines. 1887;20:17–20. [Google Scholar]
- Bell T. Characters of the order, families, and genera of the Testudinata. Zool. J. 1828;3:513–516. [Google Scholar]
- Bowen B.W, Abreu-Grobois F.A, Balazs G.H, Kamezaki N, Limpus C.J, Ferl R.J. Trans-pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. Proc. Natl Acad. Sci. USA. 1995;92:3731–3734. doi: 10.1073/pnas.92.9.3731. doi:10.1073/pnas.92.9.3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffrénil V.de, Mazin J.-M. Bone histology of the ichthyosaurs: comparative data and functional interpretation. Paleobiology. 1990;16:435–447. [Google Scholar]
- Buffrénil V.de, Schoevaert D. On how the periosteal bone of the delphinid humerus becomes cancellous: ontogeny of a histological specialization. J. Morphol. 1988;198:149–164. doi: 10.1002/jmor.1051980203. doi:10.1002/jmor.1051980203 [DOI] [PubMed] [Google Scholar]
- Ernst C.H, Barbour R.W.Turtles of the world1989Smithonian Institution Press; Washington, DC [Google Scholar]
- Fraas E. Proterochersis, eine pleurodire Schildkröte aus dem Keuper. Jahresh. Ver. Vaterl. Naturkd. Wuerttemb. 1913;80:1–30. [Google Scholar]
- Francillon-Vieillot H, Buffrénil V. de, Castanet J, Géraudie J, Meunier F.J, Sire J.Y, Zylberberg L, Ricqlès A. de. Microstructure and mineralization of vertebrate skeletal tissues. In: Carter J.G, editor. Skeletal biomineralization: patterns, processes and evolutionary trends. Van Nostrand Reinhold; New York, NY: 1990. pp. 471–530. [Google Scholar]
- Gaffney E.S. The comparative osteology of the Triassic turtle Proganochelys. Bull. Am. Mus. Nat. Hist. 1990;194:1–263. [Google Scholar]
- Gaffney E.S, Meylan P.A. The phylogeny and classification of the tetrapods. In: Benton M.J, editor. Amphibians, reptiles, birds. vol. 1. Clarendon Press; Oxford, UK: 1988. pp. 157–219. [Google Scholar]
- Gaffney E.S, Kitching J.W. The most ancient African turtle. Nature. 1994;369:55–58. doi:10.1038/369055a0 [Google Scholar]
- Gaffney E.S, Tong H, Meylan P.A. Evolution of the side-necked turtles: the families Bothremydidae, Euraxemydidae, and Araripemydidae. Bull. Am. Mus. Nat. Hist. 2006;300:1–698. doi:10.1206/0003-0090(2006)300[1:EOTSTT]2.0.CO;2 [Google Scholar]
- Haines R.W, Mohuiddin A. Metaplastic bone. J. Anat. 1968;103:527–538. [PMC free article] [PubMed] [Google Scholar]
- Joyce W.G. Phylogenetic relationships of Mesozoic turtles. Peabody Mus. Nat. Hist. Yale Univ. Bull. 2007;48:3–102. [Google Scholar]
- Joyce W.G, Gauthier J.A. Palaeoecology of Triassic stem turtles sheds new light on turtle origins. Proc. R. Soc. B. 2004;271:1–5. doi: 10.1098/rspb.2003.2523. doi:10.1098/rspb.2003.2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce W.G, Karl H.-V. The world's oldest fossil turtle: fact versus fiction. Fossil Turtle Research, vol. 1. Russ. J. Herpetol. 2006;13(Suppl.):104–111. [Google Scholar]
- Kinneary J.J. Salinity relations of Chelydra serpentina in a Long Island estuary. J. Herpetol. 1993;27:441–446. doi:10.2307/1564834 [Google Scholar]
- Lehr E, Fritz U, Obst F.J. Cuora galbinifrons picturata subsp. nov., eine neue Unterart der Hinterindischen Scharnierschildkröte. herpetofauna. Weinstadt. 1998;20:5–11. [Google Scholar]
- Linnaeus, C. 1758 Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis Tomus I. Editio Decima, Reformata, Holmiae.
- Reagan D.P. Habitat selection in the three-toed box turtle, Terrapene carolina triunguis. Copeia. 1974;2:512–527. doi:10.2307/1442543 [Google Scholar]
- Ricqlès A. de. Les mécanismes hétérochroniques dans le retour des tétrapodes au milieu aquatique. Geobios mémoire spécial. 1989;12:337–348. doi:10.1016/S0016-6995(89)80034-8 [Google Scholar]
- Ricqlès A. de, Buffrénil V. de. Bone histology, heterochronies and the return of tetrapods to life in water: where are we? In: Mazin J.-M, Buffrénil V. de, editors. Secondary adaptations of tetrapods to life in water. Verlag Dr. Friedrich Pfeil; München, Germany: 2001. pp. 289–310. [Google Scholar]
- Rougier G.W, Fuente M.S.de la, Arcucci A.B. Late Triassic turtles from South America. Science. 1995;268:855–858. doi: 10.1126/science.268.5212.855. doi:10.1126/science.268.5212.855 [DOI] [PubMed] [Google Scholar]
- Sander P.M. The Norian Plateosaurus bonebeds of central Europe and their taphonomy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1992;93:255–299. doi:10.1016/0031-0182(92)90100-J [Google Scholar]
- Sander P.M. Longbone histology of the Tendaguru sauropods: implications for growth and biology. Paleobiology. 2000;26:466–488. doi:10.1666/0094-8373(2000)026<0466:LHOTTS>2.0.CO;2 [Google Scholar]
- Scheyer, T. M. In press. Comparative bone histology of the turtle shell (carapace and plastron): implications for turtle systematics, functional morphology and turtle origins. PhD thesis, University of Bonn.
- Scheyer T.M, Sánchez-Villagra M.R. Carapace bone histology in the giant turtle Stupendemys geographicus (Pleurodira: Podocnemidae): phylogeny and function. Acta Palaeontol. Pol. 2007;52:137–154. [Google Scholar]
- Scheyer, T. M., Sander, P. M., Joyce, W. G., Böhme, W. & Witzel, U. In press. A plywood structure in the shell of fossil and living soft-shelled turtles (Trionychidae) and its evolutionary implications. Org. Divers. Evol
- Schneider J.G.Allgemeine Naturgeschichte der Schildkröten, nebst einem systematischen Verzeichnisse der einzelnen Arten und zwey Kupfern1783Johann Gotfried Müllersche Buchhandlung; Leipzig, Germany [Google Scholar]
- Stuart B.L, Parham J.F. Molecular phylogeny of the critically endangered Indochinese box turtle (Cuora galbinifrons) Mol. Phylogenet. Evol. 2004;31:164–177. doi: 10.1016/S1055-7903(03)00258-6. doi:10.1016/S1055-7903(03)00258-6 [DOI] [PubMed] [Google Scholar]
- Sukhanov V.B. An archaic turtle, Heckerochelys romani gen. et sp. nov., from the Middle Jurassic of Moscow region, Russia. Fossil turtle research, vol. 1. Russ. J. Herpetol. 2006;13(Suppl.):112–118. [Google Scholar]
- Zangerl R. The turtle shell. In: Gans C, Bellairs d'A, Parsons T.S, editors. Biology of the reptilia. Morphology A. vol. 1. Academic Press; London, UK: 1969. pp. 311–339. [Google Scholar]
- Zardoya R, Meyer A. The evolutionary position of turtles revised. Naturwissenschaften. 2001;88:193–200. doi: 10.1007/s001140100228. doi:10.1007/s001140100228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed information on the ecological/palaeoecological categorisation of turtle taxa, their authorship and specimen numbers, collections acronyms, as well as acknowledgements and additional references are given