Abstract
Mechanisms of innate and adaptive immunity play a pivotal role in the development of cancer. Chronic inflammation can drive tumor development, but antitumor immunity can also restrict or even prevent tumor growth. New data show that feed-forward signals downstream of the receptor for advanced glycation end-products (RAGE) can fuel chronic inflammation, creating a microenvironment that is ideal for tumor formation.
Chronic inflammation is a major causative factor in a wide range of human and murine malignancies. In humans, inflammation associated with the hepatitis B and C viruses is the primary cause of liver cancer, and the bacterium Helicobacter pylori plays a central role in the development of most cancers of the stomach (1, 2). Beyond infections, many autoimmune diseases are associated with an increased risk of lymphoma, and the inappropriate immune response to commensal flora in ulcerative colitis is strongly linked to colon cancer (3, 4).
Evidence now links inflammation to tumor development in both genetic tumor syndromes and in the context of chronic carcinogen exposure. Administration of nonsteroidal antiinflammatory drugs (NSAIDs) reduces the incidence of colon cancer in patients with familial adenomatous polyposis (FAP) (5) and, perhaps more remarkably, reduces the incidence of lung cancer in smokers, the principal cause of cancer-related death worldwide (6).
Studies performed in a variety of mouse models of cancer have paralleled the findings from human tumors, but the precise role of inflammation in tumor development remains incompletely understood. Several key mediators have been identified that link chronic inflammation to tumor development, yet in many cases, the pathways critical for the initiation and maintenance of chronic inflammation are unknown. On p. 275 in this issue, Gebhardt et al. [7] present evidence that signals downstream of RAGE are critical for the development of tumor-promoting inflammation in a mouse model of skin cancer.
Tumor-promoting cytokines
Production of acute inflammatory cytokines by cells of the innate immune system, including macrophages and mast cells, plays an essential role in inflammation-driven tumor development. Cytokines such as TNF, interleukin (IL)-6, and IL-1β have myriad effects in the tumor microenvironment, promoting cell growth and survival as well as angiogenesis and the recruitment of immune effector cells (2).
Which cytokines are required for tumor development depends largely on the model being examined. TNF plays an essential role in several models of cancer, and is a critical inflammatory mediator in many autoimmune diseases of both mice and humans, primarily acting via induction of NF-κB (2). IL-6 acts both as a mitogen and an angiogenic factor and has been implicated in many of the same processes as TNF. IL-6 plays an important role in carcinogen-driven liver cancer, and has recently been identified as an important driving factor in non–smoking-related lung cancer in humans (8–10). IL-1 can activate NF-κB in a manner similar to TNF, and polymorphisms in IL-1 have been linked to gastric cancer (11).
Inciting inflammation
Not surprisingly, tumor-promoting inflammation can be induced by the same pathways that respond to microbial infections, suggesting that tumors may be aberrant consequences of initially physiological immune responses.
Recent work has positioned myeloid differentiation factor 88 (MyD88), which is a critical downstream signaling molecule for both the Toll-like receptor (TLR) family of microbial pattern recognition receptors and the IL-1 and -18 receptors (IL-1R and -18R), as a central player in inflammation-driven tumorigenesis. In the diethylnitrosamine model of liver cancer, MyD88-dependent induction of IL-6 is critical for tumor formation (12). MyD88-deficient mice treated with the topical carcinogen 7,12-dimethylbenz[a]anthracene (DMBA), followed by treatment with the proinflammatory phorbol-ester TPA, developed fewer epithelial tumors than did wild-type mice (13). Surprisingly, 3-methylcholanthrene (MCA)–induced sarcomas were also reduced in MyD88-deficient mice, despite the lack of an obvious role for inflammation in this model (13).
MyD88-dependent signaling is also required in some genetic tumor models. In the APCmin/+ model of human FAP, MyD88 deficiency was associated with decreased inflammatory cytokine production within the tumor microenvironment (14). This reduction correlated with a decrease in both the number and size of spontaneously arising polyps.
Although these findings bring us one step closer to understanding the nature of the signals driving tumor-promoting inflammation, the factors responsible for engaging MyD88-dependent pathways are still obscure. TLRs can recognize a wide range of highly conserved microbial products, potentially implicating occult infections or normal flora in tumor-associated inflammation.
MyD88 may also facilitate so-called “sterile” inflammation, either through TLR-dependent recognition of endogenous adjuvants or through IL-1R signaling. IL-1R signaling is important for the initiation of neutrophil infiltration in response to necrotic cells (15), and it may also have important tumor-intrinsic effects. IL-1β was recently shown to be required for MCA-induced tumor formation, suggesting that, in this system, MyD88 may function through the IL-1R (16).
Consistent with the importance of sterile inflammation in tumor promotion, the findings of Gebhart et al. (7) in this issue implicate endogenous proteins released during cell necrosis in inciting carcinogenic inflammation. RAGE activation can occur through the recognition of at least three self-proteins released from cells during necrosis: the DNA-binding protein HMGB1 and the two calcium-binding “cytokines” S100a8 and S100a9. Intriguingly, RAGE-dependent recognition of HMGB1 has been shown to act in a costimulatory capacity for TLR-mediated responses to DNA, potentially providing a link between RAGE and MyD88 (17).
Taking off the breaks
Acute, self-limiting inflammation is generally insufficient to promote tumor formation. Failure of normal antiinflammatory mechanisms is thus an essential feature of tumor-promoting inflammation.
Although in many cases, such as infection or autoimmunity, the mechanisms that prevent the resolution of inflammation are still unclear, genetic defects in key regulatory proteins can enhance tumor formation. Loss of TIR8, which is a negative regulator of IL-1R/TLR signaling, exacerbates inflammation in the dextran sulfate sodium model of colitis, leading to a substantially increased risk of colon cancer (18–19). Similarly, loss of IL-1 receptor antagonist, a secreted protein that blocks IL-1 function, accelerates tumor onset and increases tumor aggressiveness in the DMBA/TPA model (16).
The loss of immune regulation may also aggravate disease in APCmin/+ mice. Infusion of regulatory T (T reg) cell–depleted T cells into APCmin/+ mice increases polyp formation and leads to the development of spontaneous mammary tumors (20).
One of the more intriguing elements of RAGE-dependent inflammation is the ability of RAGE to up-regulate its own ligands. Gebhart et al. (7) demonstrate that RAGE signaling in the DMBA/TPA model induces S100a8 and S100a9 synthesis in epithelial cells, likely leading to a feed-forward loop that further aggravates the inflammatory environment. Regulatory circuits limiting RAGE-mediated inflammation undoubtedly exist, but these as yet uncharacterized pathways are clearly not sufficient to prevent tumor onset in the time frame of these experiments. Interestingly, the requirement for RAGE can be bypassed by more frequent application of TPA, suggesting that other, more rapidly self-limiting inflammatory pathways can substitute for RAGE if the inflammation-inciting agent is allowed to persist.
Protective immunity
In contrast to the tumor-promoting effects of chronic inflammation, increasing evidence suggests that adaptive immunity is responsible for recognizing and rejecting malignant cells (for review see reference 21). The protective effect of adaptive immunity is most obvious in the case of tumors with viral etiologies, but immunodeficiencies in both mice and humans have also been associated with an increased incidence of many tumors without a clear viral etiology (21). Consistent with a role for adaptive immunity in regulating tumor growth, spontaneous lymphocytic infiltrates can be observed in a variety of different human cancers and, in some, these infiltrates correlate with a favorable prognosis (21–22). Once a tumor is established, immunosuppression is a common feature of the microenvironment, with most tumors infiltrated by immunosuppressive myeloid and lymphoid cells (21). The expression of antiinflammatory factors is also common in tumors, suggesting that overcoming an antitumor immune response is an important step in tumorigenesis.
The mechanism by which adaptive immune cells can control tumor growth has been studied extensively using the MCA tumor model. In this system, IFN-γ–producing T cells play an essential role in impeding tumor development. Mice with defects in T cell immunity exhibit an increased incidence of tumor formation after MCA application, and the tumors that arise in these mice are more easily rejected when transferred into immunocompetent animals than are similar tumors that arise in wild-type mice (21). These findings provide the most direct evidence to date that the immune system can restrict tumor formation.
Recent evidence also indicates that many immunocompetent animals treated with MCA harbor occult tumors that are kept in check by continuous immune surveillance (23). Although direct evidence for spontaneous, immune-mediated tumor control in humans is lacking, rare cases of tumor recurrence decades after the disappearance of the primary tumors suggest that similar mechanisms may be in play in humans as well (21).
The balance of power
In most models used to study tumor-promoting inflammation, cytokines produced by cells of the innate immune system play an indispensable role. Protective antitumor effects, in contrast, derive largely from adaptive immune cells, particularly T cells. Yet, dividing the immune response to cancer into this innate-adaptive dichotomy is too simplistic. Inflammatory cytokines can restrict the growth of certain tumors, and adaptive immunity can drive tumor-promoting inflammation in chronic infections and in autoimmunity.
Recent work has started to explain the dual role for adaptive immune cells in tumor development. Overexpression of the cytokine IL-23, for example, is highly correlated with an increased risk of tumor development (24). IL-23 expands a subset of CD4+ T cells that secrete the proinflammatory cytokine IL-17, which promotes angiogenesis, as well as the production of a range of other inflammatory factors from epithelial cells (for review see reference 25). At the same time, however, IL-23 expression in the tumor microenvironment reduces cytotoxic T cell infiltrates, potentially blocking antitumor immunity (24). IL-17–secreting T cells are found in many chronic inflammatory diseases, and may provide an important link between chronic inflammation and cancer (25).
Ultimately, the consequences of the interaction between the immune system and tumors will likely depend on the context in which the immune system is engaged. Feed-forward signals such as those initiated by RAGE lead to uncontrolled, chronic immune activation. In this setting, any potentially protective effect from the immune system is overwhelmed by tumor-promoting inflammation (Fig. 1). By understanding the mechanisms underlying tumor-associated inflammation, we can hope to circumvent these responses, thus opening the door to novel approaches to cancer treatment and prevention.
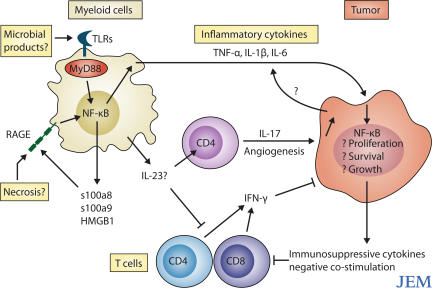
Figure 1.
Signals through innate immune receptors such as RAGE maintain chronic inflammation in the tumor microenvironment. Chronic inflammatory responses can be initiated by microbial or endogenous TLR ligands, or by RAGE ligands (s100a8, s100a9, and HMGB1) released from necrotic cells. These signals drive the expression of NF-κB–activating cytokines, such as TNF, IL-1β, and IL-6, which act as growth factors for the tumor. IL-17 produced by CD4+ T cells may also promote angiogenesis. At the same time, the tumor microenvironment disables potentially protective immune responses mediated by IFN-γ–secreting CD4+ and CD8+ T cells.
References
- 1.Karin, M., T. Lawrence, and V. Nizet. 2006. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 124:823–835. [DOI] [PubMed] [Google Scholar]
- 2.Karin, M., and F.R. Greten. 2005. NF-κB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5:749–759. [DOI] [PubMed] [Google Scholar]
- 3.Zintzaras, E., M. Voulgarelis, and H.M. Moutsopoulos. 2005. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch. Intern. Med. 165:2337–2344. [DOI] [PubMed] [Google Scholar]
- 4.Ekbom, A., C. Helmick, M. Zack, and H.O. Adami. 1990. Ulcerative colitis and colorectal cancer: a population-based study. N. Engl. J. Med. 323:1228–1233. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach, G., P.M. Lynch, R.K. Phillips, M.H. Wallace, E. Hawk, G.B. Gordon, N. Wakabayashi, B. Saunders, Y. Shen, S. Zimmerman, et al. 2000. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 342:1946–1952. [DOI] [PubMed] [Google Scholar]
- 6.Schreinemachers, D.M., and R.B. Everson. 1994. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 5:138–146. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt, C., A. Riehl, M. Durchdewald, J. Nemeth, G. Furstemberger, K. Muller-Decker, A. Enk, B. Arnold, A. Bierhaus, P.P. Nawroth, et al. RAGE signaling sustains inflammation and promotes tumor development. 2008. J. Exp. Med. 205:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda, S., H. Kamata, J.L. Luo, H. Leffert, and M. Karin. 2005. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 121:977–990. [DOI] [PubMed] [Google Scholar]
- 9.Becker, C., M.C. Fantini, C. Schramm, H.A. Lehr, S. Wirtz, A. Nikolaev, J. Burg, S. Strand, R. Kiesslich, S. Huber, et al. 2004. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 21:491–501. [DOI] [PubMed] [Google Scholar]
- 10.Gao, S.P., K.G. Mark, K. Leslie, W. Pao, N. Motoi, W.L. Gerald, W.D. Travis, W. Bornmann, D. Veach, B. Clarkson, and J.F. Bromberg. 2007. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinoma. J. Clin. Invest. 117:3846–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Omar, E.M., M. Carrington, W.H. Chow, K.E. McColl, J.H. Bream, H.A. Young, J. Herrera, J. Lissowska, C.C. Yuan, N. Rothman, et al. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 404:398–402. [DOI] [PubMed] [Google Scholar]
- 12.Naugler, W.E., T. Sakurai, S. Kim, S. Maeda, K. Kim, A.M. Elsharkawy, and M. Karin. 2007. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 317:121–124. [DOI] [PubMed] [Google Scholar]
- 13.Swann, J.B., M.D. Vesely, A. Silva, J. Sharkey, S. Akira, R.D. Schreiber, and M.J. Smyth. 2008. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc. Natil. Acad. Sci. USA. 105:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum, S., and R. Medzhitov. 2007. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 317:124–127. [DOI] [PubMed] [Google Scholar]
- 15.Chen, C.J., H. Kono, D. Golenbock, G. Reed, S. Akira, and K.L. Rock. 2007. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13:851–856. [DOI] [PubMed] [Google Scholar]
- 16.Krelin, Y., E. Voronov, S. Dotan, M. Elkabets, E. Reich, M. Fogel, M. Huszar, Y. Iwakura, S. Segal, C.A. Dinarello, and R.N. Apte. 2007. Interleukin-1β-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 67:1062–1071. [DOI] [PubMed] [Google Scholar]
- 17.Tian, J., A.M. Avalos, S.Y. Mao, B. Chen, K. Senthil, H. Wu, P. Parroche, S. Drabic, D. Golenbock, C. Sirois, et al. 2007. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8:487–496. [DOI] [PubMed] [Google Scholar]
- 18.Xiao, H., M.F. Gulen, J. Qin, J. Yao, K. Bulek, D. Kish, C.Z. Altuntas, D. Wald, C. Ma, H. Zhou, et al. 2007. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 26:461–475. [DOI] [PubMed] [Google Scholar]
- 19.Garlanda, C., F. Riva, T. Veliz, N. Polentarutti, F. Pasqualini, E. Radaelli, M. Sironi, M. Nebuloni, E.O. Zorini, E. Scanziani, and A. Mantovani. 2007. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, and inhibitory member of the interleukin-1 receptor family. Cancer Res. 67:6017–6021. [DOI] [PubMed] [Google Scholar]
- 20.Rao, V.P., T. Poutahidis, Z. Ge, P.R. Nambiar, B.H. Horwitz, J.G. Fox, and S.E. Erdman. 2006. Proinflammatory CD4+CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 66:57–61. [DOI] [PubMed] [Google Scholar]
- 21.Dunn, G.P., L.J. Old, and R.D. Schreiber. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22:329–360. [DOI] [PubMed] [Google Scholar]
- 22.Galon, J., A. Costes, F. Sanchez-Cabo, A. Kirilovsky, B. Mlecnik, C. Lagorce-Pages, M. Tosolini, M. Camus, A. Berger, P. Wind, et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313:1960–1964. [DOI] [PubMed] [Google Scholar]
- 23.Koebel, C.M., W. Vermi, J.B. Swann, N. Zerafa, S.J. Rodig, L.J. Old, M.J. Smyth, and R.D. Schreiber. 2007. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 450:903–907. [DOI] [PubMed] [Google Scholar]
- 24.Langowski, J.L., X. Zhang, L. Wu, J.D. Mattson, T. Chen, K. Smith, B. Basham, T. McClanahan, R.A. Kastelein, and M. Oft. 2006. IL-23 promotes tumor incidence and growth. Nature. 442:461–465. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli, E., M. Oukka, and V.K. Kuchroo. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350. [DOI] [PubMed] [Google Scholar]