Abstract
Atherosclerosis is a chronic inflammatory disease of the vasculature commonly leading to myocardial infarction and stroke. We show that IL-33, which is a novel IL-1–like cytokine that signals via ST2, can reduce atherosclerosis development in ApoE−/− mice on a high-fat diet. IL-33 and ST2 are present in the normal and atherosclerotic vasculature of mice and humans. Although control PBS-treated mice developed severe and inflamed atherosclerotic plaques in the aortic sinus, lesion development was profoundly reduced in IL-33–treated animals. IL-33 also markedly increased levels of IL-4, -5, and -13, but decreased levels of IFNγ in serum and lymph node cells. IL-33 treatment also elevated levels of total serum IgA, IgE, and IgG1, but decreased IgG2a, which is consistent with a Th1-to-Th2 switch. IL-33–treated mice also produced significantly elevated antioxidized low-density lipoprotein (ox-LDL) antibodies. Conversely, mice treated with soluble ST2, a decoy receptor that neutralizes IL-33, developed significantly larger atherosclerotic plaques in the aortic sinus of the ApoE−/− mice compared with control IgG-treated mice. Furthermore, coadministration of an anti–IL-5 mAb with IL-33 prevented the reduction in plaque size and reduced the amount of ox-LDL antibodies induced by IL-33. In conclusion, IL-33 may play a protective role in the development of atherosclerosis via the induction of IL-5 and ox-LDL antibodies.
Atherosclerosis is a disease of the vasculature that is characterized by chronic inflammation of the arterial wall (1). The vessel is infiltrated by macrophages and T cells, which, together with resident smooth muscle and endothelial cells (ECs), produce cytokines, growth factors, and other proinflammatory mediators in response to the presence of oxidized low-density lipoprotein (ox-LDL). B cells are rarely found in plaques, although patients do exhibit a systemic antibody response to ox-LDL (2), and B cells have been shown to be protective against atherosclerosis in murine models (3, 4). The majority of T cells present in human atherosclerotic plaques are of the CD4+ subset and predominantly produce cytokines of the Th1 subtype, such as IFNγ (5). Furthermore, a critical pathogenic role for Th1 cells has been shown in murine atherosclerosis models (6–10).
In contrast, most studies have demonstrated an atheroprotective effect of Th2 cells, with the exception of IL-4, which has produced conflicting results (1, 7). Recent work has established that deficiency of the Th2 cytokine IL-5 reduced the production of atheroprotective ox-LDL antibodies and accelerated atherosclerosis in ApoE−/− mice (11). Therefore, it is postulated that a Th1-to-Th2 switch may attenuate atherosclerosis development. However, the mechanism with which to accomplish such a switch in vivo remains obscure. We now demonstrate that the most recently identified cytokine, IL-33, can induce a Th1-to-Th2 shift in vivo in an ApoE−/− model of atherosclerosis.
IL-33 is a recently described member of the IL-1 family, which includes IL-1β and -18. Like IL-1β and -18, IL-33 was found to have strong immunomodulatory functions (12). However, unlike IL-1β and -18, which mainly promote Th1-associated responses, IL-33 predominantly induces the production of Th2 cytokines (IL-5 and -13) and increases levels of serum immunoglobulin. IL-33 was recently found to be the ligand for the orphan receptor ST2 (12). The ST2 gene encodes two isoforms of ST2 protein: ST2L, a transmembrane form, and soluble ST2 (sST2), a secreted form that can serve as a decoy receptor of IL-33. ST2L is preferentially expressed on Th2 cells, but not Th1 cells (13), and can profoundly suppress innate and adaptive immunity. ST2L is also expressed in cardiomyocytes, and serum elevations of sST2 predict mortality and heart failure in patients with acute myocardial infarction (14, 15). Recently, it was shown that IL-33/ST2 signaling is a crucial biomechanically activated system that controlled cardiomyocyte hypertrophy and cardiac fibrosis after pressure overload (16). However, the role of IL-33/ST2 in atherosclerosis has not been investigated. We now show that IL-33 administration to ApoE−/− mice induced Th2 cytokines and protective ox-LDL antibodies, which significantly reduced atherosclerotic plaque development in the aortic sinus. Conversely, mice treated with sST2 developed significantly larger atherosclerotic plaques in the aortic sinus. These results demonstrate a novel role for IL-33/ST2 in the control of Th1/Th2 balance and the generation of protective autoantibodies in atherosclerosis.
RESULTS AND DISCUSSION
IL-33 and ST2 are expressed in vascular cells and tissues
It has been reported that smooth muscle cells (SMCs) and ECs can produce or respond to IL-1 and -18 (17). Recently, RT-PCR analysis of human and mouse cDNA libraries revealed the expression of IL-33 mRNA in dermal fibroblasts, keratinocytes, and coronary artery, bronchial, and pulmonary SMCs (12). IL-33 is also expressed in ECs from chronically inflamed rheumatoid arthritis synovium and Crohn's disease intestine (18). However, the expression of IL-33 in atherosclerotic tissues has not been previously investigated.
We examined the expression of IL-33 and its receptor ST2 in murine and human vascular cells and tissues by PCR and immunohistochemistry. IL-33 and ST2 mRNA was present in the thoracic aorta of 18-wk-old C57BL/6 control mice (normal diet) and ApoE−/− (normal diet or high-fat diet) mice (Fig. 1 A) and in primary cultured human ECs and SMCs (Fig. 1 B). Quantitative PCR demonstrated a significantly higher expression of the IL-33 gene in aortas from ApoE−/− mice fed a high-fat diet versus controls (Fig. 1 C). Expression of IL-33 was also demonstrated by immunostaining in arteries and small vessels (Fig. 1, D and E). Staining of parallel sections showed a similar pattern of the IL-33 expression with an α-smooth muscle actin stain in the adventitia of larger vessels, but not in the media, and the endothelial-specific marker CD31 in small arteries (Fig. 1, D and E). The presence of IL-33 in the normal and atherosclerotic vasculature suggests its potential as a regulator of atherosclerosis under physiological or pathophysiological conditions.
Figure 1.
Expression of IL-33 and ST2 in vascular cells and tissues. The expression of mRNAs for IL-33, ST2, and β-actin were examined by RT-PCR in the thoracic aortas of 18-wk-old C57BL/6 control mice (normal diet) or ApoE−/− (normal diet or high-fat diet) mice (A; n = 3) and in primary cultured human umbilical vein ECs (HUVECs), human saphenous vein ECs (HSVECs), human saphenous vein SMCs (HSVSMCs), and human coronary artery SMCs (HCASMCs; B). (C) Quantitative PCR analysis of IL-33 gene expression in thoracic aortas of mice as described in A (expressed as a percentage of 18S endogenous control; n = 4–5). Data are the mean ± the SEM. Immunostaining and isotype controls in frozen vascular tissues of ApoE−/− mice for α-smooth muscle actin and IL-33 in the adventitia of the aorta (D), and for CD31 and IL-33 in ECs of small vessels of the heart (E). A, adventitia; M, media; L, lumen. Images shown are representative of seven sections. *, P < 0.05, Student's unpaired t test. Bars, 25 μm.
IL-33 reduces atherosclerosis development in ApoE−/− mice
6-wk-old male ApoE−/− mice were fed a high-fat diet (0.15% cholesterol and 21% lard) for 12 wk. During the last 6 wk, the mice were injected i.p. twice per week with PBS or recombinant IL-33 (1 μg/injection). Mice were killed at the end of the treatment, and intimal atherosclerotic lesion size was quantified in the aortic sinus by computerized planimetry. Mice treated with IL-33 developed a substantially smaller atherosclerotic lesion size in the aortic sinus compared with PBS-treated control mice (0.16 ± 0.02 vs. 0.42 ± 0.04 mm2; P < 0.0001; Fig. 2, A and B). However, IL-33 treatment reduced the percentage of plaque area that stained positive for F4/80+ macrophages (16.2 ± 3.9 vs. 50.3 ± 7.8; P < 0.05; Fig. 2, C and D) and reduced the number of lesion-associated CD3+ T lymphocytes (2,710 ± 363 vs. 4,665 ± 821 cells/mm2; P < 0.05; Fig. 2, C and D). Plaque stability is critically dependent on the SMC and collagen content of the fibrous cap. Therefore, we performed α-smooth muscle actin (brown) and trichrome collagen staining (blue) of plaques and found similar staining between the PBS- and IL-33–treated mice (31.6 ± 3.1 vs. 35.2 ± 6.3% and 22.3 ± 2.1 vs. 26.6 ± 2.7%, respectively; Fig. 2, C and D), indicating that IL-33 has reduced plaque size without adversely affecting SMC or collagen content. IL-33 also did not affect the final body weight of the mice (PBS-treated, 30.8 ± 0.8 g vs. IL-33–treated, 30.6 ± 0.9 g), total serum cholesterol (23.6 ± 2.7 vs. 19.9 ± 1.9 mmol/liter), HDL-c (2.3 ± 0.2 vs. 2.1 ± 0.1 mmol/liter), very (V) LDL/LDL-c (14.6 ± 2.4 vs. 15.4 ± 2.2 mmol/liter), or triglyceride (1.5 ± 0.2 vs. 1.3 ± 0.2 mmol/liter) concentrations. It is therefore unlikely that changes in lipoprotein levels account for the decreased lesion size. Schmitz et al. have demonstrated vascular changes in medium and small arteries of lung in mice treated with 0.4 μg IL-33 protein i.p. daily for 7 d (12). Histological analysis of all organs in mice at the end of this study demonstrated some lung vascular changes (mild medial hypertrophy) in only 1/12 mice. Hence, treatment with IL-33 did not appear to consistently induce lung pathological changes in our model. Overall, these results demonstrate that IL-33 effectively reduces the development of atherosclerotic plaques in the ApoE−/− model of atherosclerosis.
Figure 2.
IL-33 treatment reduced atherosclerotic plaque size and macrophage and T cell accumulation in the aortic sinus of ApoE−/− mice. ApoE−/− mice were treated with PBS (open bars) or IL-33 (filled bars). (A) Plaque size (intimal area, mm2) in the aortic sinus (n = 12–15). (B) Representative photomicrographs of hematoxylin and eosin–stained aortic sections. (C) Quantification of plaque content: F4/80+ macrophages (percentage of total plaque area; n = 11–12), T cells (number of CD3+ T cells/millimeter2, n = 10–11), SMCs (percentage of total plaque area, n = 9–10), and collagen (percentage of total plaque area, n = 9–10). Data are the mean ± the SEM. (D) Representative photomicrographs of F4/80+ macrophages (brown), CD3+ T cells (brown), α-smooth muscle actin (brown), and collagen (blue) staining in plaques of ApoE−/− mice treated with PBS or IL-33. Data shown are from two independent experiments. *, P < 0.05; ***, P < 0.001, Student's unpaired t test. Bars: (B) 400 μm; (D; F4/80+ macrophages) 25 μm; (D; CD3+ T cells) 25 μm; (D; α-smooth muscle actin) 100 μm; (D; collagen) 100 μm.
Treatment with IL-33 induces Th2 cytokines in serumand lymph node cells of ApoE−/− mice
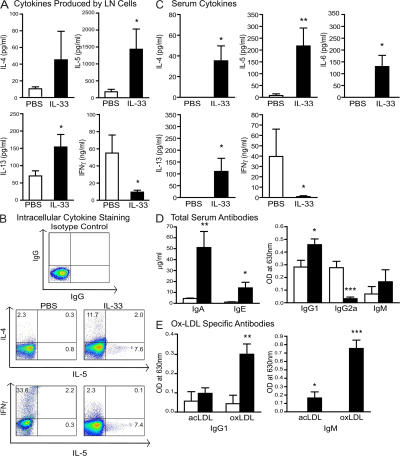
The immunological profile of the ApoE−/− mice treated with IL-33 was investigated by assessing cytokine production by lymph node cells in vitro and in serum ex vivo. First, we assessed the level of ST2 expression, which is known to be expressed on Th2 cells, but not Th1 cells (13, 19). Flow cytometric analysis of lymph node cells demonstrated that IL-33 treatment induced a significantly higher percentage of ST2+CD4+ cells compared with controls (3.6 ± 0.2 vs. 1.9 ± 0.6%; P < 0.05). To examine their cytokine secretion profile, lymph node cells were cultured in vitro with anti-CD3 antibody and culture supernatants examined by ELISA. Supernatants derived from IL-33–treated mice showed significantly elevated concentrations of IL-5 and -13, and markedly reduced IFNγ compared with the supernatants of cells from control mice treated with PBS (Fig. 3 A). IL-4 levels were low and not significantly different.
Figure 3.
IL-33 induced Th2 cytokines, Th2 antibodies, and ox-LDL–specific antibodies in serum and lymph node cells of ApoE−/− mice. ApoE−/− mice were treated with PBS (open bars) or IL-33 (filled bars). (A) Cytokine production (IL-4, -5, -13, and IFNγ) by lymph node cells restimulated with αCD3 and measured by ELISA. (B) Intracellular cytokine staining (IL-4, -5, and IFNγ) in CD4+ lymph node cells stimulated with PMA/ionomycin for 4 h. Numbers indicate the percentage of positive cells in each quadrant. (C) IL-4, -5, -6, -13 (all picogram/milliliter), and IFNγ (nanogram/milliliter) in serum measured by Luminex assay. (D) Total serum concentrations of Ig isotypes (IgA, IgE, IgG1, IgG2a, and IgM). (E) Ox-LDL–specific IgM and IgG1 antibodies in serum. Data shown are the mean ± the SEM. n = 10–13 mice/group from 2 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, Student's unpaired t test.
Recently, IL-17 was reported to be increased in plasma of unstable angina and acute myocardial infarction patients (20), suggesting a potential role for this cytokine in coronary atherosclerosis. Therefore, we examined IL-17 production in our model. Similar levels of IL-17 were produced by lymph node cells from both groups of mice (PBS-treated, 380 ± 117 vs. IL-33–treated, 441 ± 73 pg/ml), and IL-17 was below the limit of detection in serum by Luminex, thus indicating that Th17 cells were not induced by IL-33.
Intracellular cytokine staining in CD4+ lymph node cells demonstrated an increase in the percentage of IL-4+ and -5+ cells and a decrease in the percentage of IFNγ+ cells in IL-33–treated mice (Fig. 3 B). These results were paralleled in serum where Luminex analysis demonstrated that IL-33–treated mice produced markedly more IL-4, -5, -6, and -13, but no detectable IFNγ (Fig. 3 C). Levels of other cytokines (IL-1β, -2, -10, -12[p70], TNFα, and MCP-1) in serum were low or absent. These results are consistent with a switch from a Th1 to a Th2 immunological profile. Although the molecular mechanism by which IL-33 shifts the balance between Th1 and Th2 cells is currently unknown and is being addressed, it likely involves a direct expansion of a subset of peripheral ST2+ T cells.
IL-33 treatment also enhanced IL-6 synthesis, but not IL-10 production. Although IL-6 is generally regarded as a proinflammatory cytokine, IL-6–deficient LDLR−/− mice had similar atherosclerotic lesions, as the IL-6–sufficient LDLR−/− mice (12), suggesting that IL-6 may not play an important role in this model of atherosclerosis. Our data, however, are consistent with two other recent reports demonstrating that ApoE−/−xIL-6−/− mice developed larger atherosclerotic lesion than IL-6 intact ApoE−/− mice (21) and that treatment with recombinant IL-6 resulted in smaller plaque lesions in ApoE−/− mice (22). The absence of IL-10 may suggest that IL-33 treatment did not invoke a role for regulatory T cells, which have recently been shown to be protective against mouse atherosclerosis (23).
Ox-LDL–specific antibodies are induced in serumof ApoE−/− mice by IL-33 treatment
Atherosclerosis is associated with antibody formation to oxidatively modified LDL, and elevated levels of this antibody predict regression of atherosclerosis in humans (2). We assessed the humoral response to IL-33 treatment by quantifying antibody titers in serum. Th1 cells induce IgG2a production, whereas Th2 cells facilitate IgG1 and IgE synthesis. We also assessed levels of ox-LDL–specific antibodies, as this is likely to be one of the most prevalent presumptive autoantigens present in ApoE−/− mice on a high-fat diet. The IL-33–treated mice produced more total IgA, IgE, and IgG1, but less IgG2a, than control mice, demonstrating a strong Th2 bias (Fig. 3 D). Importantly, IL-33–treated mice also produced significantly elevated IgG1 antibody to ox-LDL. Interestingly, IgM antibody to both native acetyl-LDL and ox-LDL were significantly increased in IL-33–treated mice, despite similar total levels of IgM in both groups (Fig. 3 E). Antibodies to ox-LDL of the IgG2a, IgA, and IgE isotypes were absent in both groups of mice, and all LDL-specific antibodies were low or absent in PBS-treated mice.
sST2 exacerbated atherosclerosis development
Previous studies in humans have demonstrated that increased sST2 is an adverse prognostic sign in cardiovascular patients (14, 15). Although sST2 is a potentially useful biomarker in heart disease, its biological role in atherosclerosis remains unclear. To confirm that IL-33 mediates its antiatherosclerotic functions through its receptor ST2, we treated ApoE−/− mice on high-fat diet with sST2 (an Fc fusion protein), which can neutralize IL-33 activity by acting as a decoy receptor of ST2 (12). Male ApoE−/− mice aged 6 wk were fed a high-fat diet for 12 wk. During the last 6 wk, mice were injected i.p. twice per week with sST2 or normal IgG control (50 μg/injection). Mice were killed at the end of the treatment, and atherosclerotic lesion size was quantified in the aortic sinus. ApoE−/− mice treated with sST2 developed significantly larger atherosclerotic plaque in the aortic sinus compared with control IgG-treated mice (0.66 ± 0.05 vs. 0.35 ± 0.05 mm2; P = 0.0015; Fig. 4, A and B). However, this was not caused by increased immune cell infiltration, as the cellular composition of the lesions was similar between the two groups. The percentage of plaque area staining positive for F4/80+ macrophages (45.3 ± 4.7 vs. 47.0 ± 5.0%) and the number of lesion-associated CD3+ T lymphocytes (5,936 ± 745 vs. 5,632 ± 344 cells/mm2) was similar between mice treated with sST2 or control IgG (Fig. 4, C and D). SMC and collagen content did not differ between the two groups (29.1 ± 2.3 vs. 32.2 ± 3.9% and 26.2 ± 1.1 vs. 25.6 ± 1.3%, respectively; Fig. 4, C and D). Similar concentrations of total serum cholesterol (16.4 ± 1.9 vs. 20.2 ± 3.1 mmol/liter), HDL-c (2.8 ± 0.8 vs. 2.4 ± 0.3 mmol/liter), VLDL/LDL-c (22.0 ± 2.6 vs. 20.7 ± 2.4 mmol/liter), or triglyceride (1.4 ± 0.2 vs. 1.7 ± 0.3 mmol/liter) were found. It is therefore unlikely that changes in lipoprotein levels account for the aggravation of lesion size by the treatment with sST2 in these mice.
Figure 4.
sST2 exacerbated atherosclerosis development in the aortic sinus and promoted Th1 response of ApoE−/− mice. ApoE−/− mice were treated with control IgG (open bars) or sST2 (filled bars). (A) Plaque size (intimal area, millimeter2) in the aortic sinus (n = 9–10). (B) Representative photomicrographs of hematoxylin and eosin–stained aortic sections. (C) Quantification of plaque content: F4/80+ macrophages (percentage of total plaque area, n = 9–10), T cells (number of CD3+ T cells/millimeter2, n = 9–10), SMCs (percentage of total plaque area, n = 9–10), and collagen (percentage of total plaque area, n = 9–10). (D) Representative photomicrographs of F4/80+ macrophages (brown), CD3+ T cells (brown), α-smooth muscle actin (brown), and collagen (blue) staining in plaques of ApoE−/− mice treated with IgG or sST2. (E) IFNγ production (nanogram/milliliter) by lymph node cells restimulated with αCD3 and measured by ELISA (n = 9–10). IL-5 (F) and IFNγ (G; picogram/milliliter) in serum determined by Luminex assay (n = 9–10). *, P < 0.05; ***, P < 0.001, Student's unpaired t test. Bars: (B) 400 μm; (D; F4/80+ macrophages) 25 μm; (D; CD3+ T cells) 25 μm; (D; α-smooth muscle actin) 100 μm; (D; collagen) 100 μm.
Immunological analysis of the mice revealed a significant increase in IFNγ production by lymph node cells in mice treated with sST2 (Fig. 4 E). Levels of IL-4, -5, and -13 in cell culture supernatants were low or absent. In addition, a significant decrease in IL-5 (Fig. 4 F) and a marked increase in IFNγ (Fig. 4 G) were detected in serum of mice treated with sST2. Levels of other cytokines (IL-1β, -2, -4, -6, -10, -12[p70], -13, TNFα, and MCP-1) in serum were low or absent. Specific antibodies against ox-LDL could not be detected in either sST2-treated or control IgG-treated mice. These results therefore confirm that IL-33 attenuated atherosclerosis via ST2 signaling and highlight an sST2-mediated increase in IFNγ and atherosclerosis as a potential contributor to the adverse prognosis in cardiovascular patients with high sST2 in serum.
Anti–IL-5 mAb prevented the reduction in plaque sizeand the induction of ox-LDL antibodies in serum by IL-33
To investigate the mechanism of the atheroprotective effect of IL-33 further, we treated ApoE−/− mice on high-fat diet with either IL-33 alone (1 μg/injection) or IL-33 plus a neutralizing antibody to IL-5 (TRFK-5; 15 μg/injection) twice per week during the last 6 wk of high-fat diet. Coadministration of αIL-5 plus IL-33 completely reversed the protective effect of IL-33 on plaque growth (0.14 ± 0.05 vs. 0.49 ± 0.1 mm2; P = 0.0014; Fig. 5, A and B). Plaque phenotype was similar between the two groups (Fig. 5, C and D), indicating that IL-5 was not responsible for the decreased macrophage and T cell infiltration seen with IL-33 when compared with PBS treatment. However, the induction of ox-LDL–specific IgM antibodies was significantly reduced in the αIL-5–treated group (Fig. 5 E). The levels of IL-5 in serum (Fig. 5 F) were significantly lower, confirming neutralization by the αIL-5 antibody, with no effect on other cytokines such as IL-12. Similar concentrations of total serum cholesterol (20.4 ± 1.4 vs. 23.2 ± 2.6 mmol/liter), HDL-c (2.1 ± 0.7 vs. 2.1 ± 0.1 mmol/liter), VLDL/LDL-c (18.4 ± 2.2 vs. 20.9 ± 4.7 mmol/liter), or triglyceride (1.5 ± 0.1 vs. 1.3 ± 0.2 mmol/liter) were found. It is therefore unlikely that changes in lipoprotein levels account for the larger lesion size induced by the treatment with αIL-5 plus IL-33 in these mice.
Figure 5.
An anti–IL-5 mAb prevented the reduction in plaque size and the induction of ox-LDL antibodies in serum by IL-33. ApoE−/− mice were treated with IL-33 (filled bars) or IL-33/αIL-5 (striped bars). (A) Plaque size (intimal area, millimeter2) in the aortic sinus (n = 5). (B) Representative photomicrographs of hematoxylin and eosin–stained aortic sections. (C) Quantification of plaque content: F4/80+ macrophages (percentage of total plaque area, n = 5), T cells (number of CD3+ T cells/ millimeter2, n = 5), SMCs (percentage of total plaque area, n = 5), collagen (percentage of total plaque area, n = 5). (D) Representative photomicrographs of F4/80+ macrophages (brown), CD3+ T cells (brown), α-smooth muscle actin (brown), and collagen (blue) staining in plaques of ApoE−/− mice treated with IL-33 or IL-33/αIL-5. (E) Ox-LDL–specific IgM antibodies in serum (n = 5). (F) IL-5 and -12 (picogram/milliliter) in serum determined by ELISA (n = 5). *, P < 0.05; **, P < 0.01, Student's unpaired t test. Bars: (B) 400 μm; (D; F4/80+ macrophages) 25 μm; (D; CD3+ T cells) 25 μm; (D; α-smooth muscle actin) 100 μm; (D; collagen) 100 μm.
IL-5 is an important factor in the maturation and Ig-secretion of B cells (24), and IL-33–induced IL-5 secretion mediates the expansion of ox-LDL–specific antibodies in these mice. Deficiency of IL-5 has been reported to reduce the production of atheroprotective antibodies and result in accelerated atherosclerosis in ApoE−/− mice (11). Furthermore, antibodies specific for ox-LDL inhibit macrophage uptake of ox-LDL, thus reducing foam cell formation (25). In rabbit and murine models, it has been shown that immunization with ox-LDL induces antibody formation (both IgG and IgM) and protects against atherosclerosis development (26). These studies, in addition to this study with IL-33, support a protective role of antibody-mediated effects on atherosclerosis and suggest that an IL-5–induced anti–ox-LDL antibody response is likely the mechanism by which IL-33 mediates it atheroprotective effect.
This is the first study demonstrating a modulatory role for IL-33 in the inflammation of atherosclerosis. We observed a decrease in lesion size in the aortic sinus with IL-33 treatment, in addition to an induction of Th2-mediated immunity (IL-4/-5/-13), reduced Th1 responses (IFNγ), and an increase in ox-LDL–specific antibodies. Furthermore, IL-33 treatment decreased the numbers of plaque-associated macrophages and T cells infiltrating into the atherosclerotic lesions, suggesting a reduced inflammatory response to the accumulating lipid in the vessel wall. In conclusion, our study suggests that induction of Th2-associated cytokines and ox-LDL–specific antibody responses by IL-33 may represent a novel atheroprotective pathway.
MATERIALS AND METHODS
Reagents.
The IL-33 and sST2-Fc fusion proteins were prepared as previously described (27, 28). Control IgG-Fc (Sigma-Aldrich) and αIL-5 antibody (TRFK-5; Insight Bio) were obtained commercially.
Animals.
Male ApoE−/− mice on the C57BL/6 background (backcrossed 10 times; Charles River Laboratories) were bred in-house in a pathogen-free facility, weaned at 6 wk of age, and fed an atherogenic diet ad libitum (0.15% cholesterol and 21% lard; Special Diet Services). At 12 wk, mice were randomly grouped and injected i.p. twice per week for 6 wk with control PBS or murine IL-33 (1 μg/injection) or murine IL-33 (1 μg/injection), plus anti–IL-5 antibody (TRFK-5; 15 μg/injection), control IgG, or sST2 (50 μg/injection) diluted in PBS. Mice were killed at 18 wk of age. C57BL/6 mice were purchased from Harlan. Experiments were performed according to United Kingdom Home Office guidelines.
Primary cell culture.
Human umbilical vein ECs and coronary artery SMCs were purchased from TCS Cellworks Botolph Claydon. Human saphenous vein (HSV) ECs and SMCs were prepared as previously described (29).
Quantitative PCR.
RNA was extracted and purified using an RNeasy Micro kit (QIAGEN). mRNA expression was analyzed by quantitative PCR using SYBR GREEN PCR Master Mix and 18S rRNA (both from Applied Biosystems) as an endogenous control on the 7900HT RT-PCR system (Applied Biosystems). All primer sequences are available upon request.
Serum analysis.
Total serum cholesterol and triglyceride levels (millimole/liter) were measured by enzymatic assay (Roche). HDL, VLDL, and LDL cholesterol distribution were measured using a BioVision kit, and therefore values do not exactly add up to the total cholesterol measurements. Serum Ig isotypes (IgE, IgA, IgG1, IgG2a, and IgM) were measured by ELISA (1:1,000 sera dilution) and specific antibody pairs (BD Biosciences). Copper oxidation of LDL was performed as previously described (25). To quantify ox-LDL–specific antibodies, plates were coated with 100 μg/ml native acetyl- or ox-LDL, washed, and blocked, and then sera were added at an optimized 1:50 dilution and specific detection antibodies for IgA, IgE, IgG, IgG2A, and IgM (BD Biosciences) were added. Serum cytokines were analyzed in an 11-plex mouse cytokine assay (Invitrogen).
Cytokine analysis.
Peripheral lymph nodes were removed, and single-cell suspensions were obtained by passing the cells through a 70-μm cell strainer. ST2 expression was examined by flow cytometry using a FITC anti-ST2 antibody (MD Biosciences). Cells were stimulated in microwell cultures (2 × 106/well) with plate-bound anti-CD3 (2.5 μg/ml; BD Biosciences) in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Supernatants were collected at 72 h and analyzed by ELISA for IL-4, -5, -13, -17, and IFN-γ using paired antibody reagents (BD Biosciences/R&D Systems). For intracellular cytokine staining, cells were stimulated for 4 h with 50 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of 1 mg/ml Golgi-Plug (BD Biosciences). The cells were fixed, permeabilized, and stained with anti-CD4 and –IL-4, –IL-5, or -IFNγ (BD Biosciences).
Atherosclerotic lesion analysis.
Atherosclerosis was quantified in the aortic sinus, as previously described (30). In brief, the heart was sectioned parallel to the atria and sections were fixed, processed, and embedded in paraffin wax. 5 7-μm sections starting from the three valve cusps of the aortic sinus at 35-μm intervals were cut and stained with hematoxylin and eosin for plaque area measurement in each mouse using Scion Image software (Scion Corporation).
Immunohistochemical staining was performed using the following molecule-specific and isotype-control antibodies as a negative control: anti-CD3 (A0452; Dako) for T cells, anti-F4/80 (Clone CI:A3-1; Serotec) for macrophages, anti-smooth muscle actin (Clone 1A4; Sigma-Aldrich) for SMCs, anti-CD31 for ECs (BD Biosciences), and anti–IL-33 (R&D Systems). Staining was visualized using biotinylated secondary antibodies (Vector Laboratories) and detection with the ABC/DAB system. Collagen was stained using Gomori's One-Step Trichrome protocol. Macrophages, SMCs, and collagen were quantified by assessing the percentage of total plaque area stained positive for each marker, and T cells by counting the number of CD3+ T cells/millimeter2 of plaque.
Statistical analysis.
All data are the mean ± the SEM. Statistical analysis was performed using unpaired Student's t tests with Prism Software (GraphPad).
Acknowledgments
We thank Drs. G. Murphy, N. Pitman, D. Gilchrist, M. Kurowska-Stolarska, and J.A. Gracie for experimental assistance and advice, and Dr. L. Cherry for the serum lipid measurements.
This study received financial support from the Medical Research Council UK, the Wellcome Trust, and the British Heart Foundation.
The authors have no conflicting financial interests.
References
- 1.Hansson, G.K., and P. Libby. 2006. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6:508–519. [DOI] [PubMed] [Google Scholar]
- 2.Salonen, J.T., S. Yla-Herttuala, R. Yamamoto, S. Butler, H. Korpela, R. Salonen, K. Nyyssonen, W. Palinski, and J.L. Witztum. 1992. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 339:883–887. [DOI] [PubMed] [Google Scholar]
- 3.Major, A.S., S. Fazio, and M.F. Linton. 2002. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 22:1892–1898. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri, G., A. Nicoletti, B. Poirier, and G.K. Hansson. 2002. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 109:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer, O.J., A.C. van der Wal, C.E. Verhagen, and A.E. Becker. 1999. Cytokine secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J. Pathol. 188:174–179. [DOI] [PubMed] [Google Scholar]
- 6.Zhou, X., A. Nicoletti, R. Elhage, and G.K. Hansson. 2000. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 102:2919–2922. [DOI] [PubMed] [Google Scholar]
- 7.Davenport, P., and P.G. Tipping. 2003. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitman, S.C., P. Ravisankar, and A. Daugherty. 2002. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ. Res. 90:E34–E38. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, S., A.M. Pablo, X. Jiang, N. Wang, A.R. Tall, and C. Schindler. 1997. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99:2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buono, C., C.J. Binder, G. Stavrakis, J.L. Witztum, L.H. Glimcher, and A.H. Lichtman. 2005. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. USA. 102:1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder, C.J., K. Hartvigsen, M.K. Chang, M. Miller, D. Broide, W. Palinski, L.K. Curtiss, M. Corr, and J.L. Witztum. 2004. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114:427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz, J., A. Owyang, E. Oldham, Y. Song, E. Murphy, T.K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, et al. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 23:479–490. [DOI] [PubMed] [Google Scholar]
- 13.Xu, D., W.L. Chan, B.P. Leung, F. Huang, R. Wheeler, D. Piedrafita, J.H. Robinson, and F.Y. Liew. 1998. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 187:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg, E.O., M. Shimpo, S. Hurwitz, S. Tominaga, J.L. Rouleau, and R.T. Lee. 2003. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 107:721–726. [DOI] [PubMed] [Google Scholar]
- 15.Shimpo, M., D.A. Morrow, E.O. Weinberg, M.S. Sabatine, S.A. Murphy, E.M. Antman, and R.T. Lee. 2004. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 109:2186–2190. [DOI] [PubMed] [Google Scholar]
- 16.Sanada, S., D. Hakuno, L.J. Higgins, E.R. Schreiter, A.N. McKenzie, and R.T. Lee. 2007. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Invest. 117:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedgui, A., and Z. Mallat. 2006. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 86:515–581. [DOI] [PubMed] [Google Scholar]
- 18.Carriere, V., L. Roussel, N. Ortega, D.A. Lacorre, L. Americh, L. Aguilar, G. Bouche, and J.P. Girard. 2007. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA. 104:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohning, M., A. Stroehmann, A.J. Coyle, J.L. Grogan, S. Lin, J.C. Gutierrez-Ramos, D. Levinson, A. Radbruch, and T. Kamradt. 1998. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA. 95:6930–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashmi, S., and Q.T. Zeng. 2006. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 17:699–706. [DOI] [PubMed] [Google Scholar]
- 21.Schieffer, B., T. Selle, A. Hilfiker, D. Hilfiker-Kleiner, K. Grote, U.J. Tietge, C. Trautwein, M. Luchtefeld, C. Schmittkamp, S. Heeneman, et al. 2004. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 110:3493–3500. [DOI] [PubMed] [Google Scholar]
- 22.Tous, M., V. Ribas, J.C. Escola-Gil, F. Blanco-Vaca, L. Calpe-Berdiel, B. Coll, N. Ferre, C. Alonso-Villaverde, A. Rull, J. Camps, et al. 2006. Manipulation of inflammation modulates hyperlipidemia in apolipoprotein E-deficient mice: a possible role for interleukin-6. Cytokine. 34:224–232. [DOI] [PubMed] [Google Scholar]
- 23.Ait-Oufella, H., B.L. Salomon, S. Potteaux, A.K. Robertson, P. Gourdy, J. Zoll, R. Merval, B. Esposito, J.L. Cohen, S. Fisson, et al. 2006. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12:178–180. [DOI] [PubMed] [Google Scholar]
- 24.Takatsu, K. 1998. Interleukin 5 and B cell differentiation. Cytokine Growth Factor Rev. 9:25–35. [DOI] [PubMed] [Google Scholar]
- 25.Horkko, S., D.A. Bird, E. Miller, H. Itabe, N. Leitinger, G. Subbanagounder, J.A. Berliner, P. Friedman, E.A. Dennis, L.K. Curtiss, et al. 1999. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulthe, J. 2004. Antibodies to oxidized LDL in atherosclerosis development–clinical and animal studies. Clin. Chim. Acta. 348:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Komai-Koma, M., D. Xu, Y. Li, A.N. McKenzie, I.B. McInnes, and F.Y. Liew. 2007. IL-33 is a chemoattractant for human Th2 cells. Eur. J. Immunol. 37:2779–2786. [DOI] [PubMed] [Google Scholar]
- 28.Sweet, M.J., B.P. Leung, D. Kang, M. Sogaard, K. Schulz, V. Trajkovic, C.C. Campbell, D. Xu, and F.Y. Liew. 2001. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J. Immunol. 166:6633–6639. [DOI] [PubMed] [Google Scholar]
- 29.Nicklin, S.A., H. Buening, K.L. Dishart, M. de Alwis, A. Girod, U. Hacker, A.J. Thrasher, R.R. Ali, M. Hallek, and A.H. Baker. 2001. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol. Ther. 4:174–181. [DOI] [PubMed] [Google Scholar]
- 30.Daugherty, A., and S.C. Whitman. 2003. Quantification of atherosclerosis in mice. Methods Mol. Biol. 209:293–309. [DOI] [PubMed] [Google Scholar]