Abstract
Staphylococcus aureus pneumonia causes significant mortality in hospitalized or healthy individuals, and recent increases in morbidity are attributed to the rapid spread of methicillin-resistant S. aureus (MRSA) strains, which are often not susceptible to antibiotic therapy. α-Hemolysin (Hla), a secreted pore-forming toxin, is an essential virulence factor of MRSA in a mouse model of S. aureus pneumonia. We show that the level of Hla expression by independent S. aureus strains directly correlates with their virulence. Active immunization with a mutant form of Hla (HlaH35L), which cannot form pores, generates antigen-specific immunoglobulin G responses and affords protection against staphylococcal pneumonia. Moreover, transfer of Hla-specific antibodies protects naive animals against S. aureus challenge and prevents the injury of human lung epithelial cells during infection. Thus, Hla vaccination or immunotherapy may prevent S. aureus pneumonia in humans.
Staphylococcus aureus is an important human pathogen that, in addition to soft tissue and bloodborne infections, causes pneumonia in adult and pediatric populations (1). Several reports have described the growing incidence of severe S. aureus pneumonia in otherwise healthy individuals, often caused by methicillin-resistant S. aureus (MRSA) (2, 3). Further, S. aureus is one of the most common causes of ventilator-assisted pneumonia associated with significant morbidity and mortality (4). We recently developed an animal model of S. aureus–induced pneumonia in adult, immunocompetent C57BL/6J mice that closely mimics the clinicopathological features of human disease (5). Using this experimental system and the human clinical S. aureus isolate Newman (6), the contributions of surface proteins and exotoxins toward the pathogenesis of pneumonia were documented (5) by revealing virulence defects in sortase A and accessory gene regulator A mutant strains that are either unable to display cell wall–anchored surface proteins (7) or cannot express factors destined for secretion into the extracellular medium (8), respectively.
S. aureus α-hemolysin (Hla; also known as α-toxin) is the founding member of a family of bacterial pore-forming β-barrel toxins (9, 10). Its structural gene, hla, is located on the chromosome of S. aureus strains, most of which secrete the 293-residue water-soluble monomer (11). Hla is known to play a role in the pathogenesis of staphylococcal disease, as S. aureus mutants lacking hla display reduced virulence in invasive disease models. In these experimental systems, larger numbers of staphylococci are required to kill mice after either i.p. or intramammary infection (12, 13). Further supporting the role of Hla in virulence, passive immunization of mice with anti-Hla antisera affords protection from challenge both with purified toxin as well as live staphylococci in the i.p. infection model (14).
Hla is thought to engage surface receptors of sensitive host cells, thereby promoting toxin oligomerization into a heptameric prepore and insertion of a β-barrel structure with a 2-nm pore diameter into the plasma membrane (15). Hla pores form in lymphocytes, macrophages, alveolar epithelial cells, pulmonary endothelium, and erythrocytes; however, granulocytes and fibroblasts appear resistant to lysis (9, 16). Instillation of purified Hla into rabbit or rat lung tissue triggers vascular leakage and pulmonary hypertension, which has been attributed to the release of different signaling molecules (e.g., phosphatidyl inositol, nitric oxide, prostanoids, and thromboxane A2) (16–19). In agreement with the biochemical properties of Hla, mutations that abrogate hla expression in S. aureus Newman abolish virulence in the mouse pneumonia model (5). We therefore examined Hla as a target for the development of vaccines that combat S. aureus lung infection.
RESULTS AND DISCUSSION
Hla and the mortality of staphylococcal lung infections
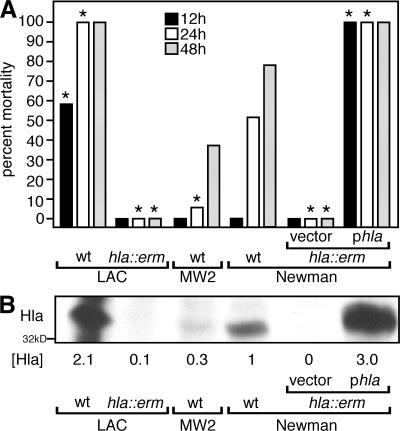
Informed by the absolute genetic requirement of hla in S. aureus strains Newman and LAC for the pathogenesis of pneumonia (5, 20), we asked whether strain-based differences in the level of Hla secretion correlate with disease severity. As a test for this conjecture, groups of 10 animals each were infected via the intranasal (i.n.) route with the community-associated MRSA (CA-MRSA) isolates LAC (USA 300, capsular polysaccharide [CP] type 5) (21, 22) and MW2 (USA400, CP type 8) (3), the methicillin-sensitive S. aureus (MSSA) isolate Newman (CP type 5), and the isogenic variants of LAC and Newman with a bursa aurealis insertion in hla (hla∷erm). The Newman hla∷erm mutant was transformed with plasmid vector (vector) or a complementing plasmid encoding Hla (phla). Animals were infected with 3–4 × 108 CFU of each isolate, and mortality from pneumonia was recorded after infection (Fig. 1) (5). Animals infected with LAC or the Newman hla∷erm variant harboring phla were highly susceptible to pneumonia and died rapidly compared with animals infected with wild-type strain Newman (P = 0.017 and 0.00001, respectively, at 12 h; and P = 0.03 for both at 24 h; Fig. 1 A). In contrast, animals infected with MW2 displayed even fewer symptoms and signs of disease and less mortality (P = 0.046 at 24 h; Fig. 1 A). As expected, the Newman hla∷erm variant carrying vector alone was completely avirulent (5), as was the LAC hla∷erm mutant (Fig. 1 A). Immunoblotting of samples derived from various S. aureus strains grown to equal cell density revealed the relative abundance of Hla secretion in the extracellular medium (Fig. 1 B); densitometry readings from each strain were obtained, normalized to the signal derived from S. aureus Newman, and recorded as [Hla] (Fig. 1 B). A comparison of the signal density of Hla in the immunoblot with animal mortality at 12, 24, and 48 h revealed correlation coefficients of 0.96, 0.96, and 0.9, respectively. Thus, not only is Hla required for the pathogenesis of S. aureus pneumonia, as we have previously demonstrated (20), but the relative level of Hla expression by distinct S. aureus strains also correlates with the virulence properties of the organism.
Figure 1.
S. aureus secretion of Hla correlates with mortality from lung infection. (A) Animals infected via the i.n. route (10 animals per group) with 3–4 × 108 wild-type S. aureus LAC, MW2, and Newman strains or the Hla-deficient strain (hla∷erm) complemented with either vector or phla revealed strain-dependent differences in mortality. LAC and Newman hla∷erm phla caused marked mortality at 12 h (P = 0.017 and 0.00001, respectively) and 24 h (P = 0.03 for both strains) compared with Newman wild-type. In contrast, infection with the MW2 strain resulted in a lower mortality compared with Newman (P = 0.046); infection with LAC hla∷erm and Newman hla∷erm demonstrated these strains to be avirulent. Statistical significance is indicated by an asterisk. (B) Strain-based differences in the level of Hla secretion. Immunoblot analysis of 18-h culture supernatants revealed increased Hla production by LAC and Newman hla∷erm phla compared with wild-type Newman, whereas MW2 produces lesser amounts of Hla. Amounts of Hla ([Hla]) secreted by each strain were quantified by evaluation of chemiluminescence signals derived from antibody binding to antigen and were recorded relative to the amount of Hla secreted by Newman.
Immunization with HlaH35L protects against S. aureus pneumonia
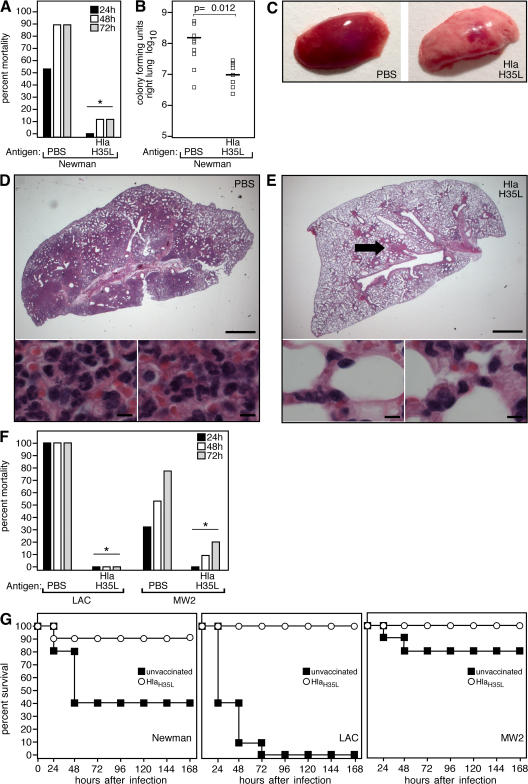
The essential role of Hla in the pathogenesis of S. aureus pneumonia raised the possibility that antibody-mediated antagonism of toxin activity may protect experimental animals from disease. To test whether Hla-specific immune responses affect the pathogenesis of staphylococcal pneumonia, 4-wk-old mice were injected i.m. with a mock control (PBS) emulsified in CFA or were immunized with 20 μg of purified HlaH35L emulsified in CFA, then boosted 10 d later with the same amount of antigen (or PBS) in IFA. HlaH35L, a variant toxin with a single amino acid substitution, cannot form cytolytic pores but retains the ability to bind its putative receptor on host target cells (14, 23). At the time of challenge, a mean HlaH35L-specific antibody titer of 1:5,601 ± 2,789 was measured in the serum of immunized animals. Upon i.n. challenge with 3–4 × 108 CFU S. aureus Newman, a significant decrease in mortality was observed in HlaH35L-immunized animals (P = 0.001; 10 animals per group; Fig. 2 A). This decrease correlated with a reduction in S. aureus CFU recovered from the lung at 24 h after infection (P = 0.012; 10 animals per group; Fig. 2 B). Gross pathological analysis of infected lung tissues revealed focal areas of consolidation in HlaH35L-immunized animals, in contrast to the diffuse consolidation observed in PBS-immunized animals (Fig. 2 C); these observations were mirrored in histopathologic sections of infected lung tissue. The majority of alveolar space in mock-immunized animals was obliterated by inflammatory exudate, immune cell infiltrate, and staphylococci (Fig. 2 D). In contrast, lesions in HlaH35L-immunized animals were discrete and surrounded by large unaffected areas of lung tissue (Fig. 2 E). To examine the effect of immunization on the pathogenesis of lung infections caused by clinically relevant S. aureus isolates, HlaH35L-immunized animals were infected via the i.n. route with LAC or MW2 (10 animals per group). Although the absolute mortality caused by these strains differed from each other, as previously demonstrated (20), significant protection was achieved in all HlaH35L-immunized animals (P = 0.00001 for LAC; P = 0.018 for MW2; Fig. 2 F). Importantly, this protection was observed over a 1-wk course after infection with the Newman, LAC, and MW2 isolates (Fig. 2 G), indicating that disease progression is blocked, not simply delayed, in presentation. Thus, active immunization targeting Hla protects animals from S. aureus pneumonia; this protection correlates with reduced microbiologic and pathological evidence of disease.
Figure 2.
Immunization with HlaH35L protects mice against S. aureus pneumonia. (A) C57BL/6J mice were immunized by i.m. injection with PBS or 20 μg HlaH35L, a mutant Hla with a single amino acid substitution that abolishes toxin activity, and challenged with S. aureus Newman. Mortality was recorded 24, 48, or 72 h after infection (P = 0.001; 10 animals per group). (B) Immunization of mice with HlaH35L reduces the growth of S. aureus Newman in infected lung tissue (P = 0.012; 10 animals per group). Horizontal bars indicate the mean of bacterial load measurements. (C) Gross pathology of S. aureus Newman–infected lung tissue from mice that were immunized with PBS or HlaH35L. (D and E) Histopathology of S. aureus Newman–infected lung tissue from mice that were immunized with PBS or HlaH35L. The arrow in E points to the focal area of consolidation in lung tissue of HlaH35L-immunized animals. Bars: (top) 0.1 cm; (bottom) 10 μm. (F) HlaH35L-immunized C57BL/6J mice were challenged via the i.n. route with S. aureus CA-MRSA strains LAC or MW2 (10 animals per group). Mortality was recorded 24, 48, or 72 h after infection. The mortality of HlaH35L-immunized animals was significantly reduced over that of mock (PBS)-immunized animals challenged with either S. aureus LAC (P = 0.00001) or MW2 (P = 0.018). Statistical significance in A and F is indicated by an asterisk. (G) Survival curves of either unvaccinated or HlaH35L-immunized animals over a 168-h time course after infection with S. aureus Newman (left), LAC (middle), or MW2 (right).
Anti-Hla prevents S. aureus injury of human lung cells
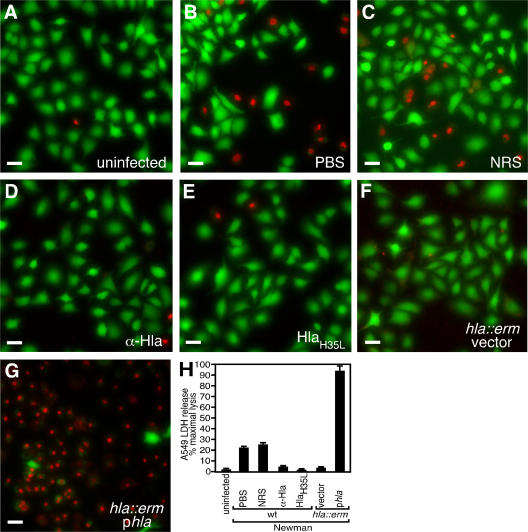
Several reports have described species-specific effects of S. aureus virulence factors in the pathogenesis of disease; among these are the phage-encoded chemotaxis inhibitory protein of S. aureus and staphylococcal complement inhibitor, immunomodulatory proteins that specifically antagonize the function of the human immune system (24). Similarly, rabbit erythrocytes are 1,000-fold more sensitive to Hla-induced cytolysis than human erythrocytes (9). To assess the contribution of Hla to the injury of human lung tissue, we analyzed the effects of S. aureus clinical isolates on human A549 alveolar epithelial cells, which have been used to model human pulmonary epithelia in a diverse array of biological and physiological studies (25, 26). When infected with S. aureus Newman, A549 injury was observed by microscopic evaluation of cells stained with a live/dead (green/red) reagent (Fig. 3). Uninfected cells reveal the stellate nature of the A549 line (Fig. 3 A); co-culture of A549 cells with live Newman in the presence of PBS (Fig. 3 B) or normal rabbit sera (NRS; Fig. 3 C) revealed cell death. Addition of rabbit anti-Hla (α-Hla) to S. aureus Newman–infected cultures led to marked reduction in A549 injury (Fig. 3 D). Furthermore, the addition of purified HlaH35L (at a concentration of 10 μg/ml) also afforded protection against staphylococcal cell injury (Fig. 3 E), presumably through its ability to bind to the A549 cell membrane and antagonize the function of the wild-type toxin (not depicted) (23). S. aureus hla∷erm variants were unable to destroy A549 cells (hla∷erm vector; Fig. 3 F), a defect that was complemented by plasmid-encoded expression of Hla (hla∷erm phla; Fig. 3 G). Cellular injury in this co-culture system was quantitated by a lactate dehydrogenase (LDH) cytotoxicity assay (Fig. 3 H) in which all conditions were scored as the percent maximal lysis obtained after detergent treatment of A549 cells. Upon demonstration of significance in the analysis of variance test (F = 284.72; P < 0.001), the significance of these studies was assessed via Dunnett's post-test, in which the critical q value (0.05) was calculated to be 2.94. Treatment of infected A549 cells with either PBS or NRS resulted in cell lysis that was 23 and 26% maximal, respectively (QNRS = 1.347). Treatment of S. aureus Newman–infected cells with either α-Hla rabbit antisera or purified HlaH35L abrogated the cytotoxic effect (Qα-Hla = 7.173; QHlaH35L = 8.045). Co-culture with a S. aureus hla∷erm variant transformed with vector failed to cause A459 injury (Qhla∷erm vector = 8.917), whereas plasmid-based expression of Hla resulted in massive cell death (Qhla∷erm p hla = 24.919). Collectively, these data demonstrate the critical role of Hla in human alveolar cell injury and suggest that antagonism of Hla markedly diminishes the ability of S. aureus to effect injury to the pulmonary epithelium.
Figure 3.
S. aureus injury of human alveolar epithelial cells is reduced by antagonism of Hla. Live (green)/dead (red) imaging of human A549 cells was captured by fluorescence microscopy 4 h after infection. A549 cells were left uninfected (A) or co-cultured with S. aureus Newman in media treated with PBS (1:1,000; B), NRS (1:1,000; C), anti-Hla rabbit sera (α-Hla; 1:1,000; D), or purified HlaH35L (10 μg/ml; E). Infections were also performed with the Newman isogenic hla insertion mutant, hla∷erm, transformed with vector (F) or phla (G). Bars, 20 μm. (H) Assessment of LDH release by A549 cells that were lysed during S. aureus infection. A significant reduction in LDH release was observed compared with PBS-treated cultures upon addition of anti-Hla sera or HlaH35L, whereas an increase in LDH release was observed upon co-culture with S. aureus hla∷erm phla. Error bars indicate mean ± SD.
Passive transfer of anti-Hla protects against S. aureus pneumonia
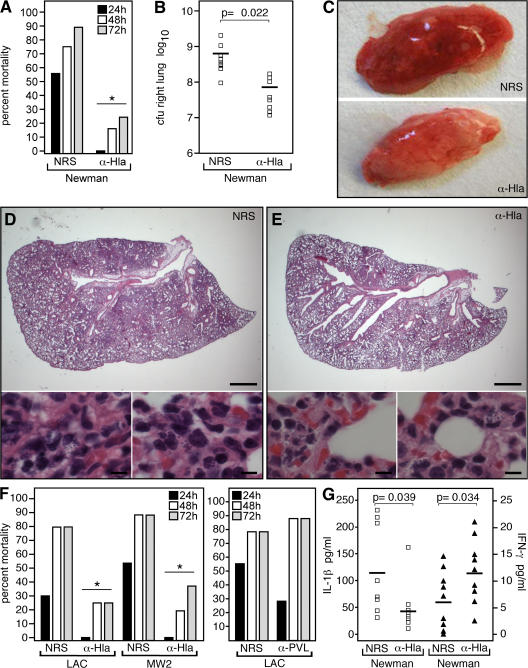
To test whether Hla-specific antibodies can protect against S. aureus lung disease, animals were passively immunized with 100 μl of either NRS or rabbit anti-Hla via i.p. injection 24 h before i.n. challenge with S. aureus Newman; the mean HlaH35L-specific serum antibody titer at the time of infection was 1:480 ± 179 (Fig. 4). Examination of pneumonia-induced mortality revealed protection via transfer of anti-Hla serum but not with control serum (P = 0.018; 10 animals per group; Fig. 4 A). Significant decreases in CFU recovered from the lungs of anti-Hla–immunized animals were observed (P = 0.022; 10 animals per group; Fig. 4 B); this protection correlated with improvements in gross (Fig. 4 C) and histopathologic (Fig. 4, D and E) features of disease. Passive immunoprotection was also effective in animals infected with the clinical isolates LAC and MW2 (P = 0.028 and 0.04, respectively; 10 animals per group; Fig. 4 F, left). Further demonstrating the specificity of this passive immunoprotection, the treatment of animals with rabbit antisera raised against Panton-Valentine leukocidin (PVL) (20), a bicomponent pore-forming secreted cytotoxin of S. aureus, did not confer protection against S. aureus LAC pneumonia (P = 0.45; 10 animals per group; Fig. 4 F, right). This result is in agreement with previous work demonstrating that PVL is dispensable for the pathogenesis of lethal MRSA and MSSA lung infections in C57BL/6J mice (20).
Figure 4.
Passive immunization of mice with anti-Hla protects against S. aureus lung infection. (A) Mice were passively immunized by i.p. injection with NRS or rabbit serum that harbored anti-Hla and were challenged via the i.n. route with S. aureus Newman (P = 0.018; 10 animals per group). Mortality was recorded 24, 48, and 72 h after infection. Passive i.p. immunization of mice with anti-Hla reduces the ability of S. aureus Newman to grow in mouse lung tissue (P = 0.022; 10 animals per group; B) and decreases the gross pathological (C) and histopathologic (D and E) lesions evident after infection. Horizontal bars in B indicate the mean. Bars: (D and E, top) 0.1 cm; (D and E, bottom) 10 μm. (F) i.p. administration of anti-Hla antisera protects animals upon challenge by i.n. inoculation with LAC (P = 0.028) or MW2 (P = 0.04; 10 animals per group; left), whereas immunization with anti-PVL immune sera does not confer protection (P = 0.45; 10 animals per group; right). Statistical significance in A and F is indicated by an asterisk. (G) Cytokine responses during lung infection are influenced by passive immunization with antibodies against Hla. Serum cytokine levels were determined 24 h after infection, revealing a decrease in IL-1β secretion in anti-Hla–immunized animals (P = 0.039) and a corresponding increase in serum IFN-γ (P = 0.034) relative to sham-immunized animals (nine animals per group). Horizonal bars indicate the mean.
To define the nature of the host response to passive immunization, we evaluated serum cytokine responses in immunized animals 24 h after infection with S. aureus Newman. When compared with mock-immunized controls, animals receiving anti-Hla sera displayed blunted secretion of IL-1β (P = 0.039; nine animals per group; Fig. 4 G), an inflammatory cytokine that is the hallmark of acute lung injury (27); anti-Hla–immunized animals also displayed increased release of IFN-γ (P = 0.034), a cytokine that promotes phagocytic uptake and killing of S. aureus by immune cells (28). Thus, immunization with anti-Hla antisera protects animals from S. aureus pneumonia, correlating with favorable alterations in the cytokine profile of the host.
For many years, S. aureus has been recognized as a prominent cause of pneumonia in intensive care units (29). The recent spread of highly virulent CA-MRSA strains in communities has caused dramatic changes in epidemiology and disease incidence, demanding the development of novel preventive and therapeutic strategies (29). S. aureus Hla is a well-characterized toxin (10). When administered in purified form to lung tissues or to alveolar epithelial cells, Hla disrupts the integrity of cellular membranes and perturbs lung function by precipitating the accumulation of inflammatory exudate within alveoli (19). We recently expanded the analysis of Hla and examined the genetic requirements of its structural gene (hla) toward the pathogenesis of pneumonia in experimental animals using pairwise comparisons of fully virulent S. aureus isolates and their isogenic hla variants (5, 20). An absolute requirement for hla in disease pathogenesis was observed in S. aureus MSSA and MRSA strains (5, 20). Thus, targeting the toxin activity of Hla via immunization would represent an exciting opportunity to improve the poor prognosis of S. aureus pneumonia. To the best of our knowledge, this work represents the first successful implementation of this strategy for lung disease, revealing that active immunization of animals with a nontoxic Hla variant as well as passive immunoprotection via transfer of Hla-specific antisera can disrupt the pathogenesis of S. aureus pneumonia.
MRSA infection triggers a complex cascade of host responses in the lung, resulting in microvascular injury and accumulation of fluid and immune cells that impair respiratory function (30). Pulmonary inflammation induced by both infectious and noninfectious stimuli causes enhanced IL-1β secretion, facilitating the recruitment of immune cells to the site of infection and predisposing to acute lung injury and systemic inflammation (27). Together, Hla- and IL-1β–mediated pathophysiologic alterations likely underlie the significant morbidity and mortality associated with S. aureus pneumonia; anti-Hla Ig appears to protect animals not only from absolute mortality but also from enhanced IL-1β secretion and associated systemic illness.
In the wake of rising antimicrobial resistance among S. aureus, it is essential to define the molecular basis of pathogenesis for each of the many different types of infections that MRSA and CA-MRSA cause (31, 32). In particular, it will be necessary to define the relative contributions of additional S. aureus virulence factors in the pathogenesis of pneumonia to derive an optimized preventative tool. The central role of Hla in lung injury appears to be unique among the pathogenic strategies of S. aureus. This organism secretes many virulence factors and typically draws on a large register of molecules with overlapping or even redundant functions. Thus, the absolute requirement for a single secreted toxin (Hla) in pathogenesis is an exception, not the rule. The role of Hla in pneumonia is underscored by the preventive value of active and passive immunization against Hla. These data reveal novel opportunities for the development of Hla vaccines or immunotherapeutics that may be useful for the prevention and treatment of S. aureus pneumonia in humans.
MATERIALS AND METHODS
Bacterial strains and culture.
For mouse lung infections, S. aureus strains (a gift of D. Missiakas, University of Chicago, Chicago, IL) were grown at 37°C in tryptic soy broth/agar to OD660 0.5. 50-ml culture aliquots were centrifuged, washed in PBS, and suspended in 750 μl PBS for mortality studies (3–4 × 108 CFU per 30-μl volume), or 1,250 μl PBS (2 × 108 CFU per 30-μl volume) for bacterial load and histopathology experiments. Inocula for S. aureus MW2 and long-term protection studies were increased to 6–8 × 108 CFU per 30-μl volume (Figs. 2 and Figs.4). For cytotoxicity studies, 5 ml of culture prepared as described was suspended in 10 ml F12K media (Invitrogen). A 100-μl suspension was used per assay well.
Plasmid construction.
The hla gene and promoter were PCR amplified from S. aureus Newman template DNA and then cloned into the pOS1 vector.
Rabbit antisera.
PCR product encoding mature HlaH35L was cloned into pGEX-6P-1 (GE Healthcare) and transformed into Escherichia coli. Purified HlaH35L was used as an immunogen for the production of rabbit antisera (provided by C. Cornelius and N. Ciletti, University of Chicago, Chicago, IL).
Animals and procedures.
Animal experiments were reviewed, approved, and supervised by the Institutional Animal Care and Use Committee at the University of Chicago. For lung infection, 7-wk-old C57BL/6J mice (The Jackson Laboratory) were anesthetized before inoculation of 30 μl of S. aureus suspension into the left nare. Animals were placed into the cage in a supine position for recovery and were observed for the time courses indicated in the figures. A small percentage of animals routinely succumbed within the first 6 h after inoculation, likely from the combined effects of aspiration and anesthesia. These animals were not included in subsequent statistical analyses.
For active immunization, 4-wk-old mice received 20 μg HlaH35L protein in CFA on day 0 via the i.m. route, followed by a boost with 20 μg HlaH35L protein in IFA on day 10. Animals were challenged with S. aureus on day 21. Sera were collected before immunization and on day 20 to assess specific antibody production. For passive immunization studies, 7-wk-old mice received 100 μl of either NRS or toxin-specific rabbit antisera via i.p. injection 24 h before challenge.
To assess the pathological correlates of pneumonia, infected animals were killed via forced CO2 inhalation before removal of both lungs. The right lung was homogenized for enumeration of lung bacterial load. The left lung was placed in 1% formalin and paraffin embedded, thin sectioned, stained with hematoxylin-eosin, and analyzed by microscopy.
ELISA.
Serum antibody titers were determined with immunoplates (MaxiSorp; Thermo Fisher Scientific) coated with 1 μg/ml HlaH35L. Serum dilutions were incubated in the appropriate plates, developed with horseradish peroxidase–conjugated secondary antibodies and Opti-EIA (BD Biosciences), and read on a spectrophotometer (GENios; Tecan).
Protein and cytokine analysis.
S. aureus cultures adjusted for OD were precipitated with trichloroacetic acid, washed in acetone, and protein solubilized in sample buffer before SDS-PAGE and anti-Hla (Toxin Technology, Inc.) immunoblot. Sera harvested 24 h after infection were assayed for cytokine content (Bioplex Mouse 8-Plex A; Bio-Rad Laboratories).
Live/dead and cytotoxcity assays.
A549 cells were washed and plated in F12K media at a density of 1.5 × 104 cells per well. For both assays, washed A549 cells were cultured with 100 μl of staphylococcal suspension per well in F12K media. After 4 h of incubation at 37°C, cells were treated with either live/dead (green/red) reagent (Invitrogen), or LDH activity was determined (Roche) according to the manufacturer's recommendations. Microscopic images of stained cells were obtained using a microscope (Eclipse TE2000U; Nikon); LDH activity was measured on a spectrophotometer.
Statistical analysis.
The statistical significance of mortality studies was determined using Fisher's exact test. The significance of bacterial recovery from lungs was calculated using the two-tailed Student's t test. The significance of LDH release was calculated using analysis of variance and Dunnett's post-test.
Acknowledgments
We thank D. Missiakas for mutants and comments on the manuscript; M. Burts and H. Kim for animal experiment support; C. Cornelius and N. Ciletti for rabbit serum; J. Kern, N. Green, A. DeDent, and S. Bond for microscopy; and the Department of Pathology and the Immunology Applications Core at the University of Chicago for histology samples and Bio-Plex support, respectively.
J.B. Wardenburg is a National Institute of Child Health and Human Development fellow of the Pediatric Scientist Development Program (grant K12-HD00850). This work was supported by the Division of Microbiology and Infectious Diseases at the National Institute for Allergy and Infectious Diseases (grant AI52747 to O. Schneewind).
The authors have no conflicting financial interests.
References
- 1.Lowy, F.D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. [DOI] [PubMed] [Google Scholar]
- 2.Gillet, Y., B. Issartel, P. Vanhems, G. Lina, F. Vandenesch, J. Etienne, and D. Floret. 2001. Severe staphylococcal pneumonia in children. [In French.] Arch. Pediatr. 8(Suppl. 4):742–746. [DOI] [PubMed] [Google Scholar]
- 3.Gillet, Y., B. Issartel, P. Vanhems, J.C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 359:753–759. [DOI] [PubMed] [Google Scholar]
- 4.Kollef, M.H., L.E. Morrow, M.S. Niederman, K.V. Leeper, A. Anzueto, L. Benz-Scott, and F.J. Rodino. 2006. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 129:1210–1218. [DOI] [PubMed] [Google Scholar]
- 5.Bubeck Wardenburg, J., R.J. Patel, and O. Schneewind. 2007. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 75:1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duthie, E.S., and L.L. Lorenz. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6:95–107. [DOI] [PubMed] [Google Scholar]
- 7.Mazmanian, S.K., G. Liu, E.R. Jensen, E. Lenoy, and O. Schneewind. 2000. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA. 97:5510–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Recsei, P., B. Kreiswirth, M. O'Reilly, P. Schlievert, A. Gruss, and R.P. Novick. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 202:58–61. [DOI] [PubMed] [Google Scholar]
- 9.Bhakdi, S., and J. Tranum-Jensen. 1991. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 55:733–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song, L., M.R. Hobaugh, C. Shustak, S. Cheley, H. Bayley, and J.E. Gouaux. 1996. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 274:1859–1866. [DOI] [PubMed] [Google Scholar]
- 11.O'Reilly, M., J.C. de Azavedo, S. Kennedy, and T.J. Foster. 1986. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb. Pathog. 1:125–138. [DOI] [PubMed] [Google Scholar]
- 12.Bramley, A.J., A.H. Patel, M. O'Reilly, R. Foster, and T.J. Foster. 1989. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect. Immun. 57:2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel, A.H., P. Nowlan, E.D. Weavers, and T. Foster. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55:3103–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menzies, B.E., and D.S. Kernodle. 1996. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect. Immun. 64:1839–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouaux, E., M. Hobaugh, and L. Song. 1997. alpha-Hemolysin, gamma-hemolysin, and leukocidin from Staphylococcus aureus: distant in sequence but similar in structure. Protein Sci. 6:2631–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElroy, M.C., H.R. Harty, G.E. Hosford, G.M. Boylan, J.F. Pittet, and T.J. Foster. 1999. Alpha-toxin damages the air-blood barrier of the lung in a rat model of Staphylococcus aureus-induced pneumonia. Infect. Immun. 67:5541–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose, F., G. Dahlem, B. Guthmann, F. Grimminger, U. Maus, J. Hanze, N. Duemmer, U. Grandel, W. Seeger, and H.A. Ghofrani. 2002. Mediator generation and signaling events in alveolar epithelial cells attacked by S. aureus alpha-toxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L207–L214. [DOI] [PubMed] [Google Scholar]
- 18.Seeger, W., M. Bauer, and S. Bhakdi. 1984. Staphylococcal alpha-toxin elicits hypertension in isolated rabbit lungs. Evidence for thromboxane formation and the role of extracellular calcium. J. Clin. Invest. 74:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeger, W., R.G. Birkemeyer, L. Ermert, N. Suttorp, S. Bhakdi, and H.R. Duncker. 1990. Staphylococcal alpha-toxin-induced vascular leakage in isolated perfused rabbit lungs. Lab. Invest. 63:341–349. [PubMed] [Google Scholar]
- 20.Wardenburg, J.B., T. Bae, M. Otto, F.R. DeLeo, and O. Schneewind. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13:1405–1406. [DOI] [PubMed] [Google Scholar]
- 21.Diep, B.A., S.R. Gill, R.F. Chang, T.H. Phan, J.H. Chen, M.G. Davidson, F. Lin, J. Lin, H.A. Carleton, E.F. Mongodin, et al. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 367:731–739. [DOI] [PubMed] [Google Scholar]
- 22.Voyich, J.M., M. Otto, B. Mathema, K.R. Braughton, A.R. Whitney, D. Welty, R.D. Long, D.W. Dorward, D.J. Gardner, G. Lina, et al. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194:1761–1770. [DOI] [PubMed] [Google Scholar]
- 23.Menzies, B.E., and D.S. Kernodle. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooijakkers, S.H., M. Ruyken, J. van Roon, K.P. van Kessel, J.A. van Strijp, and W.J. van Wamel. 2006. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell. Microbiol. 8:1282–1293. [DOI] [PubMed] [Google Scholar]
- 25.Hirst, R.A., H. Yesilkaya, E. Clitheroe, A. Rutman, N. Dufty, T.J. Mitchell, C. O'Callaghan, and P.W. Andrew. 2002. Sensitivities of human monocytes and epithelial cells to pneumolysin are different. Infect. Immun. 70:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nizet, V., R.L. Gibson, E.Y. Chi, P.E. Framson, M. Hulse, and C.E. Rubens. 1996. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64:3818–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman, R.B., J. Pugin, J.S. Lee, and M.A. Matthay. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 14:523–535. [DOI] [PubMed] [Google Scholar]
- 28.Zhao, Y.X., I.M. Nilsson, and A. Tarkowski. 1998. The dual role of interferon-gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology. 93:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micek, S.T., K.E. Kollef, R.M. Reichley, N. Roubinian, and M.H. Kollef. 2007. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob. Agents Chemother. 51:3568–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enkhbaatar, P., C. Joncam, L. Traber, Y. Nakano, J. Wang, M. Lange, R. Connelly, G. Kulp, F. Saunders, R. Huda, et al. 2007. Novel ovine model of methicillin-resistant Staphylococcus aureus-induced pneumonia and sepsis. Shock. 10.1097/shk.0b013e318158125b. [DOI] [PubMed]
- 31.Bae, T., A.K. Banger, A. Wallace, E.M. Glass, F. Aslund, O. Schneewind, and D.M. Missiakas. 2004. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. USA. 101:12312–12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stranger-Jones, Y.K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 103:16942–16947. [DOI] [PMC free article] [PubMed] [Google Scholar]