Abstract
Biomphalaria spp. serve as obligate intermediate hosts for the human blood fluke Schistosoma mansoni. Following S. mansoni penetration of Biomphalaria glabrata, hemocytes of resistant snails migrate towards the parasite, encasing the larva in a multicellular capsule resulting in its destruction via a cytotoxic reaction. Recent studies have revealed the importance of hydrogen peroxide and nitric oxide (H2O2, NO) in parasite killing [1, 2]. It is assumed H2O2 and NO production is tightly regulated although the specific molecules involved remain largely unknown. Consequently, the potential role of cell signaling pathways in B. glabrata hemocyte H2O2 production was investigated by evaluating the effects of specific inhibitors of selected signaling proteins. Results suggest that both ERK and p38 MAPKs are involved in the regulation of B. glabrata H2O2 release in response to stimulation by PMA and galactose-conjugated BSA. However, the involvement of the signaling proteins PKC, PI3 kinase and PLA2 differs between PMA- and BSA-gal-induced H2O2 production.
Keywords: Biomphalaria glabrata, hemocytes, hydrogen peroxide, NADPH oxidase, mitogen-activated protein kinase, protein kinase C, phospholipase A, phosphoinositide kinase
Introduction
The planorbid snail, Biomphalaria glabrata is an important intermediate host for larval stages of the human blood fluke, Schistosoma mansoni. Strains of B. glabrata may differ in their abilities to suppress an S. mansoni infection with some strains serving as compatible or susceptible hosts, and others demonstrating a resistant phenotype [3, 4]. When infective S. mansoni larvae penetrate a resistant host, blood cells known as hemocytes form a multilayered cellular encapsulation around the parasite resulting in larval death, usually within 24 hr post-infection [5]. Using an in vitro cellular cytotoxicity assay Hahn et al. [1, 2] demonstrated that reactive oxygen and nitrogen species, specifically H2O2 and NO, respectively, were responsible for the schistosomicidal activity. Furthermore, it was also shown that selected carbohydrates were capable of triggering B. glabrata hemocyte H2O2 production in vitro [6] suggesting an association between carbohydrate-reactive cell receptors and oxidative/nitrative responses.
Extracellular H2O2 release depends on a multi-enzyme complex, referred to as NADPH oxidase, which is comprised of the cytoplasmic components p47phox, p60 phox, p40 phox and the transmembrane proteins gp22 phox and gp91 phox. Following stimulation of the cell by interaction of cellular receptors with their activating ligands, phosphorylation of the cytoplasmic components enables them to translocate to the membrane to form a complete active enzyme complex [7]. NADPH oxidase then reduces extracellular oxygen to superoxide using NADPH as an electron donor [8]. Finally superoxide dismutase catalyzes the conversion of superoxide to oxygen and hydrogen peroxide [9].
Because free H2O2 can cause serious damage to biological membranes, whether host or pathogen, H2O2 production must be a highly regulated response. However, to date very little is known about which signaling molecules and pathways may be involved in regulating this response in B. glabrata hemocytes. Therefore, in the present study, resistant snail (BS90) hemocytes were incubated with specific inhibitors of several signaling proteins to assess their potential role in B. glabrata hemocyte H2O2 production. Results suggest that two mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK), and p38, are involved in hemocyte H2O2 production in response to both the phorbol ester PMA and BSA-galactose. However phosphoinositide-3 kinase (PI3 kinase) and phospholipase A2 (PLA2) signaling proteins appear to be involved only in BSA-gal stimulated H2O2 production, while PKC regulates PMA-induced H2O2 release.
Materials and Methods
Biomphalaria glabrata culture
B. glabrata snails (BS90 strain) were maintained on a 12h:12h light-dark cycle in 10-gal glass aquaria containing dechlorinated artificial pond water at 26°C and were fed leaf lettuce ad libitum. Snails used in all experiments ranged from 10 to 15 mm in shell diameter.
Schistosoma mansoni parasite culture and harvesting of excretory-secretory products
Miracidia of S. mansoni were isolated from eggs obtained from infected mouse liver homogenates, and cultured for 48-hr in Chernin’s balanced salt solution [CBSS; 10] at 26°C to permit their development to the mother sporocyst stage. Excretory-secretory products (ESP) used in initial experiments were harvested from the 48-hr in vitro cultured S. mansoni sporocysts as described in Humphries and Yoshino [11].
Hemocyte isolation and H2O2 assay
Hemolymph was obtained from BS90 B. glabrata snails via the headfoot retraction technique [12], pooled on parafilm, mixed and 100 μl per well aliquoted into a black-walled, clear-bottomed 96-well microtitre plate (Corning Inc. Life Sciences, Lowell, MA). This was followed by addition of 100 μl of CBSS (22°C, pH 7.2) to each well bringing the total volume to 200 μl. Hemolymph was diluted with CBSS in order to decrease adhesion of plasma proteins to the surface of the wells. Hemocytes were allowed to attach and spread for 90 min, before removal of plasma and washing with 200 μl CBSS every 15 min over the next 90 min. CBSS was then removed and replaced with either 50 μl of fresh CBSS (no inhibitor control) or CBSS containing a specific inhibitor. After a 1 hr incubation at 22°C, 50 μl of either CBSS, phorbol 12-myristate 13-acetate (PMA; 500 nM final concentration), or galactose-conjugated bovine serum albumin (BSA-gal; 200-400 nM final concentrations) were added to their respective control and inhibitor-treated wells and allowed to incubate for an additional 2 hr at 22°C prior to measuring H2O2 released. All inhibitors used in the study were purchased from Calbiochem (EMD Chemicals, San Diego, CA) and included: U0126 (Erk MAPK inhibitor), SB 202190 (p38 MAPK inhibitor), bisindolylmaleimide I (PKC inhibitor), wortmannin and LY294002 (PI3 kinase inhibitors), aristolochic acid and PACOCF3 (PLA2 inhibitors). PMA and BSA-gal were obtained from Sigma-Aldrich (St. Louis, MO). The concentration at which each inhibitor was used was determined initially through literature reviews followed by preliminary experiments.
Hydrogen peroxide concentrations were measured using Amplex® Red (Invitrogen™ Corporation, Carlsbad, CA) following a protocol derived from Bender et al. [13]. Following hemocyte treatment with CBSS, PMA or BSA-gal, plates were centrifuged at 1500 rpm at 22°C for 2 min after which supernatants were removed and transferred to a new “black-walled” 96-well plate. One-hundred μl of the reagent Amplex® Red was added to each well of supernatant and read within 5 min at 520 nm excitation/590 nm emission on a Synergy™ HT Multi-Detection Microplate Reader (BioTek Instruments, Inc., Winooski, VT).
DNA quantification and statistical analyses
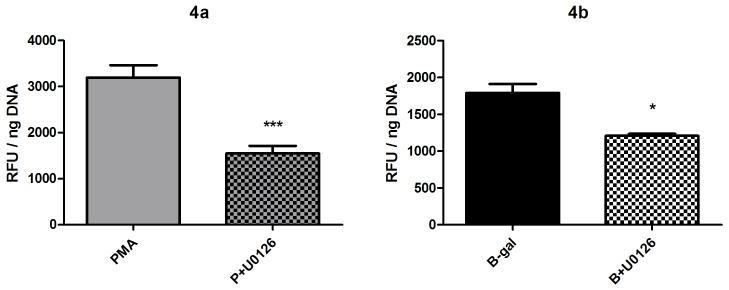
In order to control for the number of hemocytes present in each well within and between replicate experiments, hemocyte DNA levels per well were measured, thus permitting the normalization of H2O2 measurements. As a consequence data are presented as relative fluorescence units (RFU)/ ng DNA. To determine cellular DNA content, hemocytes in original 96-well plates were washed twice with 200 μl CBSS by centrifugation for 2 min at 1500 rpm. Plates were inverted to blot dry and then stored dry at -80°C overnight. Plates were then subjected to four freeze/thaw cycles over 2 hr, followed by addition of 100 μl of CyQuant® cell lysis buffer (1X; Invitrogen Corporation, Carlsbad, CA) to each well and incubated for 1 hr at 22°C. A standard curve was set up in duplicate in the same buffer using a lambda DNA standard (Invitrogen), ranging from 1-100 ng. To all wells, 100 μl Quant-iT™ PicoGreen® dsDNA reagent was added and maintained in the dark for 25 min before reading at 485 nm excitation/528 nm emission (BioTek Instruments). Each experiment was carried out a minimum of three times and statistical analyses were performed. Bartlett’s test was used to assess equality of variance, followed by One-way ANOVA and Tukey’s post-test. All data are presented as the mean +/- SEM for 3 independent experiments. However, U0126 inhibition of BSA-gal-induced H2O2 release produced data with unequal variances which were analyzed using the Kruskal-Wallis test followed by Dunn’s post-test. This data is therefore presented as median and interquartile range (Fig. 4b).
Fig. 4.
Effect of the ERK MAPK inhibitor U0126 on B. glabrata hemocyte H2O2 production. Cells were pre-incubated with U0126 (10 μM) for 1 hr prior to 2 hr stimulation with either a) PMA (500 nM) or b) BSA-gal (200 nM). Data presented in Fig. 4a. represent mean +/- SEM of 3 independent experiments. Data displayed in Fig 4b were analysed using Kruskal Wallis and are therefore presented as median and interquartile range for 3 independent experiments.
*** p≤0.001 compared with PMA, * p≤0.05 compared with BSA-gal alone
Results
Effect of Schistosoma mansoni ESP on hemocyte H2O2 production
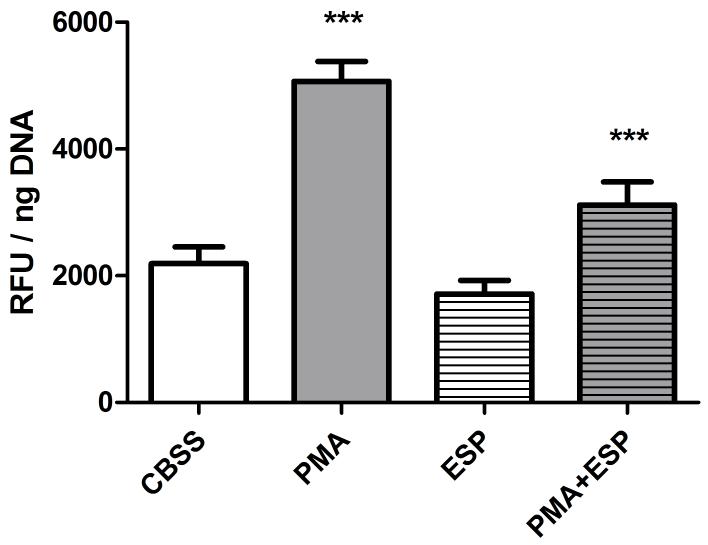
To assess B. glabrata hemocyte H2O2 release in response to S. mansoni, hemocytes were exposed to parasite excretory-secretory products (ESP, 25 μg protein/ml). Following a 2 hr exposure, there was no significant difference in H2O2 release between CBSS and ESP treated hemocytes (Fig. 1). Furthermore, a PMA-induced increase in H2O2 levels was significantly reduced when hemocytes were exposed simultaneously to ESP and PMA (1 way ANOVA p<0.0001, Tukey’s post-test p≤0.001, Fig. 1). These findings suggest that ESP may contain factors with antioxidant properties and hence could not be used to stimulate hemocyte H2O2 release in the current study. Alternatively, as the sporocyst tegument is heavily glycosylated, and galactose-conjugated bovine serum albumin (BSA-gal) had previously been shown to elicit hemocyte H2O2 release [6], BSA-gal was used as a potential stimulant in subsequent H2O2 -release assays.
Fig. 1.
Effect of S. mansoni ESP on B. glabrata hemocyte H2O2 production. Cells were treated with either PMA (500 nM), S. mansoni ESP (25 μg/ml) or both simultaneously. H2O2 levels were measured using Amplex Red reagent. Data are presented as mean +/- SEM of 3 independent experiments.
*** p≤0.001 comparing PMA with CBSS, and when comparing PMA + ESP with PMA alone.
Effect of PMA and BSA-gal on hemocyte H2O2 production
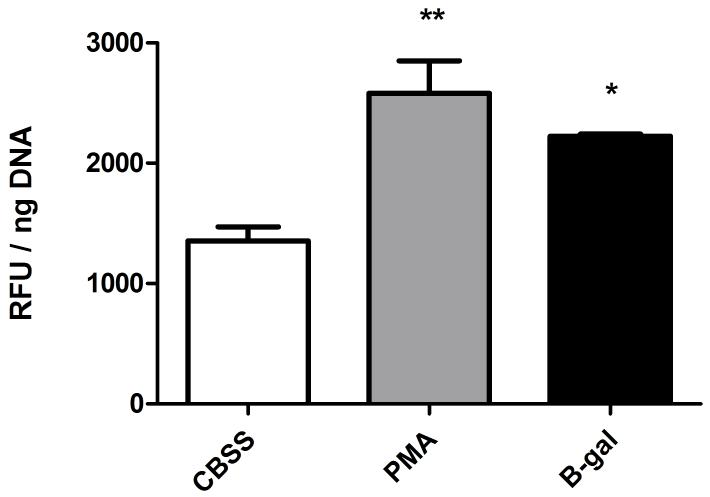
Phorbol myristate acetate (PMA) is a membrane-permeable activator of protein kinase C (PKC), capable of directly triggering a downstream signaling cascade [14, 15]. Exposure of B. glabrata hemocytes to either PMA (500 nM) or BSA-gal (200-400 nM) for 2 hr resulted in significant elevations of H2O2 production above basal levels in CBSS alone (Fig. 2). Subsequently, hemocyte H2O2 production in response to PMA and BSA-gal at their minimum stimulating concentrations was examined using specific inhibitors of cell signaling molecules associated with NADPH oxidase activation. None of the inhibitors used had significant effects on hemocyte viability compared to controls as determined by trypan blue exclusion, nor had they any significant effect on CBSS basal H2O2 levels.
Fig. 2.
Effect of PMA and B-galactose on B. glabrata hemocyte H2O2 production. Cells were treated with either PMA (500 nM) or BSA-gal (200 nM) for 2 hr, after which H2O2 levels were measured. Data are presented as mean +/- SEM of 3 independent experiments.
* p≤0.05 and ** p≤0.01 compared with CBSS
Effect of PKC inhibition
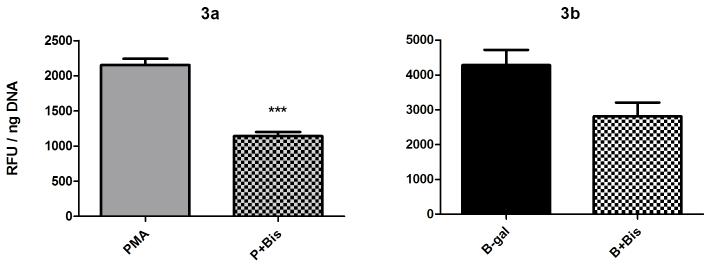
Hemocytes were incubated for 1 hr in bisindolylmaleimide I (Bis I, 250 nM) before exposure to either PMA (500 nM) or BSA-gal (200-400 nM). Results indicated that Bis I significantly reduced H2O2 release in PMA-stimulated hemocytes when compared to treatment with PMA alone (Fig. 3a; 1 way ANOVA p=0.0001, Tukey’s post-test p≤0.001). Bis I also reduced H2O2 release in response to BSA-gal but not to a significant level (Fig. 3b).
Fig. 3.
Effect of the PKC inhibitor Bisindolylmaleimide I on B. glabrata hemocyte H2O2 production. Cells were pre-incubated 1 hr with 250 nM Bis I, before stimulation with either a) PMA (500 nM) or b) BSA-gal (400 nM). H2O2 levels were then measured. Data are presented as mean +/- SEM of 3 independent experiments.
*** p≤0.01 compared with PMA alone
Effect of mitogen-activated protein kinase (MAPK) inhibition
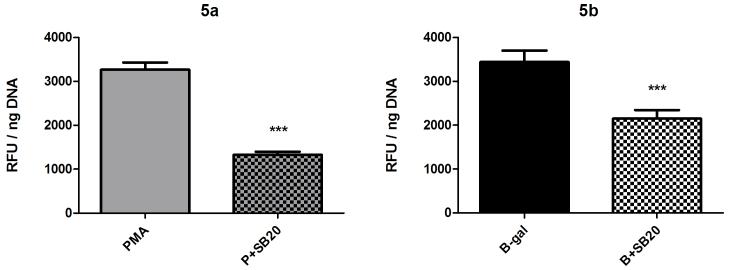
MAP kinases are a superfamily of serine-threonine kinases comprising the ERK, p38 and JNK subfamilies [16]. Because members of this superfamily are involved in the regulation of NADPH oxidase activation and subsequent ROS production [17], the putative roles of ERK and p38 in hemocyte H2O2 production were examined using the drugs U0126 and SB202190 as specific inhibitors of ERK and p38 MAPKs, respectively. U0126 treatment (10 μM) resulted in significant decreases in both PMA- and BSA-gal-induced H2O2 production (Fig. 4; PMA, 1 way ANOVA p<0.0001, Tukey’s post-test p≤0.001, BSA-gal, Kruskal-Wallis p=0.0142, Dunn’s post-test p≤0.05), while significant reductions also were recorded when hemocytes were treated with the p38 inhibitor SB202190 in conjunction with both PMA and BSA-gal (Fig. 5; 1 way ANOVA p<0.0001, Tukey’s post-test p≤0.001 for both). Amino acid alignments of the p38 MAPK ATP-binding domain, target for SB202190, was identical between B. glabrata and humans (data not shown), suggesting the inhibitor could also target B. glabrata p38 MAPK.
Fig. 5.
Effect of the p38 MAPK inhibitor SB 202190 on B. glabrata hemocyte H2O2 production. Cells were pre-incubated for 1 hr in SB 202190 (25 μM) prior to 2 hr incubation in either PMA (500 nM) or BSA-gal (200 nM). H2O2 levels were then measured. Data are presented as mean +/- SEM of 3 independent experiments.
*** p≤0.001 compared with either PMA or BSA-gal alone
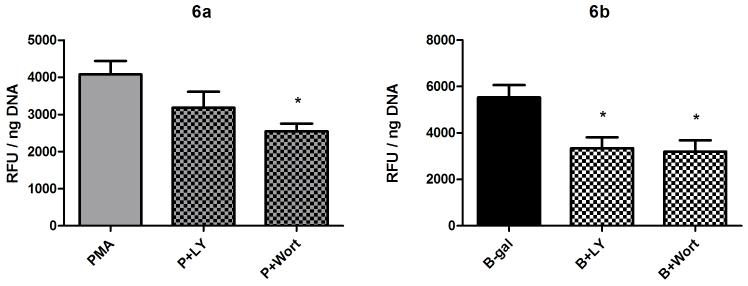
Effect of PI3K inhibition
Two inhibitors of phosphoinositide kinase (PI3K), LY294002 (50 uM) and wortmannin (500 nM), were tested for their ability to block hemocyte H2O2 production. Treatment of cells by both inhibitors resulted in a significant decrease in BSA-gal-induced H2O2 release compared to BSA-gal alone (Fig. 6b; 1 way ANOVA p=0.0115, Tukey’s post-test p≤0.05 for both inhibitors). However, only wortmannin significantly decreased PMA-induced H2O2 release (Fig. 6a; 1 way ANOVA p=0.0013, Tukey’s post-test p≤0.05), while LY294002 had no significant effect on PMA-treated cells.
Fig. 6.
Effect of the PI3 kinase inhibitors wortmannin and LY294002 on B. glabrata hemocyte H2O2 production. Cells were pre-incubated with either wortmannin (500 nM) or LY294002 (50 μM) for 1 hr prior to 2 hr stimulation with either a) PMA (500 nM) or b) BSA-gal (300 nM). Data are presented as mean +/- SEM of 3 independent experiments.
* p≤0.05 compared with either PMA or BSA-gal alone
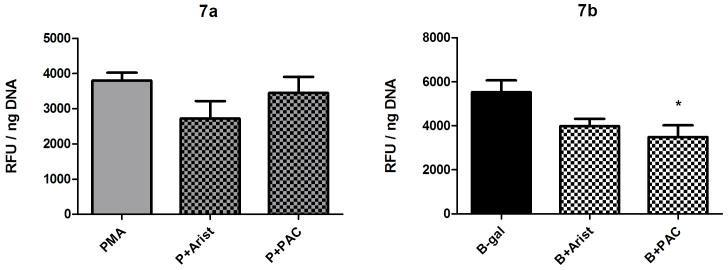
Effect of PLA2 inhibition
Since phospholipase A2 (PLA2) has been reported to play a major role in NADPH oxidase activation [18] and the generation of H2O2, inhibitors of PLA2, aristolochic acid (100 μM) and PACOCF3 (50 μM), were used to examine its potential role in hemocyte H2O2 production. In this case, neither of these inhibitors had a significant effect on PMA--induced H2O2 release (Fig. 7a), whereas PACOCF3 significantly reduced H2O2 production in B-gal-treated hemocytes (Fig. 7b; 1 way ANOVA p=0.0197, Tukey’s post-test p≤0.05).
Fig. 7.
Effect of the PLA2 inhibitors aristolochic acid and PACOCF3 on B. glabrata hemocyte H2O2 production. Cells were pre-incubated with either aristolochic acid (100 μM) or PACOCF3 (50 μM) for 1 hr prior to 2 hr stimulation with either a) PMA (500 nM) or b) BSA-gal (300 nM). Data are presented as mean +/- SEM of 3 independent experiments.
* p≤0.05 compared to BSA-gal alone.
Discussion
In the present study, the signaling proteins regulating B. glabrata hemocyte H2O2 production in response to the specific stimulants, BSA-gal and PMA, and to excretory-secretory products (ESP) of cultured larval S. mansoni were investigated. Interestingly, ESP released from early developing larval stages did not induce H2O2 release from hemocytes, but in contrast appeared to inhibit PMA-mediated peroxide production. Vermeire and Yoshino [19] recently showed that S. mansoni larval ESP possessed peroxiredoxins and H2O2-scavenging activity, and this may explain the lack of an oxidative hemocyte response, as well as ESP’s apparent antioxidant capacity in the presence of PMA. PMA and BSA-gal differ in their modes of initial activation such that PMA, as an ester, can bypass membrane receptor ligation and directly activate DAG-dependent PKCs, whereas it is proposed that BSA-gal binds a cell surface receptor which triggers a downstream signaling cascade. The results imply that the cell signaling pathways regulating PMA and BSA-gal induced H2O2 release differ from each other, however both pathways involve the MAP kinases ERK and p38. Also it was noted that although PMA-mediated stimulation of hemocyte H2O2 production at 500 nM was very consistent, the response to BSA-gal treatments between experimental replicates was more variable with minimum stimulating concentrations ranging from 200-400 nM. Biological variability (e.g., size of BSA-gal receptor (+) hemocyte subpopulations, receptor density, responsiveness to ligand binding, etc.), in part, is believed to be responsible, although one cannot rule out additional variability inherent in the H2O2 assay system.
The PKC inhibitor Bis I significantly decreased H2O2 production in response to PMA, which is not surprising as PMA activates DAG-dependent PKCs. PKC regulation of H2O2 release was likewise demonstrated in the snail Lymnaea stagnalis, when hemocytes were stimulated with either PMA or laminarin [20]. A role for PKC in vertebrate immune cell H2O2 production is well documented and it has been shown that in bovine leucocytes, NADPH oxidase-dependent superoxide production and specifically p47 phosphorylation are PKC dependent [21]. Furthermore, several different PKC isozymes have been implicated in phosphorylating p47 and inducing its translocation [22]. More detailed studies are required to determine the specific targets of PKC in the PMA-induced H2O2 signaling pathway in B. glabrata. In contrast, PKC does not appear to be significantly involved in BSA-gal-stimulated H2O2 release in B. glabrata. This was unexpected given the involvement of PKC in both PMA- and laminarin-induced H2O2 release by L. stagnalis hemocytes [20] as well as in vertebrate cells as described previously [21]. In addition, the importance of PKC in a variety of molluscan immune-related functions has become increasingly apparent in recent years [23, 24]. Perhaps the task PKC carries out in PMA-induced H2O2 release is achieved by a different signaling protein(s) such as PI3K or PLA2 in the BSA-gal induced pathway.
The significant effects of MAPK inhibitors on H2O2 levels imply that both ERK and p38 also may be regulating hemocyte H2O2 production induced by both PMA and BSA-gal.. These findings are in agreement with previous studies examining MAPK regulation of vertebrate immune cell H2O2 production. Hazan-Halevy et al. [17] showed that human neutrophil NADPH oxidase activation was dependent on an ERK/p38-activated pathway resulting in the translocation of cPLA2 and cytosolic oxidase subunits to the cell membrane. However the involvement of either of these MAPKs seems to vary depending on the type of cell studied and the nature of H2O2 stimulation. Several studies have verified that the specific role of MAPKs in NADPH oxidase activation is to phosphorylate the cytosolic oxidase components, thereby catalyzing their translocation to the membrane [17]. In contrast, the exact function of MAPKs in B. glabrata H2O2 release has not yet been determined, nor is it clear whether ERK and p38 play similar roles in both PMA- and BSA-gal-induced H2O2 release. Interestingly, in a similar study the roles of ERK and p38 MAPKs in B. glabrata hemocyte H2O2 production in response to S. mansoni sporocysts in vitro were tested and results indicated that only ERK MAPKs evoked an H2O2 response [25]. Differences in experimental setup, inhibitor concentrations and/or assay conditions may explain the disparity in results of these studies.
PI3 kinase regulation of NADPH oxidase production of superoxide has been implicated in previous studies using the inhibitors LY294002 and wortmannin. Yamamori et al. [21] showed that wortmannin inhibited superoxide production in bovine leucocytes, specifically reducing p47 activation. In the current study the inhibitory effect of both wortmannin and LY294002 on BSA-gal H2O2 release strongly suggests the involvement of PI3 kinase. However, in the case of PMA-induced peroxide generation, only wortmannin had a significant inhibitory effect, yet the two inhibitors function through a similar mechanism. Recent publications suggest a need for caution in interpreting results obtained using these inhibitors, as the same drugs have been found to differ in their effects within a single study. To illustrate, Nauc et al. [26] found that inhibition of sperm capacitation and associated phosphorylation of proteins using LY294002 may actually be independent of PI3 kinase based on the differential effects of wortmannin and LY294002. Therefore in the current study it cannot be concluded with certainty that PI3 kinase also plays a role in PMA-induced H2O2 release as only wortmannin had an inhibitory effect.
In contrast to PMA-induced peroxide release, a regulatory role for PI3 kinase in BSA-gal-induced H2O2 production in B. glabrata would appear to be consistent with this kinase’s involvement in receptor-mediated activation signals. This is paralleled in both bovine leucocytes [21] and murine macrophages [27], both of which were inhibited by PI3 kinase inhibitors, including wortmannin. Therefore the results imply that PI3 kinase is likely to be involved in BSA-gal induced H2O2 release. The questionable role of PI3 kinase in PMA-induced hemocyte H2O2 production suggest that initiation of signals through surface receptors may be important in regulating oxidative reactions through a PI3 kinase-like pathway. Contrary to our results, Zelck et al. [25] found that wortmannin at a concentration of 100 nM had no effect on H2O2 production during B. glabrata hemocyte encapsulation reactions. Differences in inhibitor effects likely were due to the fact that we measured peroxide induction and its inhibition on isolated hemocyte preparations, while Zelck et al. [25] carried out their measurements in in vitro co-cultures of hemocytes and live S. mansoni sporocysts. As noted earlier, Vermeire and Yoshino [19] found that proteins excreted/secreted during in vitro sporocyst development were rich in H2O2-scavenging peroxiredoxins that may have affected the peroxide expression levels measured in their study. Other differences, including different cell stimulating ligands (BSA-gal vs. intact sporocysts) and wortmannin concentrations used (100 nM vs. 500 nM) also could account for discrepancies.
The PLA2 enzymes comprise a large, diverse family of enzymes, classified according to size, location and calcium dependence [28]. In the current study inhibitors to different forms of PLA2 enzymes were used; aristolochic acid which inhibits secretory forms (sPLA2) and PACOCF3 which blocks the activity of both calcium-dependent and independent cytosolic PLA2 (cPLA2 and iPLA2 respectively) [29]. Neither inhibitor had any significant effect on PMA-stimulated H2O2 production. In contrast, however, inhibition of BSA-gal-induced H2O2 by PACOCF3 suggests the potential involvement of cytosolic PLA2 isozymes. This result is supported by an established role for cPLA2 in vertebrate immune cell superoxide production; cPLA2 is required for NADPH oxidase activation and/or superoxide release when human neutrophils are stimulated by a variety of reagents such as angiotensin, PMA and zymosan [17, 30].
B. glabrata H2O2 production appears to be a critical component of its immune response against S. mansoni larval stages [31]. The putative regulation of such immune responses can be estimated through in vitro PMA- and BSA-gal-stimulation of B. glabrata hemocytes. The MAP kinases, ERK and p38, are involved in cell signaling pathways downstream of both PMA and BSA-galactose, although the exact mechanisms through which each influences these pathways is presently unknown. As MAP kinases are serine-threonine kinases, it is possible they are involved in phosphorylating the cytosolic components of the putative NADPH oxidase enzyme complex, which is essential for translocation to the membrane and oxidase activation. However, these two signaling pathways markedly differ suggesting the cell signaling pathways regulating H2O2 release are specific and dependent on the mode of activation. PKC is clearly implicated in PMA-stimulation yet a similar role downstream of BSA-gal is not apparent. The reverse occurs in the case of PI3 kinase and PLA2 as these enzymes appear to signal downstream of BSA-gal but not PMA. The exact roles PI3 kinase and PLA2 play have not yet been discerned but perhaps PLA2-derived arachadonic acid activates NADPH oxidase cytosolic components downstream of BSA-gal, whereas PKC fulfils the same role in a PMA-initiated pathway in B. glabrata hemocytes.
Despite available functional evidence, to date a B. glabrata NADPH oxidase has not been identified at the molecular level. The only ESTs showing similarity to any member of the NADPH oxidase complex are similar to members of the gp91 family (CK990069, CK989379 and CK149203; National Center for Biotechnology Information). In particular, these ESTs were most similar to dual oxidases (Duox), so named because of the presence of both NADPH oxidase and peroxidase domains [32]. Recent studies demonstrate that, like other members of the gp91 family, dual oxidases also can generate superoxide [33, 34], which in turn could be converted to H2O2 through the action of superoxide dismutases known to be present in B. glabrata hemocytes [35]. At present, it cannot be said whether dual oxidase proteins are involved in B. glabrata hemocyte H2O2 production but it is possible that they fulfill such a role. Figure 8 provides a hypothetical scheme by which H2O2 production may be regulated by ERK and p38 MAPK signaling pathways. Further detailed research of B. glabrata hemocyte signaling kinases and their accessory molecules is required in order to better understand the role each is playing in the regulation of H2O2 production. This will depend on the availability of the necessary tools and reagents such as antibodies against the individual components of the NADPH oxidase enzyme complex and sensitive, specific agonists/antagonists of the putative signaling pathways.
Fig. 8.
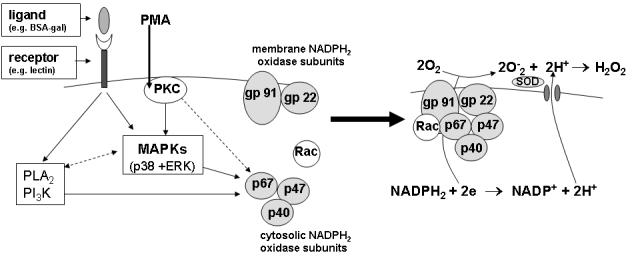
Simplified schematic diagram showing putative regulation of B. glabrata hemocyte H2O2 production stimulated by BSA-gal and PMA. Both pathways appear to involve the MAPKs ERK and p38, however their precise signaling pathways and exact role in regulating oxidative reactions are unknown as yet. It is possible that MAPKs and the other signaling kinases (e.g., PKC) may function in the direct phosphorylation and membrane translocation of cytosolic NADPH oxidase subunits, or, are acting through other pathway signaling components. Also, PI3 kinase and PLA2 appear to regulate downstream of BSA-gal only, but their specific function and position in this pathway is unknown. The regulation of B. glabrata hemocyte H2O2 production is expected to be more complex than depicted in this simplified diagram and include additional signaling molecules. Gray circled proteins indicate cytosolic and membrane-associated NADPH oxidase subunits. Solid-lined arrows indicate evidence supporting involvement of the signaling molecule in downstream events. Dash-lined arrows indicate that interactions may exist, but presently are unknown.
Acknowledgments
Supported by NIH (NIAID) Grant AI015503 to TPY and the NIH schistosome supply grant AI30026 to Dr. Fred Lewis. The authors would like to sincerely thank Dr. C. Murphy for the use of the Synergy™ HT Multi-Detection Microplate Reader.
Abbreviations
- MAPK
mitogen-activated protein kinase
- ERK
extracellular regulated kinase
- PKC
protein kinase C
- PLA2
phospholipase A
- PI3K
phosphoinositide kinase
- H2O2
hydrogen peroxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hahn UK, Bender RC, Bayne CJ. Killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata: role of reactive oxygen species. J. Parasitol. 2001;87:292–299. doi: 10.1645/0022-3395(2001)087[0292:KOSMSB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [2].Hahn UK, Bender RC, Bayne CJ. Involvement of nitric oxide in killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata. J. Parasitol. 2001;87:778–785. doi: 10.1645/0022-3395(2001)087[0778:IONOIK]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [3].Basch PF. Intermediate host specificity in Schistosoma mansoni. Exp. Parasitol. 1976;39:150–169. doi: 10.1016/0014-4894(76)90022-9. [DOI] [PubMed] [Google Scholar]
- [4].Loker ES, Bayne CJ. Immunity to trematode larvae in the snail Biomphalaria. In: Lackie AM, editor. Immune Mechanisms in Invertebrate Vectors. Oxford University Press; New York: 1986. pp. 199–220. [Google Scholar]
- [5].Loker ES, Bayne CJ, Buckley PM, Kruse KT. Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J. Parasitol. 1982;68:84–94. [PubMed] [Google Scholar]
- [6].Hahn UK, Bender RC, Bayne CJ. Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: carbohydrate-specific stimulation. Dev. Comp. Immunol. 2000;24:531–541. doi: 10.1016/s0145-305x(00)00017-3. [DOI] [PubMed] [Google Scholar]
- [7].Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- [8].Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim. Biophys. Acta. 1986;53:65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- [9].Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- [10].Chernin E. Observations on hearts transplanted from the snail Australorbis glabratus. J. Parasitol. 1963;49:353–364. [PubMed] [Google Scholar]
- [11].Humphries JE, Yoshino TP. Schistosoma mansoni excretory-secretory products stimulate a p38 signalling pathway in Biomphalaria glabrata embryonic cells. Int. J. Parasitol. 2006;36:37–46. doi: 10.1016/j.ijpara.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [12].Sminia T, Barendsen LA. A comparative morphological and enzyme histochemical study on blood cells of the freshwater snails Lymnaea stagnalis, Biomphalaria glabrata and Bulinus truncatus. J. Morphol. 1980;165:31–39. doi: 10.1002/jmor.1051650104. [DOI] [PubMed] [Google Scholar]
- [13].Bender RC, Broderick EJ, Goodhall CP, Bayne CJ. Respiratory bursts of B. glabrata hemocytes: Schistosoma mansoni - resistant snails produce more extracellular H2O2 than susceptible snails. J. Parasitol. 2005;91:275–279. doi: 10.1645/GE-415R. [DOI] [PubMed] [Google Scholar]
- [14].Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- [15].Niedel JE, Kuhn LJ, Vandenbark GR. Phorbol ester receptor copurifies with protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 1983;80:36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pearson G, Robinson F, Gibson TB, Xu B, Karandikar M, Berman K, Cobb MH. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- [17].Hazan-Halevy I, Levy T, Wolak T, Lubarsky I, Levy R, Paran E. Stimulation of NADPH oxidase by angiotensin II in human neutrophils is mediated by ERK, p38 MAP-kinase and cytosolic phospholipase A2. J. Hypertens. 2005;23:1183–1190. doi: 10.1097/01.hjh.0000170381.53955.68. [DOI] [PubMed] [Google Scholar]
- [18].Dana R, Leto TL, Malech HL, Levy R. Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J. Biol. Chem. 1998;273:441–445. doi: 10.1074/jbc.273.1.441. [DOI] [PubMed] [Google Scholar]
- [19].Vermeire JJ, Yoshino TP. Antioxidant gene expression and function in in vitro-developing Schistosoma mansoni mother sporocysts: possible role in self-protection. Parasitology. 2007 doi: 10.1017/S0031182007002697. in press. [DOI] [PubMed] [Google Scholar]
- [20].Lachini AH, Davies AJ, Mackintosh D, Walker AJ. β-1, 3-glucan modulates PKC signaling in Lymnaea stagnalis defence cells: a role for PKC in H2O2 production and downstream ERK activation. J. Exp. Biol. 2006;209:4829–40. doi: 10.1242/jeb.02561. [DOI] [PubMed] [Google Scholar]
- [21].Yamamori T, Inanami O, Nagahata H, Cui Y-D, Kuwabara M. Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett. 2000;467:253–258. doi: 10.1016/s0014-5793(00)01167-4. [DOI] [PubMed] [Google Scholar]
- [22].Fontayne A, Dang PM-C, Gougerot-Pocidalo M-A, El Benna J. Phosphorylation of p47phox Sites by PKCα, βπ, δ, and ζ: Effect on Binding to p22phox and on NADPH Oxidase Activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- [23].Walker AJ, Plows LD. Bacterial lipopolysaccharide modulates protein kinase C signaling in Lymnaea stagnalis haemocytes. Biol. Cell. 2003;95:527–33. doi: 10.1016/j.biolcel.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [24].Wright B, Lacchini AH, Davies AJ, Walker AJ. Regulation of nitric oxide production in snail (Lymnaea stagnalis) defence cells: a role for PKC and ERK signaling pathways. Biol. Cell. 2006;98:265–278. doi: 10.1042/BC20050066. [DOI] [PubMed] [Google Scholar]
- [25].Zelck UE, Gege BE, Schmid S. Specific inhibitors of mitogen-activated protein kinase and PI3-K pathways impair immune responses by hemocytes of trematode intermediate hosts. Dev. Comp. Immunol. 2007;31:321–331. doi: 10.1016/j.dci.2006.06.006. [DOI] [PubMed] [Google Scholar]
- [26].Nauc V, de Lamirande E, Leclerc P, Gagnon C. Inhibitors of phosphoinositide 3-kinase, LY294002 and Wortmannin, affect sperm capacitation and associated phosphorylation of proteins differently: Ca2+-dependent divergences. J. Androl. 2004;25:573–585. doi: 10.1002/j.1939-4640.2004.tb02828.x. [DOI] [PubMed] [Google Scholar]
- [27].Suh C-L, Stull ND, Li XJ, Tian W, Price MO, Grinstein S, Yaffe MB, Atkinson S, Dinaur MC. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcγIIA receptor-induced phagocytosis. J. Exp. Med. 2006;203:1915–1925. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- [29].Tessier C, Hichami A, Khan NA. Implication of three isoforms of PLA2 in human T-cell proliferation. FEBS Lett. 2002;520:111–116. doi: 10.1016/s0014-5793(02)02779-5. [DOI] [PubMed] [Google Scholar]
- [30].Dana R, Malech HL, Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem. J. 1994;297:217–223. doi: 10.1042/bj2970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bayne CJ, Hahn UK, Bender RC. Mechanisms of molluscan host resistance and of parasite strategies for survival. Parasitology. 2001;123:S159–167. doi: 10.1017/s0031182001008137. [DOI] [PubMed] [Google Scholar]
- [32].De Deken X, Wang D, Many M-C, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- [33].Forteza R, Salathe M, Miot F, Forteza R, Conner GF. Regulated Hydrogen Peroxide Production by Duox in Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- [34].Shao MXG, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Goodall CP, Bender RC, Broderick EJ, Bayne CJ. Constitutive differences in Cu/Zn superoxide dismutase mRNA levels and activity in hemocytes of Biomphalaria glabrata (Mollusca) that are either susceptible or resistant to Schistosoma mansoni (Trematoda) Mol. Biochem. Parasitol. 2004;137:321–328. doi: 10.1016/j.molbiopara.2004.06.011. [DOI] [PubMed] [Google Scholar]