Abstract
Tissue engineering and regenerative medicine is an exciting research area that aims at regenerative alternatives to harvested tissues for transplantation. Biomaterials play a pivotal role as scaffolds to provide three-dimensional templates and synthetic extracellular-matrix environments for tissue regeneration. It is often beneficial for the scaffolds to mimic certain advantageous characteristics of the natural extracellular matrix, or developmental or would healing programs. This article reviews current biomimetic materials approaches in tissue engineering. These include synthesis to achieve certain compositions or properties similar to those of the extracellular matrix, novel processing technologies to achieve structural features mimicking the extracellular matrix on various levels, approaches to emulate cell-extracellular matrix interactions, and biologic delivery strategies to recapitulate a signaling cascade or developmental/would-healing program. The article also provides examples of enhanced cellular/tissue functions and regenerative outcomes, demonstrating the excitement and significance of the biomimetic materials for tissue engineering and regeneration.
Keywords: Biomimetic, Tissue engineering, Regeneration, Polymer scaffold, Matrix, Biomaterial, Nano, Controlled release
1. Introduction
Tissue engineering is an interdisciplinary and multidisciplinary field that aims at the development of biological substitutes that restore, maintain, or improve tissue function [1-4]. In a typical tissue engineering approach, to control tissue formation in three dimensions (3D), a highly porous scaffold is critical. In addition to defining the 3D geometry for the tissue to be engineered, the scaffold provides the microenvironment (synthetic temporary extracellular matrix) for regenerative cells, supporting cell attachment, proliferation, differentiation, and neo tissue genesis [2, 3, 5, 6]. Therefore, the chemical composition, physical structure, and biologically functional moieties are all important attributes to biomaterials for tissue engineering.
To fulfill the diverse needs in tissue engineering, various materials have been exploited as scaffolds for tissue regeneration. Although certain metals are excellent choice for medical implants due to their superior mechanical properties [7], they are disadvantageous for scaffold applications because of the lack of degradability in a biological environment [5]. Certain inorganic/ceramic materials such as hydroxyapatite (HAP) or calcium phosphates, having good osteoconductivity and being studied for mineralized tissue engineering, are also limited due to poor processability into highly porous structures and brittleness. In contrast, polymers have great design flexibility because the composition and structure can be tailored to the specific needs, and therefore have been extensively studied in various tissue engineering applications, including bone tissue engineering [4, 5, 8, 9].
To serve as the temporary extracellular matrix (ECM) for regenerative cells, it may be beneficial for the scaffold to emulate certain advantageous features of the natural ECM. However, because the neo tissue genesis in tissue engineering process is not exactly the same as a developmental or wound healing program it is likely unnecessary and impractical for a scaffold to entirely duplicate the ECM. A natural ECM or its derivatives may actually not be the ideal scaffold for tissue engineering applications because tissue engineering should be an accelerated regeneration process compared to the natural development program. Mature tissue matrix often does not possess the highly interconnected macro- or micro-pore structures to allow for quick and uniform cell population throughout, which is essential for a tissue engineering/repair process. Therefore certain artificially designed scaffold features (such as porosity, pore size, interpore connectivity, etc.) are necessary for optimal tissue engineering applications (accelerated tissue regeneration). In addition, there are always concerns over the possible immune rejection and pathogen transmission when a natural ECM is used.
Based on the above discussion, a biomimetic scaffold for tissue engineering should be an artificially designed scaffold that mimics certain advantageous features of the natural ECM to facilitate cell recruiting/seeding, adhesion, proliferation, differentiation and neo tissue genesis.
2. Biomimetic Materials Synthesis
A biomimetic biomaterial for tissue engineering can be any scaffolding material that mimics one or multiple characteristics of the natural ECM. This section is intended to illustrate the biomimetic concept intentionally applied to the materials synthesis using a few examples, but not to exhaustively list all those belonging to this category.
2.1. Biomimetic Biodegradability
Biodegradability is generally required for a tissue engineering scaffold material, and the degradation rate also needs to match the neo tissue formation rate to ideally serve the template purpose [3]. For example, linear aliphatic polyesters such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymers poly(lactic acid-co-glycolic acid) (PLGA) are frequently used as polymers for scaffold fabrication because of their wide range of biodegradability in addition to their well accepted biocompatibility [10-13]. In contrast, poly(ethylene glycol) (PEG) does not have the needed biodegradability, but is a biocompatible hydrogel material that has similar mechanical characteristics to certain soft tissues such as cartilage [14, 15]. One way to overcome this deficiency is to synthesize copolymers of PEG with PLA, PGA or PLGA [16].
Another way to impart biodegradability, i.e. a biomimetic way, is to synthesize a PEG-based polymeric biomaterial exhibiting degradation by specific enzymes, i.e., matrix metalloproteases (MMPs). This approach mimics the enzymatic biodegradability of collagen and other natural ECM components [17]. These materials are often telechelic BAB block copolymers of PEG (the A block) and short oligopeptides that are cleavage sequences for targeted enzymes (the B block). These copolymers capped at each end with reactive groups are polymerized to form crosslinked hydrogel networks. Such hydrogels can be specifically degraded by the cell secreted MMPs (such as collagenase).
2.2. Biomimetic Mechanical Properties
Many tissues such as cardiac muscle, heart valves, and blood vessels have distinct elastomeric properties. Engineering these tissues has been a continuous effort in the tissue engineering community [18-22]. To achieve the needed elastomeric properties, various elastomeric materials have been evaluated as the scaffolds to engineer these tissues. In addition to elastic materials derived from the natural ECM [22], poly(ε-cprolactone) (PCL) and polyurethanes (PU) are some of the typical synthetic polymers. PCL is a semicrystalline polymer, has a very low glass-transition temperature (around −62°C), and thus is highly elastic at room or body temperature. Although PCL degrades by various mechanisms under physiological conditions [23, 24], the degradation rate is too slow, making it less attractive [3]. Segmented PU allow for structural variations to achieve elastomeric properties [25]. A major limitation of PU for biomedical applications is the involvement of toxic precursors (such as toluene diisocyanates) in the synthesis. Progress has been made in the development of biodegradable PU or urethane-based polymers using less toxic diisocyanates [26]. These polymers have been explored for vascular and other tissue engineering applications [27-29].
In addition to using existing polymer technologies to achieve needed elastomeric properties of various tissues, there are biomimetic approaches to develop elastomeric materials. Elastin is an insoluble, polymeric, ECM protein that provides various tissues in the body with the properties of extensibility and elastic recoil. Elastin is synthesized as tropoelastin (~70kDa), a soluble precursor to insoluble elastin [30]. Tropoelastin has a repeating domain structure, alternating between hydrophobic and crosslinking regions. Tropoelastin and elastin-derived repeating peptides are capable of self-assembling (coacervation) to form insoluble forms of aggregates at an elevated temperature. Utilizing recombinant technologies, Dan Urry pioneered the research on elastin-like polypeptides (ELPs). The artificial polypeptides are derived from the pentapeptide repeat Val-Pro-Gly-Xaa-Gly (VPGXG) found in the hydrophobic domain of tropoelastin as reviewed elsewhere [31, 32]. Certain elastin-mimetic triblock (hydrophobic-hydrophylic-hydrophobic) copolymers have also been developed to self-assemble into thermoreversible hydrogels [33]. ELPs are promising scaffolding materials for certain tissue engineering applications [34]. Inspired by the natural elastomeric proteins, a small non-mechanical protein GB1 (the streptococcal B1 immunoglobulin-binding domain of protein G) was recently engineered into a polyprotein (GB1)8 and was found to have excellent elastomeric properties [35], which could be a candidate material for tissue engineering scaffolds.
For certain tissues such as tendons and ligaments, tensile modulus and strength are critically important. Silk from silkworm cocoon is a natural fibrous protein material, and has been used for textile production and surgical sutures for centuries because of its excellent tensile mechanical properties. It could be an excellent scaffolding material for tendon and ligament tissue engineering [36]. Silk in its natural form is composed of a filament core protein, silk fibroin, and a glue-like coating consisting of a family of sericin proteins. There is some concern over the toxicity of natural silk [37]. Sericin in the natural silk materials has been found to cause adverse immune responses and is disadvantageous for tissue engineering applications [38]. Upon sericin removal, regenerated silk fibroin has shown improved biocompatibility [39]. Silk fibroins are characterized as natural block copolymers composed of hydrophobic blocks and hydrophilic blocks of amino acids. The hydrophobic blocks tend to form β-sheets or crystals through hydrogen bonding and hydrophobilc interactions, resulting in the tensile strength of silk fibroins [39]. Genetic engineering techniques have been utilized to synthesize recombinant spider silk fibroin-mimetic polymers, possessing excellent mechanical properties [40] and improved cell adhesion capacity [41].
3. Nano-fibrous Materials
Many extracellular proteins have a fibrous structure with diameters on the nano-meter or sub-micrometer scales. For example, collagen (the most abundant ECM protein in the human body) possesses a fibrous structure with fiber bundle diameter varying from 50 to 500 nm [42, 43]. To engineer materials mimicking the nano-fibrous ECM proteins, a few technologies have been developed with varying degrees of success.
3.1. Electrospinning
Electrospinning has existed in the literature for more than 100 years [44]. It was utilized to fabricate industrial and household nonwoven fabric products in the early 1930s [45]. The technique has been rejuvenated recently as a very active research area because of its ability to produce fibers with diameters down to the sub-micron or nanometer range, significantly smaller than that can be achieved by other textile technologies such as melt spinning. The basic equipment setup includes a polymer solution (or melt) reservoir with a spinneret, an electric field (at a high voltage), and a grounded target collector. Under the electric field, the pulling force overcomes the surface tension of the polymer solution (or melt) and generates a charged jet that travels in a straight line for a certain distance. It then bends due to electrical instability to follow a whipping and spiraling path, reducing the jet diameter. The solvent evaporates on the way and the jet solidifies to form a non-woven fibrous mat on the grounded collector [46, 47]. An electrically grounded rotating drum can be used as the collector to achieve preferred orientation if desired [48, 49].
A variety of natural macromolecules (e.g. collagen, silk fibroin, and fibrinogen) and synthetic polymers (e.g. PGA, PLLA, PLGA, and PCL) have been processed into fine nonwoven fabrics for tissue engineering research [48, 50-60]. Various cells have been reported to attach, proliferate, and differentiate into or maintain their functional phenotypes on these electrospun nano-fibrous materials [48, 54, 58, 59, 61, 62]. However, significant challenges still exist in using this technique to fabricate complex 3D scaffold shapes or to generate designed internal pore structures. In addition, the electrospun fibers using FDA approved biodegradable polymers (e.g. PLLA and PLGA) often have diameters in the micrometer range.
3.2. Self-assembly
Self-assembly is loosely defined as the autonomous organization of components into patterns or structures without human intervention [63]. Such patterns or structures are typically formed and maintained by non-covalent bonds [64]. Although non-covalent bonds such as hydrogen bonds, ionic bonds, and hydrophobic interactions are rather weak in isolation, when in concert they govern the self-assembly of biological macromolecules. One example of molecules that readily self-assemble into higher order structures in the nature is phospholipids, which are the principal component of the plasma membrane in cells. In aqueous solution, phospholipids readily self-assemble to form vesicles, micelles, and tubules. Schnur et al. studied the molecular self-assembly using lipid tubules [65]. Various other molecules have also been utilized in molecular self-assembly.
To emulate the triple helical structure of collagen, peptide-amphiphiles (PAs) have been synthesized by Fields, Tirrell and coworkers [66-68]. A PA consists of a collagen sequence Gly-Val-Lys-Gly-Asp-Lys-Gly-Asn-Pro-Gly-Trp-Pro-Gly-Ala-Pro connected to a long-chain mono- or di-alkyl ester lipid. The collagen sequence peptide head group forms the triple helical structure, while the lipophilic tail region will associate by hydrophobic interactions to induce and/or stabilize the three-dimensional structure of the collagen sequence peptide head group [68]. Melanoma cells were cultured on this PA in order to determine its biological activity in relation to adhesion, spreading and signal transduction. Neither the peptide nor the tail alone produced significant adhesion; however, the self-assembled triple helical structure of the PA significantly promoted cell adhesion [69]. However, such lipids were not demonstrated to form supramolecular fibers.
Beyond above-discussed simple units, PA molecules are known to self assemble into sheets, spheres, rods, disks or channels depending on the shape, charge, and environment in which they are placed [70, 71]. Stupp and coworkers have developed nano-fiber forming PAs, having five structural features [72]. They include: a long alkyl tail that conveys hydrophobic characteristics to drive the self assembly; four consecutive cysteine residues that form disulfide bonds to stabilize the structure; a linker region containing three glycine residues to provide the hydrophilic head group the flexibility from the rigid cross-linked regions; a phosphorylated serine residue that interacts strongly with calcium ions intended to enhance mineralization; and an Arg-Gly-Asp (RGD) peptide to aid in cell adhesion. The PAs can form cylindrical micelle tubes (“nano-fibers”) with dimensions of approximately 5-8 nm in diameter and over 1 μm in length. The PA molecules are perpendicular to the nano-fibers with the hydrophobic portion in the interior and the hydrophilic portion on the surface. This is different from the natural collagen fibers, in which collagen molecular helices are aligned parallel to the fiber axis. Such nano-fibers can be formed through treating PAs with dithiothreitol at a pH of 8 and then reducing the pH to 4. As the solution is acidified the PAs quickly become insoluble and form nano-fibers of a gel. Some of the self-assembled hydrogels are being explored for cell entrapment utilizing polyvalent metal ions in the media, and one of them has been shown to allow for MC3T3-E1 osteoblastic cells to survive for 3 weeks [73].
Zhang and coworkers have created self-assembled structures using ionic self-complementary oligopeptides [74]. These oligopeptides contain alternating blocks of hydrophobic and hydrophilic amino acids. In water, they freely organize themselves into stable β-sheet structures. When exposed to monovalent alkaline cations or physiological conditions, the oligopeptides can assemble into hydrogels with interwoven nano-fibers with a diameter of 10-20 nm [74, 75]. Self-assembly has also been shown by molecules other than peptides or PAs [76]. For example, Liu et al. reported the self-assembly of polyphenylene dendrimers into micrometer-long nano-fibers [77, 78]. Perutz demonstrated the formation of nanotubes from polyglutamines [79-81].
Tissue engineering scaffolds should ideally have sufficiently large pores to allow for cell incorporation, migration, and proliferation. One of the limitations of the current self-assembled scaffolds is their inability to controllably form macro sized pores. Another may be the limitation to form mechanically stable 3D geometry. Their degradation also needs to be addressed. Nevertheless, self-assembled materials are often hydrogels, which are convenient for injection.
3.3. Phase Separation
Phase separation occurs when a homogeneous multi-component system becomes thermodynamically unstable under certain conditions and tends to separate into a multi-phase system in order to lower the system free energy [3]. The phase separation of polymer solutions has been explored in our laboratory and others to generate porous structures as tissue engineering scaffolds [11, 82-88]. When phase separation occurs, a polymer solution separates into two phases, a polymer-rich phase (with a high polymer concentration) and a polymer-lean phase (with a low polymer concentration). After the solvent is removed, the polymer-rich phase solidifies. Depending upon the system and phase separation conditions the resulting materials are different in physical form: powder, closed-pore foam, or open-pore foam [3]. Only the open-pore foam materials are suitable for tissue engineering applications.
A novel phase separation technique has been developed in our laboratory to fabricate nano fibrous matrices from synthetic biodegradable polymers [89, 90]. For example, PLLA solutions are thermally induced following a series of processes to phase separate. The solvent is directly sublimated or first exchanged with a different solvent and then sublimated to fabricate the desired nano-fibrous matrices (Figure 1) [89]. However, these matrices themselves have similar limitations to those of a collagen matrix, self-assembled PA gel, or electrospun mat, lacking of inter-connected macro-pores.
Figure 1.
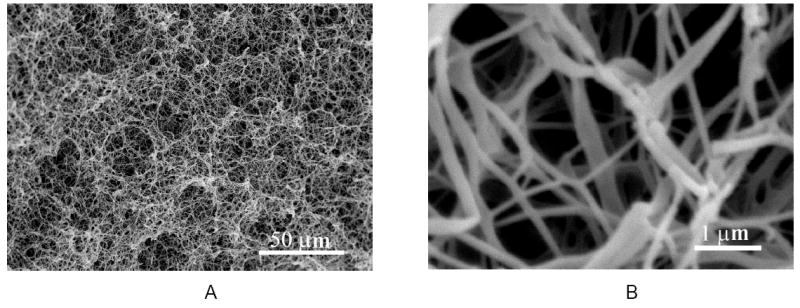
SEM micrographs of a PLLA nano-fibrous matrix prepared from 2.5% (wt/v) PLLA/THF solution at a phase separation temperature of 8°C: A) 500x; B) 20,000x. From Ma and Zhang [89], Copyright © 1999 by John Wiley & Sons. Reprinted by permission of John Wiley & Sons.
To improve the 3D structure of nano-fibrous scaffolds for cell seeding and distribution, mass transport, vascular invasion, and tissue organization, techniques have been developed in our laboratory to build pre-designed macro-/micro-pore networks in nano-fibrous matrices [91]. For example, larger water-soluble fibers (diameter from 100 μm to 1 mm) are prepared from sugar as a geometrical porogen (pore-generating material) element, and are assembled into various 3D structures (such as helicoidal network). PLLA solution is then cast into this 3D assembly and is thermally induced to phase separate for nano-fibrous matrix formation. After the solvent removal, the sugar fiber assembly is dissolved away using water to achieve nano-fibrous scaffolds with predesigned helicoidal tubular pore network (Figure 2 A&B). Similarly, we have combined a technique to generate inter-connected spherical pore network [92] with the nano-fiber techniques to fabricate nano-fibrous scaffolds with interconnected spherical macro pores (Figure 2 C&D) [93, 94].
Figure 2.
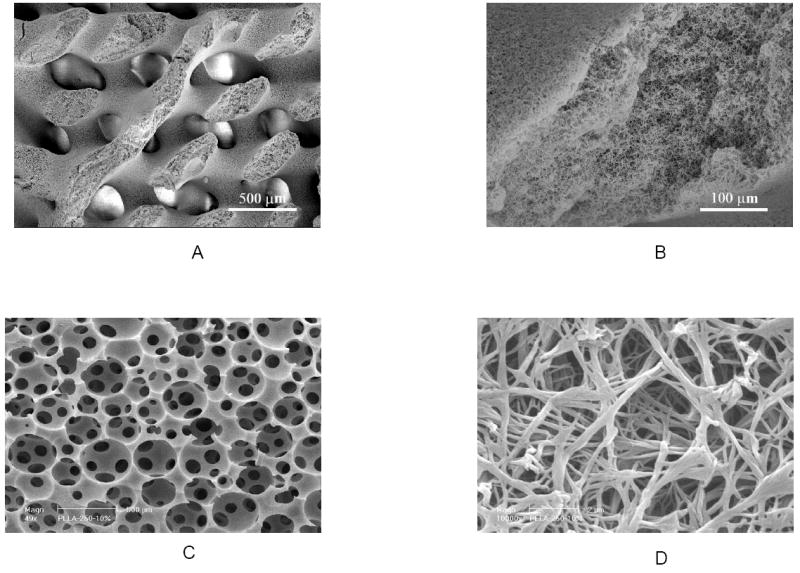
PLLA nano-fibrous scaffolds with designed macro-pore network. A-C) PLLA nano-fibrous scaffolds with helicoidal tubular macropore network prepared from PLLA/THF solution and a helicoidal sugar fiber assembly: A) SEM micrograph at an original magnification of 35x, and B) Original magnification of 250x; C&D) PLLA nano-fibrous scaffolds with interconnected spherical pore network prepared from PLLA/THF solution and a sugar sphere assembly: C) SEM micrograph at an original magnification of 50x, and D) Original magnification of 10,000x. (A&B) From Zhang and Ma [91], Copyright © 2000 by John Wiley & Sons. (C&D) From Wei and Ma [94], Copyright © 2006 by John Wiley & Sons. Reprinted by permission of John Wiley & Sons.
One of the common shortcomings of the many scaffold fabrication techniques is the lack of control over 3D shapes. To tackle this problem, computer-assisted-design and computer-assisted-manufacture (CAD/CAM) techniques, a.k.a. solid freeform fabrication (SFF) or rapid prototyping, that have been widely used in modern manufacture industry, are adopted by the field of tissue engineering [3, 95-98]. However, the existing SFF techniques also have their limitations such as limited material selections, inadequate resolution, and structural heterogeneity due to the “pixel assembly” nature of these fabrication processes [2]. To overcome these shortcomings, a reverse SFF fabrication technique, integrating phase-separation methodology, has been recently developed in our laboratory to fabricate nano-fibrous scaffolds based on reversed images of CT scans or histological sections of human anatomical parts (Figure 3). This technique allows us to fabricate nano-fibrous scaffolds with patient defect specific shapes and designed macro/micro pore networks. Such scaffolds mimic the body parts at several size levels – from nano-fibrous ECM architecture, to macro/micro pores, and to the patient anatomical shapes.
Figure 3.
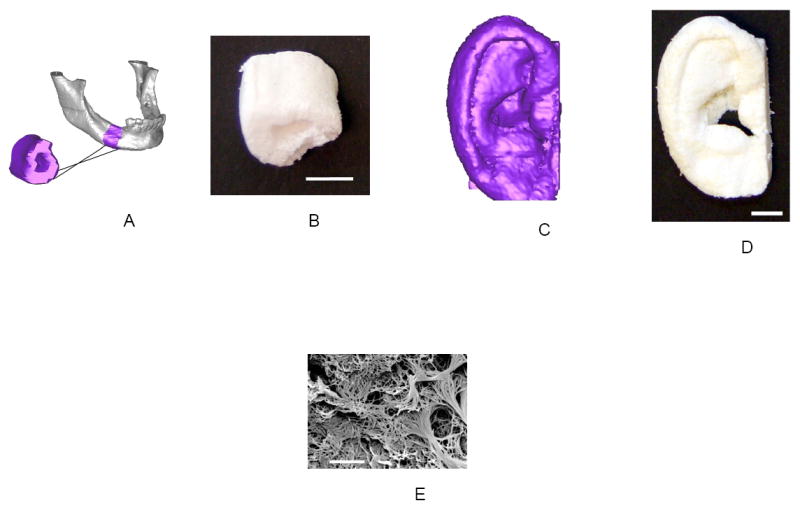
Scaffolds created from 3D reconstructions of CT-scans or histological sections. (a) Human mandible reconstruction from CT-scans (The purple segment shows the reversed image of the bone fragment to be engineered); (b) Resulting nano-fibrous scaffold of the mandible segment (scale bar; 10 mm); (c) Human ear reconstruction from histological sections; (d) Resulting nano-fibrous scaffold of the human ear (scale bar; 10 mm); (e) The nano-fibrous pore wall morphology (scale bar; 5 μm). From Chen, Smith and Ma [100], Copyright © 2006 by Elsevier.
3.4. Biological Effects of Nano-fibrous Architecture
Using the macro-porous and nano-fibrous scaffolds, we investigated the biological effects of the nano-fibrous scaffold architecture. The nano-fibrous scaffolds were found to adsorb 4.2-fold more human serum proteins than solid-walled scaffolds (control scaffolds with smooth pore wall morphology) [99]. Moreover, the profile of serum proteins adsorbed to the nano-fibrous scaffold was different from that adsorbed to the solid-walled scaffolds (Figure 4). While the amount of one protein (arrow) absorbed to both types of scaffolds similarly, some other proteins (arrow heads) exclusively adsorbed onto the nano-fibrous scaffold. Furthermore, Western blot analysis showed that the nano-fibrous scaffolds adsorbed significant levels of fibronectin and vitronectin from serum, while these cell-adhesion proteins were barely detectable on the solid-walled scaffolds [99].
Figure 4.
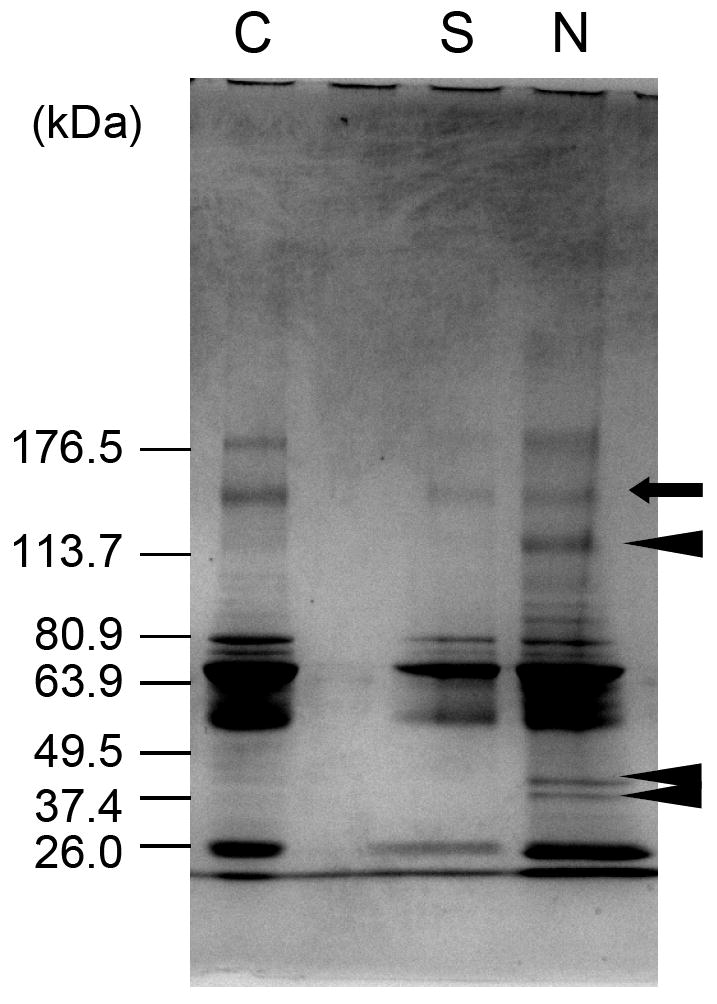
Protein adsorption profile on nano-fibrous and solid-walled scaffolds. Polyacrylamide gels stained with coomassie blue, lane C – bovine serum proteins, lane S – adsorbed bovine serum proteins on the solid-walled scaffold, lane N – adsorbed bovine serum proteins on the nano-fibrous scaffold. From Woo, Chen and Ma [99], Copyright © 2003 by John Wiley & Sons. Reprinted by permission of John Wiley & Sons.
The new nano-fibrous scaffolds were evaluated using osteoblastic progenitor cells (MC3T3-E1). Cells attached on nano-fibrous scaffolds at a level of 70% higher than that on solid-walled scaffolds [99]. The nano-fibrous architecture also enhanced the osteoblastic marker genes’ expression (Figure 5), indicating increased differentiation of the osteoblastic cells [100]. To further examine the effect of the synthetic nano-fibrous matrix on the osteoblastic phenotype, collagen fiber formation was inhibited using dehydroproline. It is known that the interaction of type I collagen with α2β1 integrin signals the expression of genes associated with the osteoblast phenotype, such as OCN and BSP [101, 102]. Expression of α2 integrin was reduced to less than 10% by dehydroproline in the cells cultured on the solid-walled (SW) scaffolds, whereas mRNA levels for α2 integrin were not altered in cells cultured on nano-fibrous scaffolds (Figure 6). These results suggested that the signals, from α2-containing integrin dimers for expression of OCN and BSP on cells, could be partially sustained in cells seeded on nano-fibrous scaffolds in the absence of cell-secreted collagen fibers.
Figure 5.
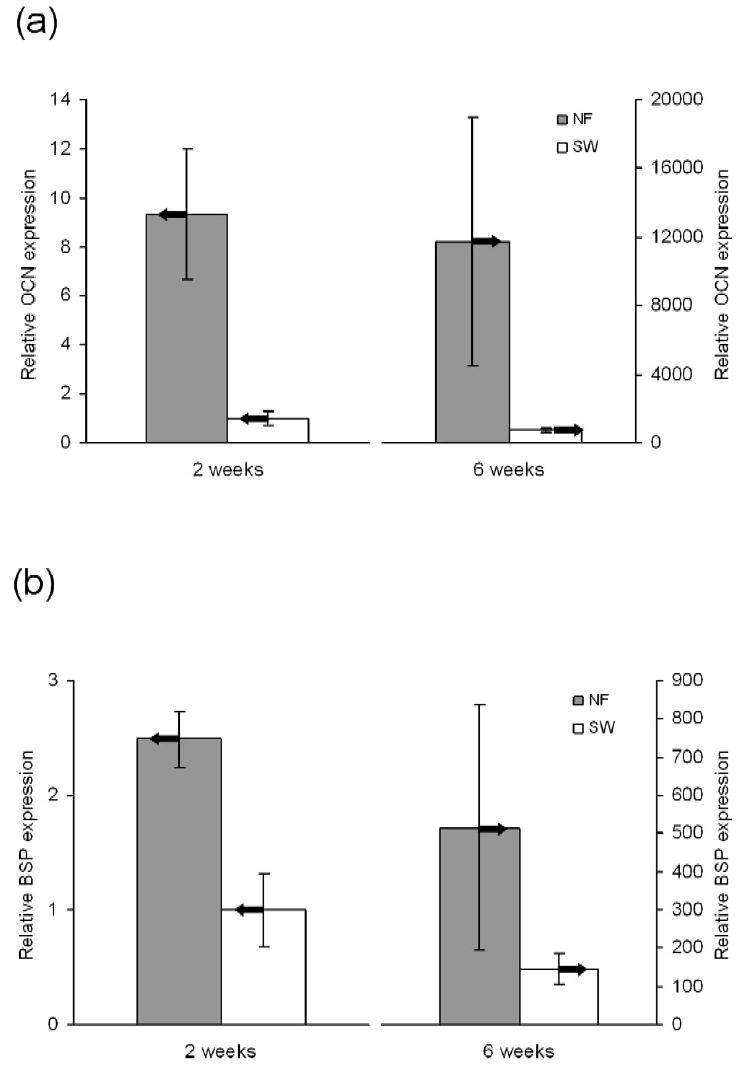
Relative levels of bone marker gene expression on NF and SW scaffolds after 2 and 6 weeks of culture under differentiation conditions. (A) Osteocalcin (OCN) expression. (B) Bone sialoprotein (BSP) expression. From Chen, Smith and Ma [100], Copyright © 2006 by Elsevier.
Figure 6.
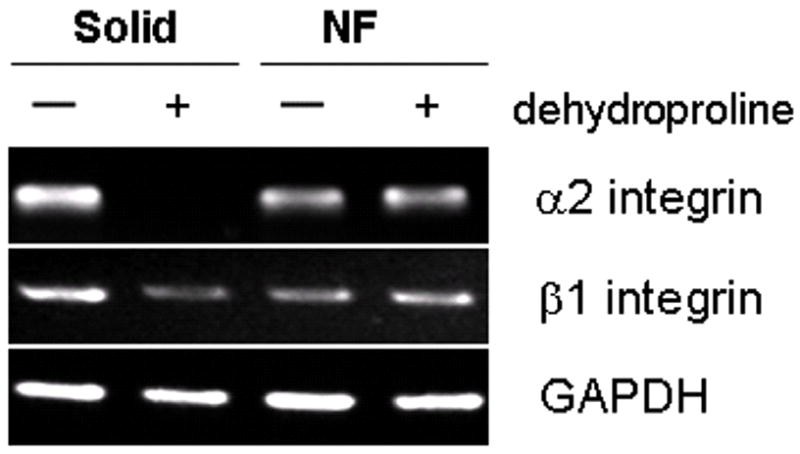
Effect of dehydroproline, an inhibitor of collagen fiber formation, on gene expression in neonatal mouse calvarial osteoblasts grown on the scaffolds. From Woo, Jun, Chen, Seo, Baek, Ryoo, Kim, Somerman and Ma [103], Copyright © 2006 by Elsevier.
Significantly more mineral deposition was observed in the nano-fibrous scaffolds than in the control (solid-walled) scaffolds. Calcium assay revealed 13-fold greater amount of mineral deposition in the nano-fibrous scaffolds than in the control scaffolds [103]. In a rat calvarial bone defect regeneration model, the amount of new bone formation was a few times greater in the nano-fibrous scaffolds than in the solid-walled scaffolds (data to be published). We also found that the nano-fibrous architecture facilitated the differentiation of both adult and embryonic stem cells (data to be published). These data demonstrate that the nano-fibrous polymer scaffolds advantageously mimicked the natural ECM to enhance tissue regeneration while circumventing the potentially adverse immune response and pathogen transmission when using natural ECM materials.
4. Composite and Nano-composite Materials
Although most materials used in tissue engineering scaffolds this far are in their pure form (single component), one polymer often does not meet all the needs for various tissue engineering applications. Natural bone matrix is a typical example of organic/inorganic composite material consisting of collagen and mineral (apatites). This natural composite material has an excellent balance between strength and toughness, superior to either of its individual components. As an example of biomimetic scaffolds, polymer/inorganic composite scaffolds will be reviewed below as advantageous scaffolds for bone tissue engineering.
Being similar to the major inorganic component of natural bone, the inorganic compound such as hydroxyapatite (HAP) or a calcium phosphate in a composite scaffold provides good osteoconductivity while the polymer provides the continuous structure and design flexibility to achieve the high porosity and high surface area, which are necessary for anchorage-dependent cells such as osteoblasts to survive and differentiate [5]. By blending and phase separation techniques, polymer/inorganic composite (PLLA/HAP and PLGA/HAP) scaffolds have been developed (Figure 7 A&B) with improved mechanical properties and osteoconductivity [11, 86]. The HAP-containing scaffolds improve osteoblastic cell seeding uniformity and show significantly enhanced expression of mature bone marker genes such as osteocalcin and bone sialoprotein over plain polymer scaffolds. Bone tissue formation throughout the scaffold has been demonstrated [86]. Several other groups fabricated PLGA/HAP or PLGA/PCL/HAP composite scaffolds using a salt-leaching technique [104-107]. Although the salt-leaching technique has limitations in generating highly porous and well-connected pore structures, they also consistently demonstrated the improved osteoconductivity over the PLGA scaffolds. Our recent data indicate that HAP in the composite scaffolds significantly improves the protein adsorption capacity, suppresses apoptotic cell death, and provides a more favorable microenvironment for bone tissue regeneration [108].
Figure 7.
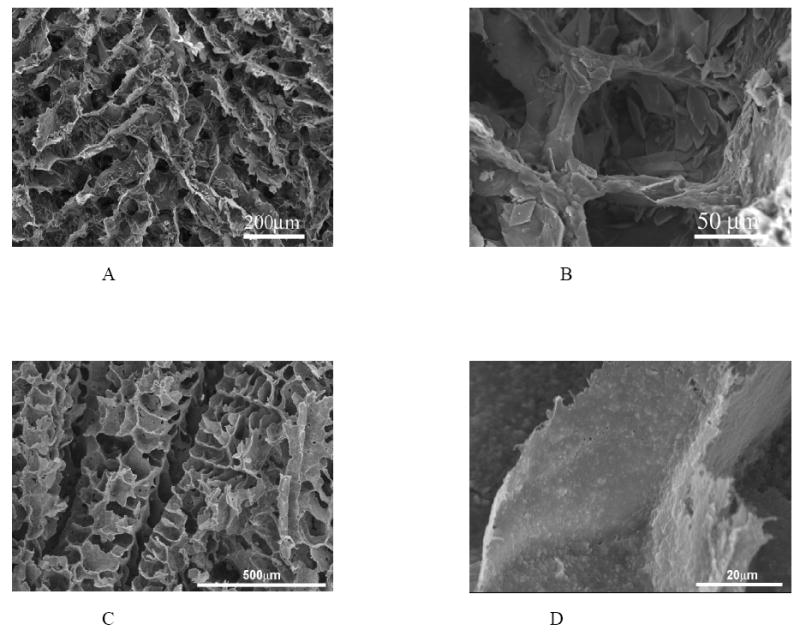
SEM micrographs of PLLA/mHAP (A, B) and PLLA/nHAP (C, D) composite scaffolds fabricated using phase separation. (A, B) From Zhang and Ma [11], Copyright © 1999 by John Wiley & Sons. Reprinted with permission of John Wiley & Sons; (C, D) From Wei and Ma [109], Copyright © 2004 by Elsevier.
In addition to mimicking the organic/inorganic nature of the bone matrix, polymer/nano-HAP scaffolds have also been developed to mimic the nano-sized features of the natural bone mineral (Figure 7 C&D) [109]. The nano-HAP/polymer composite scaffolds not only improved the mechanical properties, but also significantly enhanced protein absorption over micro-sized HAP/polymer scaffolds [109]. The enhanced protein adsorption improves cell adhesion and function [99, 108].
Considering that protein-scaffold and cell-scaffold interactions occur at the scaffold pore surfaces, a biomimetic approach has been developed to grow bone-like apatite nano particles on pre-fabricated porous polymer scaffolds in a simulated body fluid (SBF) to efficiently modify the internal pore wall surfaces with bone-like apatite without altering the bulk structures and properties of the scaffolds (Figure 8 A&B) [82, 110, 111]. The apatite generated via the biomimetic process in an SBF is partially carbonated HAP more similar to the natural bone apatite (calcium deficient Ca/P~1.5) than the stoichiometric HAP crystals (Ca/P=1.67). The partially carbonated apatites should degrade faster than the stoichiometric HAP crystals and serve as a better scaffold component in terms of new bone tissue modeling and remodeling. The growth of apatite crystals is significantly affected by the polymer materials, porous structure, ionic concentration of the SBF as well as the pH value [112].
Figure 8.
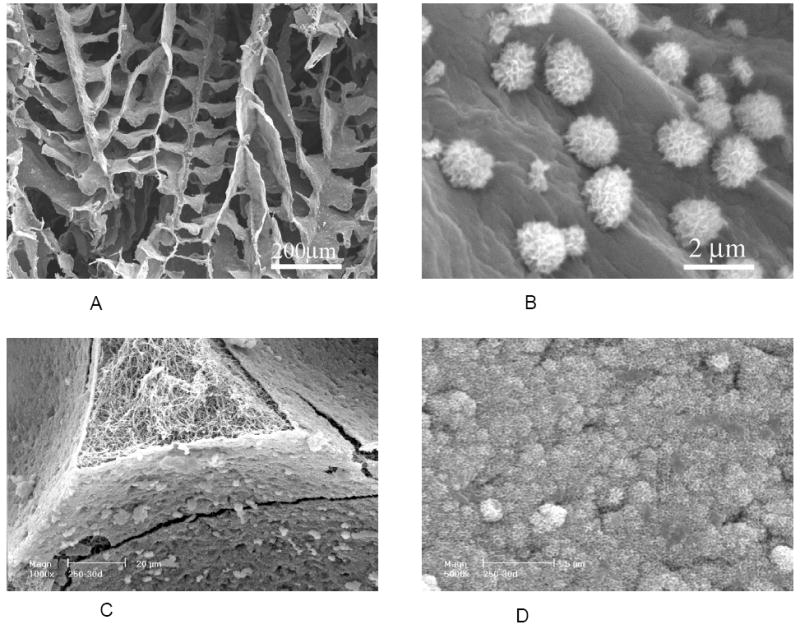
SEM micrographs of PLLA/apatite composite scaffold prepared by a biomimetic process in a simulated body fluid (SBF). (A, B) PLLA scaffolds phase separated in dioxane; (C, D) PLLA nano-fibrous scaffolds prepared by sugar template leaching and phase separation in THF. Scaffolds were incubated in 1.5x SBF at 37 °C for 30 days. (A, B) From Zhang and Ma [82], Copyright © 1999 by John Wiley & Sons; (C, D) From Wei and Ma [94], Copyright © 2006 by John Wiley & Sons. Reprinted with permission of John Wiley & Sons.
To further mimic the nano-fibrous organic component (collagen) and the partially carbonated nano apatite of the bone matrix, macroporous nano-fibrous scaffolds were investigated for bone-like apatite deposition. A large number of nano apatite particles were formed, and even an uniform and dense layer of nano apatite were found to cover the entire internal pore wall surfaces without clogging the macro pores after a reasonably long time of incubation in an SBF (Figure 8 C&D) [94].
5. Surface Modification
The surface of scaffolding materials is important in tissue engineering because the surface can directly affect cellular response and ultimately the tissue regeneration [2, 5]. An ideal tissue engineering scaffolds should mimic ECM and positively interact with cells, including enhanced cell adhesion, growth, migration, and differentiated function. Although a variety of synthetic biodegradable polymers have been used as tissue engineering scaffolding materials, they often lack biological recognition. The use of composite materials such as growing bone-like apatite on scaffold pore surfaces as discussed above is one of the effective ways to improve cellular interactions.
In general, either bulk or surface modification can be employed to achieve the desired positive cellular interactions of a polymer scaffold. Bulk modification is typically realized by copolymerization or functional group attachment to the polymer chain before scaffold fabrication. For example, Langer’s group synthesized poly(L-lactic acid-co-L-lysine) and chemically attached RGD peptide to the lysine residue of the copolymer to enhance cell adhesion [113, 114]. However, bulk chemical modification usually changes the processing and mechanical properties of the polymers, which often is a disadvantage.
Different from bulk modification, surface modification can be carried out after a porous scaffold has been fabricated. It therefore does not usually affect the scaffold structure and mechanical properties significantly. Nitschke et al. utilized low pressure ammonia plasma treatment for the modification of poly(3-hydroxybutyrate) (PHB) thin films [115]. The introduction of amine function was used for subsequent protein immobilization. The plasma treatment of PHB induced a durable conversion from a hydrophobic into a hydrophilic surface without significantly altering the morphology. Hollinger’s group proposed a surface modification method using ammonia plasma treatment, followed by the attachment of poly(L-lysine) and RGD peptide [116]. The surface-modified polymer films enhanced osteoprogenitor cell attachment. Because of the limited plasma penetration, this method can only be used for thin films or very thin 3D structures.
Most of the surface modification work in the literature has been focusing on the modification of device surfaces or thin films [117-120]. Our group recently developed a few techniques to effectively modify the internal pore surfaces of 3D porous polymer scaffolds. For example, a pre-fabricated 3D PLLA scaffold was immersed in a gelatin solution in a solvent mixture (e.g., dioxane and water) [121]. Then the scaffold was moved to a non-solvent of the scaffold material (water in this case). The gelatin molecules were successfully immobilized (entrapped) on the pore surfaces of the scaffolds. Presumably, the immersion of the scaffold in the gelatin solution in the solvent mixture swelled the pore surfaces to a certain degree, allowing the gelatin molecules to at least partially penetrate into the swelled pore surfaces. The immersion in the non-solvent afterwards solidified the pore surfaces, resulting in the entrapment of the gelatin molecules. This is a facile and permanent surface modification technique for complex 3D porous scaffolds. The entrapped gelatin (or other molecules if desired) can not be washed off in water or in an aqueous tissue culture medium [121].
A porogen-induced surface modification technique has also been developed in our lab recently [122]. In this technique, the modifying agent is prepared as the porogen. The surface modification is accomplished during the scaffold fabrication, again using gelatin as an example of surface modifying agent. First, the gelatin spheres were fabricated and assembled into a 3D negative replica of the scaffold to be fabricated. A polymer solution in a solvent mixture (e.g., PLLA in water/THF mixture) was then cast on such a porogen template. After phase separation and porogen removal, a nano-fibrous PLLA scaffold was generated with an interconnected spherical pore network. The PLLA nanofibers in this scaffold was modified with a layer of gelatin molecules, which effectively enhanced the cell spreading, proliferation, and ECM secretion [122]. Presumably, the solvent mixture (water/THF) of the PLLA solution ensured the entrapment of the gelatin molecules during the casting and phase-separation because of the solubility of gelatin in water.
An electrostatic layer-by-layer self-assembly process has also been utilized to modify 3D nano-fibrous scaffolds [123]. A macro-porous and nano-fibrous polymer scaffold was first fabricated. The scaffold was then activated in an aqueous poly(diallyldimethylammonium chloride) (PDAC) solution to obtain positively charged pore surfaces. The scaffold was subsequently immersed in a solution of negatively charged macromolecules (e.g. gelatin). Then the scaffold was alternately immersed in the solutions of the positively charged molecules (e.g., PDAC) and negatively charged molecules (e.g., gelatin). This technique not only allow for surface modification but also provide a controlled way to regulate the surface charge type and the thickness of the surface modification layer [123]. Such surface modification improved the hydrophilicity (lowered the contact angle) and enhanced cell adhesion and proliferation.
For convenience, less expensive molecules such as gelatin or collagen have been used as model biomolecules to demonstrate the surface modification techniques. However these techniques are not limited to gelatin or collagen, other biologically functional molecules (such as proteins, peptides, growth factors, and so forth) can be readily employed to modify polymer scaffold pore surfaces using these technologies.
Although this review paper focuses on bioimimetic scaffold fabrication and modification, it should be pointed out that the recent development in the area of genetic engineering may provide excellent peptide molecules to be used in scaffold surface modification. Sarikaya’s group uses combinatorial biology protocols to display peptide libraries either on the cell surface or on phages, to select short peptides specific to a variety of materials [124, 125]. The selected peptides are further engineered into multiple repeats or variations to tailor their function. The engineered peptides and their derivatives via such a molecular biomimetic approach has high potentials to be used for scaffold surface modification. Similarly, findings in the interactions between cell surface receptors and ECM ligands will continue to provide inspirations for biomimetic surface modification of scaffolds.
6. Bioactive Molecule Delivery
In addition to scaffolds, biological signaling is a key component for cell function and tissue regeneration. Endogenous signaling molecules are often not sufficient in type and/or quantity for the repair of defects of a large size, where the addition of exogenous signaling molecules is required. Because of the sensitivity of cells to the concentration of biological molecules and the short half-lives of these molecules, the successful application of biological factors in tissue engineering critically depends on the delivery technologies.
Controlled release using microspheres has been shown to be effective in retaining the bioactivities of various therapeutic agents [126-128]. However, microspheres, when used alone, often coalesce or migrate, which may not allow the controlled spatial delivery. In tissue engineering, it is desirable to controlled release biological molecules within the scaffold [2, 3, 129-134]. Biological factors have been directly added into a polymer solution or emulsion to fabricate scaffolds [135, 136]. There also have been attempts to modify a scaffold with biological factors using certain coating techniques [137]. These methods can achieve certain slow release characteristics, but the control over release kinetics is limited. The gas foaming process can entrap PLGA microspheres in a porous scaffold [138, 139]. In addition to the lack of control over pore size and shape, the release kinetics of imbedded microspheres in the matrix walls are uncontrollably changed from that by the microspheres.
We have recently developed technologies to immobilize nano-spheres onto the pore surface of scaffolds, such as the macro-porous and nano-fibrous scaffolds [140, 141]. Single or multiple biological factors can be released in a spatially and temporally controlled fashion (Figure 9). The release kinetics of each factor can be individually controlled using a specific nanosphere formulation (Figure 10).
Figure 9.
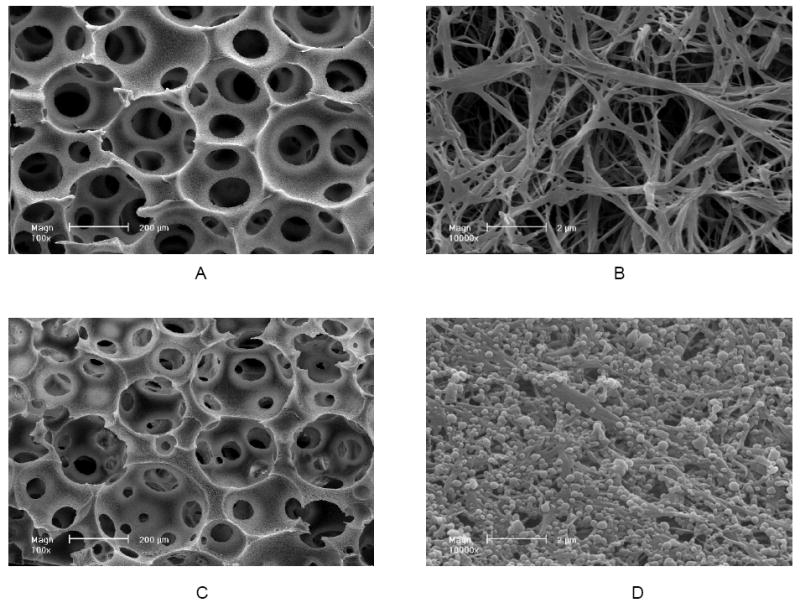
Scanning electron micrographs of nanosphere (NS) incorporated PLLA nano-fibrous scaffolds: (A, B) PLLA nano-fibrous scaffolds before nanosphere incorporation at 100x (A) and 10,000x (B); (C, D) PLLA nano-fibrous scaffolds after nanosphere incorporation at 100x (C) and 10,000x (D). From Wei, Jin, Giannobile and Ma [140], Copyright © 2007 by Elsevier.
Figure 10.
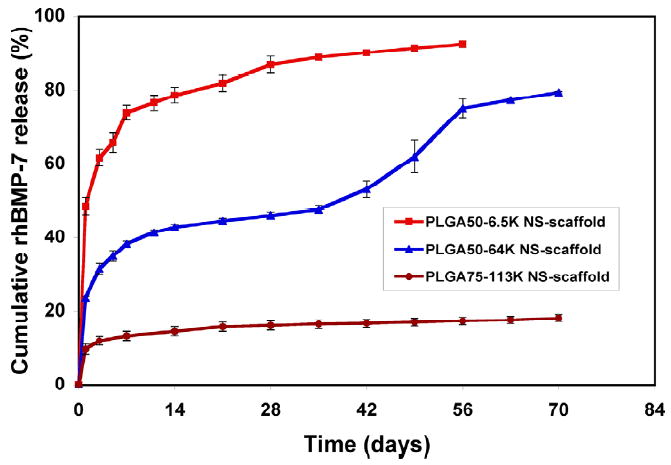
In vitro release kinetics of rhBMP-7 from nanospheres immobilized on nano-fibrous scaffolds: In 10 mM PBS with a BMP-7 loading of 200 ng/scaffold. Each data point represents a mean ± standard deviation (n=3). From Wei, Jin, Giannobile and Ma [140], Copyright © 2007 by Elsevier.
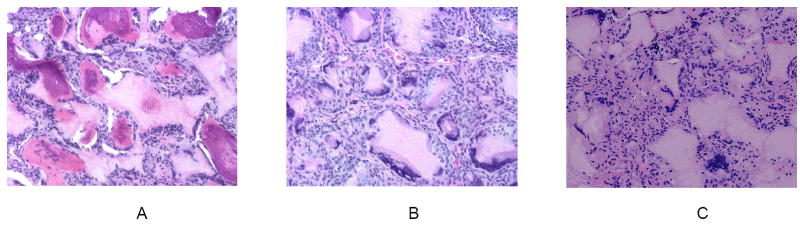
A rat subcutaneous implantation model has been successfully used for bone regeneration with BMP7 loaded nanospheres (BMP7-NS) incorporated in a nano-fibrous PLLA scaffold. Four scaffolds (without cells) were implanted subcutaneously onto the dorsum of each rat. After 6 weeks, H & E staining indicates that there was significant bone formation in the BMP7-NS containing scaffold (Figure 11 A), but only fibrous tissue in the scaffold without BMP7 (Figure 11 B), or in the same scaffold pre-soaked in a BMP7 solution (Figure 11 C) [140]. This result was also supported by von Kossa staining and radiographic measurements [140]. The results indicated that incorporation of BMP7 into nanospheres, which were then immobilized onto a scaffold, preserved the biological activity of BMP7 and could prolong the duration to induce ectopic bone formation.
Figure 11.
BMP7-NS incorporated nano-fibrous PLLA scaffolds were implanted subcutaneously onto the dorsa of rats for 6 weeks: (A) Significant bone formation in the BMP7-NS containing scaffolds (H & E staining); (B) Fibrous tissue in the scaffolds without BMP7 (H & E); (C) Fibrous tissue in scaffolds pre-soaked in a BMP7 solution (H & E). From Wei, Jin, Giannobile and Ma [140], Copyright © 2007 by Elsevier.
Similarly, platelet-derived growth factor (PDGF) incorporated in PLGA nanospheres was released in a controlled fashion [141]. The released PDGF was demonstrated to be biologically active in vitro [141]. After implanted in Spague-Dawley rats, angiogenesis was evaluated using Factor VIII staining and hematoxylin counterstaining. Angiogenesis and corresponding pericyte formation occurred in a PDGF-dose dependent manner. In the control group (scaffolds with empty NS), there was negligible new blood vessel formation. In the scaffolds containing PDGF-NS, there was appreciable new blood vessel formation, increasing with PDGF dose (unpublished data).
Although protein delivery was used as an example in this section, these approaches can also be taken to deliver other biological molecules such as DNA or siRNA etc.
7. Conclusions
Tissue engineering experienced an exponential growth during the last decade from an immerging conceptual stage into a fast developing and multifaceted field. Due to the intrinsic interdisciplinary and multidisciplinary nature of this field, the fast evolution and development of the field of tissue engineering have benefited from the convergence of the progresses in each and every area of the field. On the one hand, the explosively growing life sciences (including stem cell biology, genomics, proteomics and so forth) have substantially expanded the knowledge base of tissue regeneration. The line between tissue engineering and regenerative biology are becoming blurring and a comprehensive field of regenerative medicine is developing. The new discoveries and understandings in life sciences become the inspirations for biomaterials scientists to learn from the nature and to advantageously mimic the biological systems on multiple levels. On the other hand, the exciting nanoscience and nanotechnology are driving the revolution of materials science and engineering. These new and emerging technologies are enabling the materials scientists and engineers to design and fabricate novel scaffolds incorporating various biomimetic characteristics often at the genetic, molecular, and nanometer scales. The author envisions a continuous expansion of biomimetic approaches in the scaffolding materials design in the decades to come, which will substantially advance the field of tissue engineering and regenerative medicine.
Because of space and time limitations, the author did not try to provide a complete or exhaustive review. The author would also like to acknowledge the fact that the figures and examples are frequently selected from the author’s own research because of availability and convenience. It is hoped that the contents reviewed in this article illustrate the importance of biomimetic approaches in tissue engineering materials and that the readers will be able to find more information from the cited references and related publications.
Acknowledgments
The author gratefully acknowledges the past and present support to research in biomimetic scaffolds from the NIH (DE014755, DE015384, GM075840, DE017689), The Whitaker Foundation (RG-99-013), The University of Michigan (Nano Materials Initiative Grant), NASA (NNC04AA21A), NIH Training Grants and NSF Graduate Research Fellowships (for postdoctoral fellows and graduate students).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Ma PX. Scaffolds for tissue fabrication. Materials Today. 2004;7:30–40. [Google Scholar]
- 3.Ma PX. Tissue Engineering. In: Kroschwitz JI, editor. Encyclopedia of Polymer Science and Technology. Third. Vol. 12. John Wiley & Sons, Inc.; Hoboken, NJ: 2005. pp. 261–291. [Google Scholar]
- 4.Rice MA, Dodson BT, Arthur JA, Anseth KS. Cell-based therapies and tissue engineering. Otolaryngol Clin North Am. 2005;38:199–214. doi: 10.1016/j.otc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Annals of biomedical engineering. 2004;32:477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 6.Ma PX, Elisseeff J. Scaffolding in Tissue Engineering. ed. volume. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 7.Catledge SA, Vohra YK, Bellis SL, Sawyer AA. Mesenchymal stem cell adhesion and spreading on nanostructured biomaterials. J Nanosci Nanotechnol. 2004;4:986–9. doi: 10.1166/jnn.2004.137. [DOI] [PubMed] [Google Scholar]
- 8.Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, Kaplan D. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688–98. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Hu J, Lang L, Wang X, Zhang P, Jing X, Wang X, Chen X, Lelkes PI, Macdiarmid AG, Wei Y. Synthesis and characterization of electroactive and biodegradable ABA block copolymer of polylactide and aniline pentamer. Biomaterials. 2007;28:1741–51. doi: 10.1016/j.biomaterials.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Ma PX, Langer R. Degradation, structure and properties of fibrous poly(glycolic acid) scaffolds for tissue engineering. In: Mikos AG, Leong KW, Radomsky ML, Tamada JA, Yaszemski MJ, editors. Polymers in Medicine and Pharmacy. MRS; Pittsburgh, PA: 1995. pp. 99–104. [Google Scholar]
- 11.Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–55. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Ma PX. Degradation behavior of porous poly(a-hydroxyl acids)/hydroxyapatite composite scaffolds. Polymer Preprint. 2000;41:1618–1619. [Google Scholar]
- 13.Chen VJ, Ma PX. The effect of surface area on the degradation rate of nano-fibrous poly(L-lactic acid) foams. Biomaterials. 2006;27:3708–3715. doi: 10.1016/j.biomaterials.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Elisseeff JH, Lee A, Kleinman HK, Yamada Y. Biological response of chondrocytes to hydrogels. Annals of the New York Academy of Sciences. 2002;961:118–22. doi: 10.1111/j.1749-6632.2002.tb03062.x. [DOI] [PubMed] [Google Scholar]
- 15.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng. 2004;86:747–55. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 16.Han DK, Hubbell JA. Synthesis of polymer network scaffolds from L-lactide and poly(ethylene glycol) and their interaction with cells. Macromolecules. 1997;30:6077–6083. [Google Scholar]
- 17.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 18.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Langer R, Vacanti JP, Mayer JE., Jr Tissue-engineered heart valve leaflets: does cell origin affect outcome? Circulation. 1997;96:II–102. 7. [PubMed] [Google Scholar]
- 19.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, Vacanti JP, Mayer JE., Jr Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115:536–45. doi: 10.1016/S0022-5223(98)70315-0. discussion 545-6. [DOI] [PubMed] [Google Scholar]
- 20.Berglund JD, Nerem RM, Sambanis A. Incorporation of intact elastin scaffolds in tissue-engineered collagen-based vascular grafts. Tissue Eng. 2004;10:1526–35. doi: 10.1089/ten.2004.10.1526. [DOI] [PubMed] [Google Scholar]
- 21.Stephan S, Ball SG, Williamson M, Bax DV, Lomas A, Shuttleworth CA, Kielty CM. Cell-matrix biology in vascular tissue engineering. J Anat. 2006;209:495–502. doi: 10.1111/j.1469-7580.2006.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 23.Pitt CG, Gratzl MM, Kimmel GL, Surles J, Schindler A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2:215–20. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- 24.Woodward SC, Brewer PS, Moatamed F, Schindler A, Pitt CG. The intracellular degradation of poly(epsilon-caprolactone) Journal of Biomedical Materials Research. 1985;19:437–44. doi: 10.1002/jbm.820190408. [DOI] [PubMed] [Google Scholar]
- 25.Lin HB, Sun W, Mosher DF, Garcia-Echeverria C, Schaufelberger K, Lelkes PI, Cooper SL. Synthesis, surface, and cell-adhesion properties of polyurethanes containing covalently grafted RGD-peptides. J Biomed Mater Res. 1994;28:329–42. doi: 10.1002/jbm.820280307. [DOI] [PubMed] [Google Scholar]
- 26.Skarja GA, Woodhouse KA. In vitro degradation and erosion of degradable, segmented polyurethanes containing an amino acid-based chain extender. Journal of Biomaterials Science, Polymer Edition. 2001;12:851–73. doi: 10.1163/156856201753113060. [DOI] [PubMed] [Google Scholar]
- 27.Guan JJ, Sacks MS, Beckman EJ, Wagner WR. Synthesis,characterization, and cytocompatibility of efastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. Journal of Biomedical Materials Research. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JY, Beckman EJ, Piesco NP, Agarwal S. A new peptide-based urethane polymer: synthesis, biodegradation, and potential to support cell growth in vitro. Biomaterials. 2000;21:1247–58. doi: 10.1016/s0142-9612(00)00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alperin C, Zandstra PW, Woodhouse KA. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials. 2005;26:7377–86. doi: 10.1016/j.biomaterials.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 30.Bellingham CM, Lillie MA, Gosline JM, Wright GM, Starcher BC, Bailey AJ, Woodhouse KA, Keeley FW. Recombinant human elastin polypeptides self-assemble into biomaterials with elastin-like properties. Biopolymers. 2003;70:445–55. doi: 10.1002/bip.10512. [DOI] [PubMed] [Google Scholar]
- 31.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. Journal Of Physical Chemistry B. 1997;101:11007–11028. [Google Scholar]
- 32.Chilkoti A, Christensen T, MacKay JA. Stimulus responsive elastin biopolymers: Applications in medicine and biotechnology. Curr Opin Chem Biol. 2006;10:652–7. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin- mimetic polypeptide sequences. Adv Drug Deliv Rev. 2002;54:1057–73. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 34.Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27:91–9. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y, Li H. Polyprotein of GB1 is an ideal artificial elastomeric protein. Nat Mater. 2007;6:109–14. doi: 10.1038/nmat1825. [DOI] [PubMed] [Google Scholar]
- 36.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–41. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 37.Kourie JI, Shorthouse AA. Properties of cytotoxic peptide-formed ion channels. Am J Physiol Cell Physiol. 2000;278:C1063–87. doi: 10.1152/ajpcell.2000.278.6.C1063. [DOI] [PubMed] [Google Scholar]
- 38.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–16. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 39.Santin M, Motta A, Freddi G, Cannas M. In vitro evaluation of the inflammatory potential of the silk fibroin. J Biomed Mater Res. 1999;46:382–9. doi: 10.1002/(sici)1097-4636(19990905)46:3<382::aid-jbm11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Guerette PA, Ginzinger DG, Weber BH, Gosline JM. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science. 1996;272:112–5. doi: 10.1126/science.272.5258.112. [DOI] [PubMed] [Google Scholar]
- 41.Bini E, Foo C, Huang J, Karageorgiou V, Kitchel B, Kaplan D. RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacromolecules. 2006 Nov;7(11):3139–45. doi: 10.1021/bm0607877. [DOI] [PubMed] [Google Scholar]
- 42.Hay ED. Cell biology of extracellular matrix. 2. volume. New York: Plenum Press; 1991. [Google Scholar]
- 43.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton WJ. Method of Dispersing Fluids. 705,691. US patent. 1902
- 45.Formhals A. Process and apparatus for preparing artificial threads. 1,975,504. US Patent. 1934
- 46.Deitzel JM, Kosik W, McKnight SH, Beck Tan NC, DeSimone JM, Crette S. Electrospinning of polymer nanofibers with specific surface chemistry. Polymer. 2001;43:1025–1029. [Google Scholar]
- 47.Reneker DH, Chun I. Nanometer diameter fibers of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. [Google Scholar]
- 48.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 49.Theron A, Zussman E, Yarin AL. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology. 2001;12:384–390. [Google Scholar]
- 50.Boland ED, Wnek GE, Simpson DG, Pawlowski KJ, Bowlin GL. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly(glycolic acid) electrospinning. Journal of Macromolecular Science-Pure and Applied Chemistry. 2001;38:1231–1243. [Google Scholar]
- 51.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 52.Wnek GE, Carr ME, Simpson DG, Bowlin GL. Electrospinning of nanofiber fibrinogen structures. Nano Letters. 2003;3:213–216. [Google Scholar]
- 53.Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–24. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of Biomedical Materials Research. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 55.Huang L, McMillan RA, Apkarian RP, Pourdeyhimi B, Conticello VP, Chaikof EL. Generation of synthetic elastin-mimetic small diameter fibers and fiber networks. Macromolecules. 2000;33:2989–2997. [Google Scholar]
- 56.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J Control Release. 2003;89:341–53. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 57.Chew SY, Wen J, Yim EK, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6:2017–24. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- 58.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–10. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 59.Chua KN, Lim WS, Zhang P, Lu H, Wen J, Ramakrishna S, Leong KW, Mao HQ. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537–47. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 60.Duan B, Wu L, Li X, Yuan X, Li X, Zhang Y, Yao K. Degradation of electrospun PLGA-chitosan/PVA membranes and their cytocompatibility in vitro. J Biomater Sci Polym Ed. 2007;18:95–115. doi: 10.1163/156856207779146105. [DOI] [PubMed] [Google Scholar]
- 61.Li W-j, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(e-caprolactone) scaffolds. Journal of Biomedical Materials Research, Part A. 2003;67A:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 62.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 63.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–21. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 64.Roemer D, Buescher HH, Hill RC, Pless J, Bauer W, Cardinaux F, Closse A, Hauser D, Huguenin R. A synthetic enkephalin analogue with prolonged parenteral and oral analgesic activity. Nature. 1977;268:547–9. doi: 10.1038/268547a0. [DOI] [PubMed] [Google Scholar]
- 65.Schnur JM, Price R, Schoen P, Yager P, Calvert JM, Georger J, Singh A. Lipid-based tubule microstructures. Thin Solid Films. 1987;152:181–206. [Google Scholar]
- 66.Yu YC, Tirrell M, Fields GB. Minimal lipidation stabilizes protein-like molecular architecture. Journal Of The American Chemical Society. 1998;120:9979–9987. [Google Scholar]
- 67.Berndt P, Fields GB, Tirrell M. Synthetic Lipidation Of Peptides And Amino-Acids - Monolayer Structure And Properties. Journal Of The American Chemical Society. 1995;117:9515–9522. [Google Scholar]
- 68.Yu YC, Roontga V, Daragan VA, Mayo KH, Tirrell M, Fields GB. Structure and dynamics of peptide-amphiphiles incorporating triple-helical proteinlike molecular architecture. Biochemistry. 1999;38:1659–68. doi: 10.1021/bi982315l. [DOI] [PubMed] [Google Scholar]
- 69.Fields GB, Lauer JL, Dori Y, Forns P, Yu YC, Tirrell M. Protein-like molecular architecture: biomaterial applications for inducing cellular receptor binding and signal transduction. Biopolymers. 1998;47:143–51. doi: 10.1002/(SICI)1097-0282(1998)47:2<143::AID-BIP3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 70.Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of lipid bilayers and vesicles. Biochim Biophys Acta. 1977;470:185–201. doi: 10.1016/0005-2736(77)90099-2. [DOI] [PubMed] [Google Scholar]
- 71.Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2004;39:125–31. doi: 10.1016/j.colsurfb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5133–8. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomater. 2005;1:387–97. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci U S A. 2000;97:6728–33. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. PNAS. 1993;90:3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith LA, Beck JA, Ma PX. Nanofibrous Scaffolds and Their Biological Effects. In: Kumar C, editor. Nanotechnologies for the Life Sciences. Tissue, Cell and Organ Engineering. Wiley-VCH Verlag GmbH & Co.; Weinheim: 2006. pp. 188–215. [Google Scholar]
- 77.Liu DJ, De Feyter S, Cotlet M, Wiesler UM, Weil T, Herrmann A, Mullen K, De Schryver FC. Fluorescent self-assembled polyphenylene dendrimer nanofibers. Macromolecules. 2003;36:8489–8498. [Google Scholar]
- 78.Liu DJ, Zhang H, Grim PCM, De Feyter S, Wiesler UM, Berresheim AJ, Mullen K, De Schryver FC. Self-assembly of polyphenylene dendrimers into micrometer long nanofibers: An atomic force microscopy study. Langmuir. 2002;18:2385–2391. [Google Scholar]
- 79.Perutz MF. Glutamine repeats and neurodegenerative diseases. Brain Res Bull. 1999;50:467. doi: 10.1016/s0361-9230(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 80.Perutz MF, Pope BJ, Owen D, Wanker EE, Scherzinger E. Aggregation of proteins with expanded glutamine and alanine repeats of the glutamine-rich and asparagine-rich domains of Sup35 and of the amyloid beta-peptide of amyloid plaques. Proc Natl Acad Sci U S A. 2002;99:5596–600. doi: 10.1073/pnas.042681599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perutz MF, Windle AH. Cause of neural death in neurodegenerative diseases attributable to expansion of glutamine repeats. Nature. 2001;412:143–4. doi: 10.1038/35084141. [DOI] [PubMed] [Google Scholar]
- 82.Zhang R, Ma PX. Porous poly(L-lactic acid)/apatite composites created by biomimetic process. J Biomed Mater Res. 1999;45:285–93. doi: 10.1002/(sici)1097-4636(19990615)45:4<285::aid-jbm2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 83.Nam YS, Park TG. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. Journal of Biomedical Materials Research. 1999;47:8–17. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 84.Zhang R, Ma PX. Processing of polymer scaffolds: Phase separation. In: Atala A, Lanza R, editors. Methods of Tissue Engineering. Academic Press; San Diego, CA: 2001. pp. 715–724. [Google Scholar]
- 85.Ma PX, Zhang R. Micro-tubular architecture of biodegradable polymer scaffolds. Journal of Biomedical Materials Research. 2001;56:469–477. doi: 10.1002/1097-4636(20010915)56:4<469::aid-jbm1118>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 86.Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly(alpha- hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54:284–293. doi: 10.1002/1097-4636(200102)54:2<284::aid-jbm16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 87.Chen VJ, Ma PX. Phase Separation for Polymer Scaffolds. In: Ma PX, Elisseeff J, editors. Scaffolding in Tissue Engineering. CRC Press; Boca Raton, FL: 2006. pp. 121–133. [Google Scholar]
- 88.Chen VJ, Wei G, Ma PX. Nanostructured scaffolds for tissue engineering and regeneration. In: Nalwa HS, editor. Handbook of Nanostructured Biomaterials and Their Applications in Nanobiotechnology. II. American Scientific Publishers; Stevenson Ranch, California: 2005. pp. 415–435. [Google Scholar]
- 89.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 90.Ma PX, Zhang R. Fibrillar Matrices. 6,146,892. US Patent. 2000
- 91.Zhang R, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. J Biomed Mater Res. 2000;52:430–8. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 92.Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7:23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 93.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25:2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 94.Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. Journal of Biomedical Materials Research: Part A. 2006;78:306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 95.Giordano RA, Wu BM, Borland SW, Cima LG, Sachs EM, Cima MJ. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. Journal of Biomaterials Science, Polymer Edition. 1996;8:63–75. doi: 10.1163/156856297x00588. [DOI] [PubMed] [Google Scholar]
- 96.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Engineering. 2002;8:1–11. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 97.Lin CY, Kikuchi N, Hollister SJ. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J Biomech. 2004;37:623–36. doi: 10.1016/j.jbiomech.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 98.Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:354–62. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 99.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. Journal of Biomedical Materials Research. 2003;67A:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 100.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973–9. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 101.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–94. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 102.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184:207–13. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 103.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, Kim GS, Somerman MJ, Ma PX. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28:335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 104.Marra KG, Szem JW, Kumta PN, DiMilla PA, Weiss LE. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. Journal of Biomedical Materials Research. 1999;47:324–35. doi: 10.1002/(sici)1097-4636(19991205)47:3<324::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 105.Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Hydroxyapatite fiber reinforced poly(alpha-hydroxy ester) foams for bone regeneration. Biomaterials. 1998;19:1935–43. doi: 10.1016/s0142-9612(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 106.Attawia MA, Herbert KM, Laurencin CT. Osteoblast-like cell adherance and migration through 3-dimensional porous polymer matrices. Biochem Biophys Res Commun. 1995;213:639–44. doi: 10.1006/bbrc.1995.2179. [DOI] [PubMed] [Google Scholar]
- 107.Laurencin CT, Attawia MA, Elgendy HE, Herbert KM. Tissue engineered bone-regeneration using degradable polymers: the formation of mineralized matrices. Bone. 1996;19:93S–99S. doi: 10.1016/s8756-3282(96)00132-9. [DOI] [PubMed] [Google Scholar]
- 108.Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28:2622–30. doi: 10.1016/j.biomaterials.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–57. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Ma PX, Zhang R. Porous composite materials. 6,281,257. US Patent. 2001
- 111.Ma PX, Zhang R. Porous composite materials. 6,867,240. US Patent. 2005
- 112.Zhang R, Ma PX. Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromol Biosci. 2004;4:100–11. doi: 10.1002/mabi.200300017. [DOI] [PubMed] [Google Scholar]
- 113.Barrera D, Zylstra E, Lansbury P, Langer R. Synthesis and RGD peptide modification of a new biodegradable copolymer system: Poly(lactic acid-co-lysine) Journal of American Chemical Society. 1993;115:11010–11011. [Google Scholar]
- 114.Cook AD, Hrkach JS, Gao NN, Johnson IM, Pajvani UB, Cannizzaro SM, Langer R. Characterization and development of RGD-peptide-modified poly(lactic acid-co-lysine) as an interactive, resorbable biomaterial. J Biomed Mater Res. 1997;35:513–23. doi: 10.1002/(sici)1097-4636(19970615)35:4<513::aid-jbm11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 115.Nitschke M, Schmack G, Janke A, Simon F, Pleul D, Werner C. Low pressure plasma treatment of poly(3-hydroxybutyrate): toward tailored polymer surfaces for tissue engineering scaffolds. J Biomed Mater Res. 2002;59:632–8. doi: 10.1002/jbm.1274. [DOI] [PubMed] [Google Scholar]
- 116.Hu YH, Winn SR, Krajbich I, Hollinger JO. Porous polymer scaffolds surface-modified with arginine-glycine-aspartic acid enhance bone cell attachment and differentiation in vitro. Journal of Biomedical Materials Research Part A. 2003;64A:583–590. doi: 10.1002/jbm.a.10438. [DOI] [PubMed] [Google Scholar]
- 117.Ratner BD. Characterization of biomaterial surfaces. Cardiovascular Pathology. 1993;2:87S–100S. [Google Scholar]
- 118.Amiji M, Park K. Surface modification of polymeric biomaterials with poly(ethylene oxide), albumin, and heparin for reduced thrombogenicity. J Biomater Sci Polym Ed. 1993;4:217–34. doi: 10.1163/156856293x00537. [DOI] [PubMed] [Google Scholar]
- 119.Ruckenstein E, Li ZF. Surface modification and functionalization through the self-assembled monolayer and graft polymerization. Adv Colloid Interface Sci. 2005;113:43–63. doi: 10.1016/j.cis.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 120.Morra M. Biomolecular modification of implant surfaces. Expert Rev Med Devices. 2007;4:361–72. doi: 10.1586/17434440.4.3.361. [DOI] [PubMed] [Google Scholar]
- 121.Liu X, Won Y, Ma PX. Surface modification of interconnected porous scaffolds. Journal of Biomedical Materials Research: Part A. 2005;74:84–91. doi: 10.1002/jbm.a.30367. [DOI] [PubMed] [Google Scholar]
- 122.Liu X, Won Y, Ma PX. Porogen-induced surface modification of nano-fibrous poly(L-lactic acid) scaffolds for tissue engineering. Biomaterials. 2006;27:3980–7. doi: 10.1016/j.biomaterials.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 123.Liu X, Smith L, Wei G, Won Y, Ma PX. Surface engineering of nano-fibrous poly(l-lactic acid) scaffolds via self-assembly technique for bone tissue engineering. Journal of Biomedical Nanotechnology. 2005;1:54–60. [Google Scholar]
- 124.Sarikaya M, Tamerler C, Jen AK, Schulten K, Baneyx F. Molecular biomimetics: nanotechnology through biology. Nat Mater. 2003;2:577–85. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 125.Tamerler C, Sarikaya M. Molecular biomimetics: utilizing nature’s molecular ways in practical engineering. Acta Biomater. 2007;3:289–99. doi: 10.1016/j.actbio.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 126.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 127.Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–52. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 128.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388:860–2. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 129.Saltzman WM, Olbricht WL. Building drug delivery into tissue engineering. Nat Rev Drug Discov. 2002;1:177–86. doi: 10.1038/nrd744. [DOI] [PubMed] [Google Scholar]
- 130.Hu Y, Hollinger JO, Marra KG. Controlled release from coated polymer microparticles embedded in tissue-engineered scaffolds. Journal of Drug Targeting. 2001;9:431–8. doi: 10.3109/10611860108998777. [DOI] [PubMed] [Google Scholar]
- 131.Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, Ota H, Nozaki K, Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nature Biotechnology. 2001;19:332–335. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- 132.Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, Chen J, Vunjak-Novakovic G, Kaplan DL. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J Biomed Mater Res A. 2006;78:324–34. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- 133.Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537–46. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cushing MC, Liao JT, Anseth KS. Activation of valvular interstitial cells is mediated by transforming growth factor-beta1 interactions with matrix molecules. Matrix Biol. 2005;24:428–37. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 135.Kim H, Kim HW, Suh H. Sustained release of ascorbate-2-phosphate and dexamethasone from porous PLGA scaffolds for bone tissue engineering using mesenchymal stem cells. Biomaterials. 2003;24:4671–9. doi: 10.1016/s0142-9612(03)00358-2. [DOI] [PubMed] [Google Scholar]
- 136.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nature Medicine. 1999;5:753–9. doi: 10.1038/10473. see comments. [DOI] [PubMed] [Google Scholar]
- 137.Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–20. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 138.Cooper AI. Polymer synthesis and processing using supercritical carbon dioxide. Journal of Materials Chemistry. 2000;10:207–234. [Google Scholar]
- 139.Hile DD, Amirpour ML, Akgerman A, Pishko MV. Active growth factor delivery from poly(D,L-lactide-co-glycolide) foams prepared in supercritical CO(2) J Control Release. 2000;66:177–85. doi: 10.1016/s0168-3659(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 140.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano- fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–10. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]