Abstract
Background/Aims
The temporal association between diabetes mellitus and pancreatic cancer is poorly understood. We compared temporal patterns in diabetes prevalence in pancreatic cancer and controls.
Methods
We reviewed medical records of pancreatic cancer cases residing ≤120 miles of Rochester seen at Mayo Clinic between 1/15/1981 and 7/9/2004 and approximately two matched controls/case residing locally. We abstracted all outpatient fasting blood glucose (FBG) up to 60 months before index i.e., date of cancer diagnosis for cases and grouped them into 12-month intervals; 736 cases and 1875 controls had ≥1 outpatient FBG in the medical record. Diabetes was defined as any FBG ≥126 mg/dl or treatment for diabetes and as new-onset when criteria for diabetes were first met ≤24 months before index, with at least one prior FBG <126 mg/dl.
Results
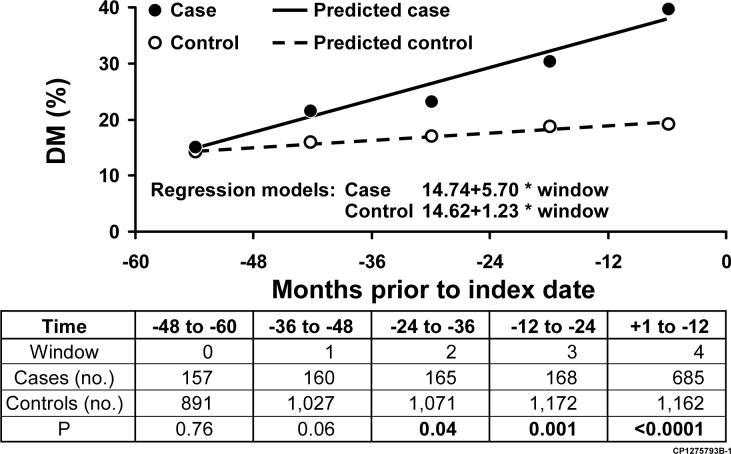
A higher proportion of pancreatic cancer cases compared to controls met criteria for diabetes at any time in the 60 months before index (40.2% vs 19.2%, p<0.0001). The proportions were similar in the −60 to −48 (p=0.76) and −48 to −36 (p=0.06) month time periods; however, a greater proportion of cases than controls met criteria for diabetes in the −36 to −24 (p=0.04), −24 to −12 (p<0.001) and −12 to 0 (p<0.001) month time periods. Diabetes was more often new-onset in cases versus controls (52.3% vs 23.6%, p<0.0001).
Conclusions
Diabetes has a high (40%) prevalence in pancreatic cancer and is frequently new-onset. Identification of a specific biomarker for pancreatic cancer-induced diabetes may allow screening for pancreatic cancer in new-onset diabetes.
The association between diabetes mellitus (DM) and pancreatic cancer (PaC) has long been recognized. While long-standing DM is thought to be an etiologic factor for PaC, new-onset DM may be a manifestation of the cancer 1. Previous epidemiologic studies of the association between DM and PaC 2 have generally used either a case-control design where the prevalence and duration of DM in persons with and without PaC are compared or a cohort design where subjects are followed forward for survival free of PaC regardless of the DM status. A recent meta-analysis 2 of 17 case–control and 19 cohort (or nested case–control) studies published between 1966 and 2005 found that the combined age- and sex-adjusted odds ratio (OR) for PaC associated with DM was 1.82 (95% confidence interval (95% CI) 1.66−1.89). However, subjects with DM duration of ≤4 years had a 50% greater risk of PaC compared to subjects with DM duration of ≥5 years (OR 2.1 vs 1.5; P = 0.005). Since this meta-analysis was published, four more epidemiologic studies from North America 3-6, including one from our group, have reported a strong association of PaC with recent-onset DM.
Based on such data some authors have suggested that recognition of DM as an early manifestation of PaC could lead to diagnosis of surgically resectable PaC 7. However, in most case-control studies, the prevalence of DM in patients with PaC (5−20%) is significantly but not substantially higher than that found in the control group, and only a subset of PaC patients have new-onset DM. Additionally, recent studies 8, 9 in which subjects with new-onset DM were screened for PaC found that, of the few PaCs identified, most cancers were already at an advanced stage and hence unresectable. These data would suggest that new-onset DM associated with PaC (PaCDM) is not sufficiently frequent or early manifestation of PaC to be a clinically relevant marker of undiagnosed PaC. To evaluate the utility of new onset DM as a marker of early PaC, a better understanding of the relationship between DM and PaC is required.
It is important to note that the approaches used in most published epidemiologic studies to identify DM and to determine its date of onset have important limitations. In the recent meta-analysis of 36 epidemiologic studies only 3 used fasting glucose or oral glucose tolerance tests to identify subjects with DM at baseline; the other studies used proxy or self report or review of medical records or a combination of these methods. Often the length of medical record available for review is very limited, and in some studies is limited solely to the acute hospitalization 7. The date of onset and duration of DM in PaC in most previous epidemiologic studies has been based on self report by patient or proxy, date of first hospitalization for DM, or date of earliest clinical mention of DM in the medical records 7. These approaches have obvious limitations in that they underestimate the prevalence of DM, and among those with identified DM, they likely lead to an emphasis on long-standing DM.
The goals of the present study were to confirm the association between PaC and new-onset DM and determine the prevalence and temporal association of DM in PaC compared to age- and sex-matched subjects without PaC. To overcome the problems noted above we reviewed the hospital and ambulatory medical records (including all laboratory fasting blood glucose [FBG] values) for 60 months before index which was defined as the date of PaC diagnosis for cases and approximately the same date for matched controls. Using this approach we were able to identify not only subjects with a prior clinical diagnosis and/or treatment for DM, but also those who met glycemic criteria for DM (FBG ≥126 mg/dl) but were undiagnosed. In addition the date of onset of DM was based on the date subjects met glycemic criteria for DM rather than the date clinical diagnosis of DM was assigned. This extended period of observation and access to laboratory data allowed us to more accurately examine intra-individual patterns over time in subjects with and without PaC.
METHODS
The study was approved by the Mayo Clinic Foundation Institutional Review Board.
PaC case ascertainment
The Mayo Diagnostic Index was searched using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) 10 codes for PaC (157.0 through 157.9, excluding 157.4 (Malignant neoplasm of islets of Langerhans). The search was restricted to subjects seen at Mayo Medical Center (MMC), Rochester, Minnesota (MN), between 1/15/1981 and 7/9/2004 and living within 120 miles of Rochester. We manually reviewed the medical records of the 1820 subjects thus identified to confirm the details of clinical presentation, results of clinical work-up.Only those who were seen within 30 days of PaC diagnosis and had definite PaC (histologically confirmed, n=988 (84%)) or probable PaC (pancreatic mass with obstructive jaundice, n=184 (16%)) were included in the study. The most common reason for physician or patient not obtaining histologic diagnosis of PaC was old age as elderly patients often opted to have no treatment for their cancer; median age of subjects without histology was 78 years versus 68 years for those with histology (p<0.001). Details of date of birth, sex and date of diagnosis of PaC were abstracted from the medical records of the 1172 subjects with definite or probable PaC.
Selection of Control Subjects without PaC
Rochester, MN is the county seat of Olmsted County. For each of the confirmed PaC cases, we selected 2 Olmsted County residents who did not have PaC matched on cases' sex (same), birth date (± 1 year) and seen in the year of case's PaC diagnosis. As was done with cases, we limited controls to residents whose first MMC encounter was before 30 days from index.
Abstraction of Fasting Outpatient Blood Glucose Values
We electronically retrieved all out-patient FBG values for 60 months before the index date on 1172 PaC cases and 2344 subjects without PaC. Further analyses were restricted to the 736 PaC cases and 1875 subjects without PaC who had at least one outpatient FBG values.
Greater proportion of controls had at least one FBG compared to cases (80% vs 63%, p<0.0001). Within controls, females were more likely to have at least one FBG than males (86.47% vs 81.92%, p=0.0152); controls with FBG tended to older than controls without FBG (69.42±11.4 vs 65.8±13.5, (p-value<=0.0001).Within cases, similar proportion of females and males had at least one FBG value (62.9% vs 62.71%, p=0.9478); cases with FBG tended to older than cases without FBG (68.5±11.2 vs 67.1±12.7, p=0.0480).
Abstraction of Demographic and Clinical Data
The inpatient and outpatient medical records of the 736 subjects with PaC and 1875 without PaC were manually reviewed to abstract the following: body mass index (BMI = wt in kg/height in meters squared) at each FBG for 60 months before index and at index, parental history of diabetes, parental history of pancreatic cancer, and smoking history classified as ever/never. In those subjects with any clinical diagnosis of DM or DM-related condition (e.g., hyperglycemia), the medical records were also reviewed to determine, for each glucose value, whether it was drawn while on anti-diabetic medication. In PaC subjects, we noted the presence and duration of any of the cancer-related symptoms of abdominal pain, back pain, jaundice, weight loss and anorexia.
Definition of Time Windows
Data on outpatient FBG values and BMI from cases and controls were grouped into 12-month time periods from 60 months before index date until the index date. In patients without an FBG at event date, an FBG value up to 30 days after was included as long as no surgical intervention had been performed before measurement of FBG. Data on outpatient fasting blood glucose values and BMI from cases and controls were grouped into the following time intervals (windows): +1 to − 12 months, −12 to −24 months, −24 to −36 months, −36 to −48 months, and −48 to −60 months.
Definition of Diabetes and its Duration
Study subjects were defined as having DM if at least one FBG value was ≥126 mg/dl or they were on prescription anti-diabetic medications. All but 19 subjects (13 cases and 6 controls) with DM in our study had FBG values of ≥126 mg/dl. In patients meeting DM criteria, the date that criteria for DM were met was noted. Duration of DM was classified as a) long standing if subjects met criteria for DM more than 24 months before index, b) new-onset if the first date subjects met criteria for DM was within 24 months before index and they had ≥1 previous FBG value <126 mg/dl, and c) of uncertain duration if subjects first met criteria for DM within 24 month before index but had no previous FBG values or if patients whose first elevated FBG value of ≥126 mg/dl was in the <24 month window which was followed by non-elevated values without being on treatment.
Statistical analyses
All analyses were conducted using SAS 9.1.3 (SAS Institute Inc, Cary, North Carolina). The subsets of cases and controls and our approaches to the following questions were as follows:
What is the prevalence of DM in subjects with and without PaC? Among subjects who had at least one FBG value in the 60 months before index, we determined the proportion of cases and controls that met criteria for DM at any time in the 60 months prior to index date.
Are there differences in clinical profile of diabetic subjects with and without PaC? We compared the demographics, smoking history, BMI, parental history of DM, and amount of weight loss in diabetic subjects with and without PaC. Weight loss was calculated as the difference between weight 3−5 years before index and weight at index.
Is there any temporal association between PaC and DM? We compared the proportion of cases and controls that met criteria for DM in each time period limiting the analysis to those subjects with at least one FBG value in that period. An individual could be in multiple windows. Unadjusted proportions of subjects meeting criteria for DM in each time period were calculated, and a Cochran-Armitage trend test was employed to investigate patterns over time. Predicted proportions of DM over time were modeled separately for cases and controls using regression models. Additionally, in each time period, logistic regression models were run to determine if PaC case status, sex, age at index, parental history of DM, smoking status, or average BMI predicted DM status.
What proportion of DM in cases and controls is new-onset? We analyzed all FBG data to determine who met criteria for DM and in which time period. Each individual was classified as either having DM or as being non-diabetic. We then classified the DM as long-standing, new-onset and of uncertain duration, as described above.. A chi-square statistic compared the proportions of cases and controls that 1) met criteria for DM and 2) qualified as new-onset DM.
What is the duration of cancer-related symptoms in PaC? In PaC subjects we noted median duration of cancer-related symptoms of abdominal pain, back pain, jaundice, weight loss and anorexia. We also noted the duration of the first cancer-related symptom.
RESULTS
The demographic and clinical characteristics of the 736 cases and 1875 controls with at least one FBG value at MMC are provided in Table 1.
Table 1.
Characteristics of Subjects with at least one FBG
| Cases (n=736) | Controls (n=1875) | p | |
|---|---|---|---|
| Mean Age (yr ± SD) at index date | 68.6 ± 11.3 | 68.7 ± 11.4 | 0.81 |
| Mean fasting blood glucose (mg/dl ± SD) | 126.5 ± 50.5 | 104.7 ± 31.1 | <0.0001 |
| Body Mass Index (kg/m2 ± SD)* | 26.7 ± 5.4 | 27.3 ± 5.3 | 0.01 |
| Gender: Male, n (%) | 404 (54.9) | 992 (52.9) | 0.36 |
| Smoking, n (%) | <0.0001* | ||
| Never | 242 (34.1) | 799 (43.8) | |
| Ever | 467 (65.9) | 1025 (56.2) | |
| Unknown | 27 | 51 | |
| Parental History of Diabetes, n (%) | 0.96* | ||
| Positive | 117 (17.4) | 311 (17.5) | |
| Negative | 556 (82.6) | 1469 (82.5) | |
| Unknown | 63 | 95 | |
| Parental History of Pancreas Cancer, n (%) | 0.05* | ||
| Positive | 17 (2.5) | 24 (1.4) | |
| Negative | 652 (97.5) | 1688 (98.6) | |
| Unknown | 67 | 163 |
Excluding subjects in whom history of exposure is unknown
Prevalence of DM in Cases and Controls
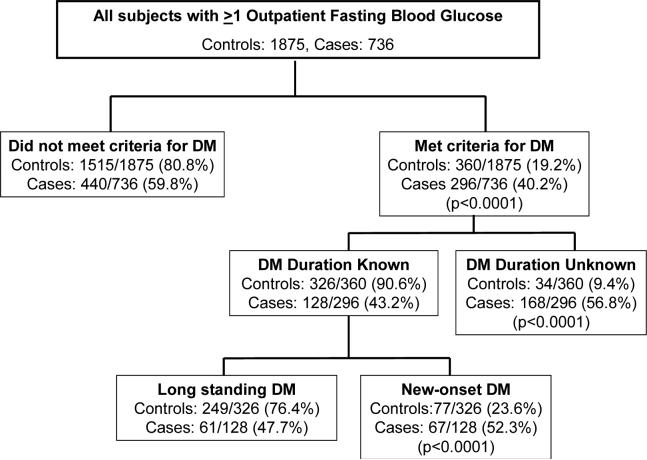
At any time in the 60 month period before index, a greater proportion of PaC subjects met criteria for DM compared to controls, (296/736 (40.2%) vs 360/1875 (19.2%), p<0.0001). The proportion of subjects who met criteria for DM without any clinical mention of DM was greater in PaC cases versus non-PaC controls (107/1172 (9.1%) vs 136/2141 (6.4%), p=0.003).
Clinical Profile of Diabetic Subjects with and without PaC (Table 2)
Table 2.
Comparison of Clinical Profile of Diabetic Cases and Controls
| Cases with Diabetes (n=296) | Controls with Diabetes (n=360) | p | |||
|---|---|---|---|---|---|
| N# | % | N# | % | ||
| Sex (male) | 296 | 55.7 | 360 | 60.3 | 0.24 |
| Parental history of DM | 264 | 23.5 | 333 | 30.3 | 0.06 |
| Parental history of PaC | 269 | 3.4 | 319 | 1.3 | 0.10 |
| Ever smoked (yes) | 294 | 69.4 | 345 | 60.6 | 0.02 |
| N# | Mean (SD) | N# | Mean (SD) | ||
| Age at index (yr) | 296 | 69.7 (9.7) | 360 | 71.5 (10.1) | 0.02 |
| BMI at index (kg/m2) | 252 | 27.0 (5.8) | 98 | 29.5 (6.0) | 0.002 |
| BMI 3−5 yr before index | 81 | 28.8 (4.8) | 245 | 29.7 (6.1) | 0.19 |
| Weight Loss (kg) * | 60 | 6.4 (8.3) | 72 | 1.1 (6.7) | <0.0001 |
Refers to number of patients with informative data on the characteristic evaluated
Weight loss (kg) = Weight 3−5 yr before index - Weight at index
Compared to controls with DM, cases with DM were more likely to be smokers (69.4% vs 60.6%, p=0.02), be younger (69.7 ± 9.7 vs 71.5 ± 10.1, p=0.02), have lower BMI at index (27.0 ± 5.8 vs 29.5 ± 6.0, p=0.002), and experience greater weight loss (6.4 ± 8.3 Kg vs 1.1 ± 6.7, p <0.0001). In the time period 36−60 months before index, cases and controls had similar BMI.
Temporal association between PaC and DM (Figure 1)
Figure 1.
Observed and Predicted Prevalence of DM at 12-Month Intervals Prior to Index
The proportions of cases and controls who met criteria for DM were similar in the −48 to −60 and −36 to −48 month time intervals. By contrast, the proportion of cases who met criteria for DM was higher than that for controls in the time intervals −24 to −36, −12 to −24, and +1 to −12 months. Factors which predicted DM status in each window were age, parental history of DM, and BMI. PaC case status was significant only in the −12 to +1 time frame.
Proportion of DM that is new-onset (Figure 2)
Figure 2.
Proportion of New-onset DM in Controls vs Cases
Among diabetic cases and controls in whom duration of DM was known, DM was more likely to be new-onset (within 24 months before index) in subjects with PaC compared to subjects without PaC (52.3% vs 23.6%, p<0.0001).
Duration of symptoms in PaC
Duration of cancer symptoms was unknown in 28 (3%) of the 736 PaC subjects. Twenty three patients were asymptomatic at the time of diagnosis of PaC (i.e., PaC was detected incidentally either on imaging or at autopsy). In the symptomatic patients, the median duration of symptoms in months was 2.75 (range 0.5−36) for weight loss, 2 (range 0.5−36) for abdominal pain, 2 (range 0.5−28) for back pain, 1.75 (range 0.5−18) for anorexia and 0.5 (0.5 −4) for jaundice. The median time from onset of first cancer-related symptom to diagnosis of PaC was 2 months (range 0.5 to 36 months).
DISCUSSION
Though there have been many epidemiologic studies of the association between PaC and DM 2, few have closely evaluated the relationship between new-onset DM and PaC. Many have excluded PaC occurring within a year of diagnosis of PaC and not all have stratified the risk based on duration of DM 2. This is the first epidemiologic study in which FBG values were abstracted from medical records for 5 years preceding diagnosis of PaC and DM diagnosed if, in that time period, they were either on anti-diabetic medications and/or met glycemic criteria for DM (FBG ≥126 mg/dl). Using this approach we show that the prevalence of DM in PaC (40%) is substantially higher than that reported in previous epidemiologic studies (5−20%) and similar to the prevalence of DM in studies that have screened PaC subjects for DM using FBG measurement 11, 12 or oral glucose tolerance test (OGTT) (45−64%) 13, 14. We also show that the DM in over half the diabetic PaC patients is new-onset, i.e., ≤24 months prior to cancer diagnosis. The findings provide strong epidemiologic evidence to support the notion that if PaC-associated DM (PaCDM) can be distinguished from primary type 2 DM, older subjects with new-onset DM may benefit from screening for asymptomatic PaC.
The best estimates of the prevalence of type 2 DM in the general population are available from the National Health and Nutrition Examination Surveys (NHANES). In NHANES 1999−2000, 15% (CI 12.6−17.5) of 6,596 United States subjects ≥60 years reported a previous diagnosis of DM by a physician or health care professional; an additional 4.2% had a fasting plasma glucose of ≥126 mg/dl. In our study, in which prior treatment of DM or an FBG of ≥126 mg/dl was used to define DM, 19.2% of control subjects without PaC met criteria for DM, a figure remarkably similar to the combined prevalence of diagnosed and undiagnosed DM in the NHANES 1999−2000 study.
While the prevalence of DM in non-cancer subjects in our study remained stable throughout the study period, for PaC subjects it was temporally associated with the diagnosis of cancer. The prevalence of DM in PaC and non-cancer subjects was similar >36 months before index date. However, a marked and continuous increase in prevalence of DM was seen from 24 to 36 months before diagnosis until the date of diagnosis of PaC. Eventually 40.2% of PaC met criteria for DM compared to 19.2% of non-cancer subjects. The high prevalence of DM in PaC in our study is very similar to the 46% prevalence of DM at diagnosis of PaC in an earlier study of 130 PaC patients using the same criteria 12. In yet another study of 532 incident PaC studied at diagnosis of cancer using the same criteria, 47% had DM 11. In small studies using OGTT to diagnose DM in PaC, the reported prevalence is up to 64% 14, 15. Thus, when screened for DM using FBG measurements or OGTT nearly half the patients with PaC have DM. Remarkably the prevalence of DM in PaC is much higher than in other well known diabetogenic states such as morbid obesity 16, polycystic ovarian syndrome 17 and pregnancy 18.
The reason for the low prevalence of DM (<20%) reported from studies using medical records and self (or proxy) report to identify DM is that a high proportion of DM in PaC remains undiagnosed. In the NHANES 1999−2000 study, ∼25% of type 2 DM in the general population is undiagnosed. In our study, the proportion of subjects who were identified as having DM based on FBG of ≥126 mg/dl but who did not have a clinical mention of DM was greater in cases versus controls (107/1172 (9.1%) vs 136/2141 (6.3%), p =0.003). It is not surprising that a greater proportion of PaC had undiagnosed DM. The time-interval between onset of type 2 DM and its clinical diagnosis is 4 to 7 years 19. In PaC the cancer becomes symptomatic before the DM is diagnosed. Additionally, we found that in patients with symptomatic PaC, physicians often do not record a new diagnosis of DM because the focus of medical attention is on the newly diagnosed PaC and not on the newly elevated fasting glucose values.
A limitation of our study is that a significant proportion of cases was seen only once, close to their date of PaC diagnosis and did not have prior FBG values in our institutional medical records. While these cases contributed to the analyses of prevalence of DM in PaC, they were uninformative in terms of understanding the temporal association of DM and PaC. However, the results of our analyses of temporal trends were unchanged when we restricted our study to cases that had been seen at least once ≥6 months prior to their PaC diagnosis, presumably when they did not have cancer specific symptoms (data not shown).
The two published meta-analyses of the epidemiologic studies on the association between DM and PaC 2, 20 have both found a positive but weak association between long-standing DM and PaC; the OR for DM ≥4 years in the most recent meta-analysis was 1.5 (95% CI 1.3−1.8). In our study the prevalence of DM in cases and controls was similar >36 months prior to PaC diagnosis. Many previous studies 21-27 have also failed to observe a significant association between DM and PaC after exclusion of cases in which DM was diagnosed 1−5 years before PaC diagnosis. Thus, while a weak association may exist between long-standing DM and PaC, it is likely to have limited clinical utility, both in terms of understanding the pathogenesis of PaC or its early detection.
The very high prevalence of new-onset DM in PaC suggests that the incidence of PaC would be higher in subjects with new-onset DM than in the general population. We have found in a recent study that subjects with new-onset DM have an 8-fold higher likelihood of being diagnosed with PaC within 3 years of meeting criteria for DM compared to the general population 3. Other investigators have screened subjects with new-onset DM with cancer-related symptoms such as jaundice, abdominal pain, weight loss and increased levels of Ca 19−9 for PaC 8, 9 and found a high prevalence of PaC (5.2 to 13.6%), but its resectability rate was low. This is essentially because cancer-related symptoms occur shortly before the diagnosis of PaC. In our study the median duration of symptoms prior to diagnosis of cancer was only 2 months. This suggests that the strategy to use cancer-related symptoms as a means to suspect PaC in new-onset DM is unlikely to detect resectable cancer 8, 9.
Since PaC patients seldom exhibit disease-specific symptoms until late in the course of the disease, early detection of small tumors will require screening of asymptomatic subjects for PaC. Screening for asymptomatic PaC is a challenge because of lack of a high-risk group and lack of a biomarker of early PaC. Screening un-selected populations for asymptomatic PaC will not be cost effective. For example, the age-adjusted incidence of PaC in subjects ≥50 years of age is 38/100,00028. If a test with 99% sensitivity and 99% specificity for PaC is used to screen 100,000 subjects ≥50 years of age, the test would identify nearly all PaCs in the population screened (n=37); but the test would also falsely identify another 1000 subjects as having PaC. Thus screening for asymptomatic PaC will require at least two “sieves” to enrich the population to allow cost-effective screening 29. We believe that new-onset DM can serve as the first sieve.
Our study shows that in many PaC patients, onset of DM occurs when they would have no cancer-related symptoms. Thus new-onset DM is the only clue to the presence of asymptomatic PaC. Previous authors have suggested that subjects with new-onset DM who are lean and/or lack a family history of DM should be targeted for screening 7. When we compared the profile of diabetic PaC subjects with controls to identify pre-morbid demographic indicators of PaCDM, we found statistical but not clinically useful differences between the two groups in BMI, proportion of subjects who were smokers or those with parental history of pancreatic cancer. Therefore, we believe that the success of any strategy to use hyperglycemia and DM to identify undiagnosed PaC will depend largely on our ability to differentiate PaCDM from type 2 DM using a serologic marker.
Only a prospective study can determine the clinical validity of the observation that older subjects with new-onset DM would benefit from further screening for PaC. However, since prevalence of PaC in new-onset DM is <1%, a large number of subjects with new-onset DM would have to be followed prospectively to have sufficient incident PaC cases in the study. Screening for PaC in new-onset DM will become practical only if we can accurately distinguish PaCDM from type 2 DM. To increase the prevalence of PaC in new-onset DM from 1% to 10%, a biomarker would have to have a specificity of 93% for PaCDM. Our hope is that the mediator of DM in PaC will also serve as a marker to distinguish PaCDM from type 2 DM.
The very high prevalence of diabetes in PaC and its close temporal association with the diagnosis of cancer provide strong epidemiologic evidence to support the notion that PaC causes DM. Further support for this hypothesis is provided by small clinical studies in which resection of the tumor has been shown to improve glucose tolerance and reverse the metabolic defect 13, 30, 31. That PaCDM may be a paraneoplastic phenomenon caused by tumor secreted products is also suggested by the experimental observations that PaC cell line supernatants are metabolically active. They have been shown to induce glucose intolerance in SCID mice 32, alter glucose metabolism in the liver 33 and skeletal muscle 34, 35 and cause selective amylin secretion from ß islet cells 36. This provides hope that the mediator of PaCDM can be identified.
In summary, our study shows DM is present in a high proportion of patients with PaC and is often of recent onset. Based on available evidence we believe that if the PaC-associated DM can be distinguished from type 2 DM, older subjects with new-onset DM would benefit from further screening for PaC.
Author Funding
Dr Chari's research was funded by grants from NIH (R01 CA 100685 and P50 CA 10270) and the Lustgarten Foundation
Dr Leibson's research was funded by grants from SmithKline Beecham Pharmaceuticals
Dr Petersen's and Dr de Andrade's research was funded by grants from NIH (R01 CA 100685 and P50 CA 10270)
The grants and sponsors did not influence design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interests exist
REFERENCES
- 1.Wang F, Herrington M, Larsson J, Permert J. The relationship between diabetes and pancreatic cancer. Mol Cancer. 2003;2:4. doi: 10.1186/1476-4598-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chari S, Leibson CL, de Andrade M, Rabe KG, Ransom JE, Petersen GM. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousseau MC, Parent ME, Pollak MN, Siemiatycki J. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer. 2006;118:2105–9. doi: 10.1002/ijc.21600. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Vittinghoff E, Bertenthal D, Corley D, Shen H, Walter LC, McQuaid K. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol. 2006;4:1366–72. doi: 10.1016/j.cgh.2006.06.024. quiz 1301. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev. 2006;15:1458–63. doi: 10.1158/1055-9965.EPI-06-0188. [DOI] [PubMed] [Google Scholar]
- 7.Noy A, Bilezikian JP. Clinical review 63: Diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab. 1994;79:1223–31. doi: 10.1210/jcem.79.5.7962312. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa Y, Tanaka M, Inoue K, Yamaguchi K, Chijiiwa K, Mizumoto K, Tsutsu N, Nakamura Y. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94:2344–9. doi: 10.1002/cncr.10493. [DOI] [PubMed] [Google Scholar]
- 9.Damiano J, Bordier L, Le Berre JP, Margery J, Dupuy O, Mayaudon H, Bauduceau B. Should pancreas imaging be recommanded in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes & Metabolism. 2004;30:203–7. doi: 10.1016/s1262-3636(07)70111-8. [DOI] [PubMed] [Google Scholar]
- 10.Martinez A, Weaver C, Lopez J, Bhathena SJ, Elsasser TH, Miller MJ, Moody TW, Unsworth EJ, Cuttitta F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology. 1996;137:2626–32. doi: 10.1210/endo.137.6.8641217. [DOI] [PubMed] [Google Scholar]
- 11.Pannala RLJ, Bamlet WR, Basu A, Petersen GM, Chari ST. Very high prevalence of new-onset diabetes and impaired fasting glucose in pancreatic cancer- Results of a case-control study. Gastroenterology. 2007;132:A118. [Google Scholar]
- 12.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–5. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 13.Permert J, Larsson J, Fruin AB, Tatemoto K, Herrington MK, von Schenck H, Adrian TE. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60–8. doi: 10.1097/00006676-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–93. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist HJ, Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–7. [PubMed] [Google Scholar]
- 16.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama. 1999;282:1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 17.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 18.Coustan DR, Nelson C, Carpenter MW, Carr SR, Rotondo L, Widness JA. Maternal age and screening for gestational diabetes: a population-based study. Obstet Gynecol. 1989;73:557–61. [PubMed] [Google Scholar]
- 19.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4−7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–9. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 20.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta- analysis. JAMA. 1995;273:1605–9. [PubMed] [Google Scholar]
- 21.Gold EB, Gordis L, Diener MD, Seltser R, Boitnott JK, Bynum TE, Hutcheon DF. Diet and other risk factors for cancer of the pancreas. Cancer. 1985;55:460–7. doi: 10.1002/1097-0142(19850115)55:2<460::aid-cncr2820550229>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Bueno de Mesquita HB, Maisonneuve P, Moerman CJ, Walker AM. Aspects of medical history and exocrine carcinoma of the pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1992;52:17–23. doi: 10.1002/ijc.2910520105. [DOI] [PubMed] [Google Scholar]
- 23.Cuzick J, Babiker AG. Pancreatic cancer, alcohol, diabetes mellitus and gallbladder disease. Int J Cancer. 1989;43:415–21. doi: 10.1002/ijc.2910430312. [DOI] [PubMed] [Google Scholar]
- 24.Jain M, Howe GR, St Louis P, Miller AB. Coffee and alcohol as determinants of risk of pancreas cancer: a case-control study from Toronto. Int J Cancer. 1991;47:384–9. doi: 10.1002/ijc.2910470313. [DOI] [PubMed] [Google Scholar]
- 25.Lin RS, Kessler A multifactorial model for pancreatic cancer in man. Epidemiologic evidence. Jama. 1981;245:147–52. [PubMed] [Google Scholar]
- 26.Norell S, Ahlbom A, Erwald R, Jacobson G, Lindberg-Navier I, Olin R, Wiechel KL. Diabetes, gall stone disease, and pancreatic cancer. Br J Cancer. 1986;54:377–8. doi: 10.1038/bjc.1986.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, Schwartz AG, Brown LM, Greenberg RS, Schoenberg JB, Pottern LM, Hoover RN, Fraumeni JF., Jr Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–7. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SEER 2007 Fast Facts: Pancreatic Cancer@ www.seer.cancer.gov/ statistics.
- 29.Chari ST. Detecting Pancreatic Cancer Early: Problems and Prospects. Semin Oncol. 2007;34:284–294. doi: 10.1053/j.seminoncol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–50. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 31.Fogar P, Pasquali C, Basso D, Sperti C, Panozzo MP, Tessari G, D'Angeli F, Del Favero G, Plebani M. Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res. 1994;14:2827–30. [PubMed] [Google Scholar]
- 32.Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res. 1995;15:2585–8. [PubMed] [Google Scholar]
- 33.Basso D, Valerio A, Brigato L, Panozzo MP, Miola M, Lucca T, Ujka F, Zaninotto M, Avogaro A, Plebani M. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas. 1997;15:132–8. doi: 10.1097/00006676-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Basso D, Millino C, Greco E, Romualdi C, Fogar P, Valerio A, Bellin M, Zambon CF, Navaglia F, Dussini N, Avogaro A, Pedrazzoli S, Lanfranchi G, Plebani M. Altered glucose metabolism and proteolysis in pancreatic cancer cell conditioned myoblasts: searching for a gene expression pattern with a microarray analysis of 5000 skeletal muscle genes. Gut. 2004;53:1159–66. doi: 10.1136/gut.2003.024471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Adrian TE. A factor from pancreatic and colonic cancer cells stimulates glucose uptake and lactate production in myoblasts. Biochem Biophys Res Commun. 1999;260:626–33. doi: 10.1006/bbrc.1999.0955. [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Larsson J, Abdiu A, Gasslander T, Westermark P, Adrian TE, Permert J. Dissociated secretion of islet amyloid polypeptide and insulin in serum- free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol. 1997;21:157–64. doi: 10.1007/BF02822387. [DOI] [PubMed] [Google Scholar]