Abstract
Lipid rafts play an important role in signal integration and in the cellular activation of a number of cytokine and growth factor receptors. It has recently been demonstrated that flotillin proteins are recruited to lipid raft microdomains upon cellular activation and play a role in neural cell regeneration, receptor signaling and lymphocyte activation. However, little is known about the relevance of the flotillin proteins during T cell responses to chemoattractant stimulation. To this end, cytoplasmic and lipid raft fractions from human T cells were analyzed for flotillin protein redistribution prior to and after CXCL12 stimulation. Flotillin-1, but not flotillin-2, redistributes to lipid rafts upon CXCR4 ligation. Moreover, in CXCL12-treated T cells, flotillin-1 also associates with several raft proteins including LAT, CD48 and CD11a but not Lck. In addition, an increase in CXCR4 association with flotillin-1 in lipid rafts was observed after chemokine treatment. RNAi technology was also utilized to inhibit the expression of flotillin-1, resulting in an inhibition of CXCL12-mediated signaling, function and CXCR4 recruitment into lipid rafts. Together, these data suggest that the increased association of cellular flotillin-1 with lipid raft microdomains during chemokine exposure may play an important role in chemokine receptor signaling and receptor partitioning with lipid rafts.
Keywords: Chemotaxis, CXCL12, CXCR4, Flotillin-1, Lipid rafts
Introduction
Lipid rafts are liquid ordered cholesterol- and ganglio-side-enriched membrane domains in the plasma membrane [1, 2] that play a major role in intracellular signaling in many different cell types. The involvement of lipid rafts has been implicated in many important cellular processes, including the generation and maintenance of cellular activation and signaling polarization, adhesion, and migration. Rafts are believed to spatially restrict the activation of signaling molecules that govern critical aspects of cellular activation and migration. Upon activation or receptor cross-linking, many signaling and stimulatory proteins are recruited to or modified in lipid rafts in order to convey efficient intracellular signals [3]. A number of proteins have been shown to be constitutively associated with lipid raft domains on T cells, including glycosylphosphatidylinositol (GPI)-anchored proteins such as CD48, CD59, and Thy-1, and palmitoylated molecules such as CD4, CD8, LAT, PAG, and more recently the flotillin proteins [3-5]. In addition, CD2, although not usually considered a constituent of rafts, has been found in association with rafts, and its presence there is linked to T cell receptor (TCR) signal transduction [6]. CD3 is also found in the detergent-insoluble (raft) fraction of the T cell, and acts as a costimulatory molecule for signal transduction via the TCR [7]. Although the precise functional role for flotillin proteins in rafts remains unclear, it has been recently hypothesized that flotillin-1 and flotillin-2 may serve as structural lipid raft components that assist in raft assembly, similar to the role caveolins play in the scaffold development of caveolae [4, 5, 8].
Flotillin-1, also known as Reggie-2, is a 48-kDa protein that has been shown to be constitutively present in the lipid rafts of human T cells and associates with a number of other raft proteins [4, 5]. Flotillin-2, also known as epidermal surface antigen (ESA) or Reggie-1, is a 42-kDa protein also associated with lipid rafts and caveolae [5]. These flotillin molecules have been shown to predominantly localize in catecholaminergic nerves in the rat brain during axon growth and regeneration [9] and within the neuronal lesions of Alzheimer’s disease patients [10, 11]. In addition, flotillins also appear to play a role in insulin signaling and glucose transport via interactions with c-cbl, cbl-associated protein (CAP) and the raft-associated GLUT4 receptor in adipose tissue [12]. The flotillin proteins are also associated with lipid raft microdomains in a number of different cell types including neurons, erythrocytes, adipocytes, platelets and T and B lymphocytes [4, 5, 8, 10-13]. Cellular activation via T or B cell receptor cross-linking results in the relocalization of flotillin-1 to the sites of receptor engagement within lipid rafts [4, 5, 13]. Flotillin proteins have also been found in lipid rafts in association with prion proteins in human T cells and are subsequently released via lipid-rich vesicles [14]. More recently, it has been shown that flotillin-1 movement into lipid rafts appears to be mediated via a Golgi-independent pathway [15]. Lang and coworkers [16] have reported that both flotillin-1 and flotillin-2 colocalize with activated GPI-anchored cell adhesion molecules in non-caveolar or non-raft micropatches in rat neurons. However, despite several reports proposing a possible role for flotillins in immune cells, adipocytes and neuronal cell activation and signaling, no reports have been published to date directly demonstrating a functional role for flotillin proteins in immune cell activation or bioactivity.
Chemokine receptors comprise a superfamily of seven-transmembrane-spanning G protein-coupled receptors that, upon binding of their specific chemokine ligand(s), activate several biochemical pathways resulting in inositol turnover, phospholipase activation, intracellular calcium mobilization, activation of several kinases, Rac phosphorylation and actin polymerization. Lipid rafts and cholesterol have been recently shown to play an important role in the signal transduction and function of chemokine receptors [17-23]. Membrane cholesterol depletion impedes lipid raft redistribution and the recruitment of kinases and adhesion molecules to the cell side facing the chemoattractant source. Several chemokine receptors have been shown to partition to lipid rafts localized at the migrating edge of cells, suggesting an important role for lipid rafts and raft-associated proteins in chemokine activity [18, 19]. We have recently reported that the chemokine receptors CXCR4 and CCR5 require bioactive cholesterol and localization within lipid rafts to mediate optimal ligand binding and receptor signaling [17, 20-22]. This raft localization was found to be critical in that the absence of cellular cholesterol resulted in an almost complete loss of chemokine ligand binding and activity. In a recent paper by Jiao and colleagues [23], ligand binding to CXCR1 promoted lipid raft partitioning of the receptors and facilitates the activation of heterotrimeric G proteins, although, unlike our studies with CXCR4 and CCR5, these authors failed to demonstrate a role for cholesterol in the ligand binding to CXCR1. All of the current literature suggests that lipid rafts facilitate spatial signaling in migrating cells by concentrating the gradient-sensing machinery, including chemokine receptors and adhesion molecules.
Given the localization of flotillin proteins within lipid raft microdomains and the important role of lipid rafts and cholesterol in chemokine-mediated cell migration, we initially examined the relationship between lipid rafts and flotillin proteins in response to chemokine signaling and the functional role of such interactions in chemokine bioactivity. Our results reveal that flotillin-1 plays a critical role in CXCR4 signaling and function and suggest a possible role in the partitioning of CXCR4 to lipid raft microdomains on human T cells.
Results
CXCL12-induced redistribution of flotillin-1 with lipid rafts on T cells
It has recently been shown that flotillin-1 is predominantly associated with lipid rafts in human T and B cells and that the localization of flotillin-1 in rafts increases upon antigen receptor, Thy1 or the ganglioside raft marker, GM1, ligation [4, 5]. Our initial studies focused on the ability of the CXCR4 ligand, CXCL12, to induce the redistribution of cellular flotillin-1 to lipid raft microdomains. Upon treatment of T cells with CXCL12 (100 ng/mL) for 5 min, lipid rafts were separated and then examined by Western blot analysis to assess the distribution of flotillin-1 in both raft and non-raft fractions. The results shown in Fig. 1B demonstrate that CXCL12 treatment of primary human T cells and Jurkat cells results in a significant redistribution of the cellular flotillin-1 from the cytosol and plasma membrane (fractions 7–9) to the lipid raft microdomains (fractions 2 and 3) of the human T cells. In anti-CD3 mAb- and IL-2-activated T cells (Fig. 1B), no significant increase or change in flotillin-1 localization was observed in response to the chemokine CXCL12. In fact, the activation of human T cells resulted in a higher level of partitioning of flotillin-1 with lipid rafts, similar to the levels observed for Jurkat T cells by Nel and colleagues [4]. However, in their studies, all of the cellular flotillin-1 was found to be associated with lipid raft fractions, while we have found that only a portion of the cellular flotillin-1 was associated with lipid rafts.
Figure 1.
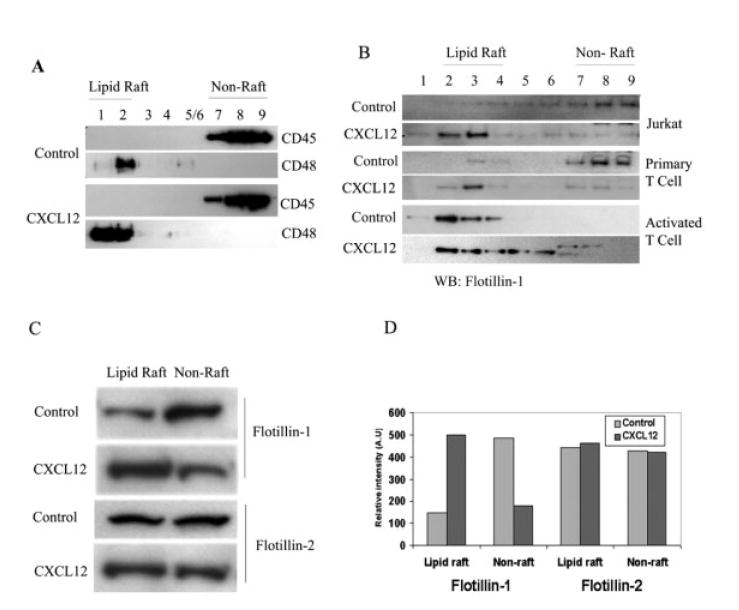
Flotillin-1 but not flotillin-2 redistributes to lipid rafts upon CXCR4 signaling. (A) Immunoblotting analysis of fractions of sucrose gradient separation of lipid rafts and cytosolic-membrane fractions from control versus chemokine-treated T cells. Total cell lysates in MES buffer as described in Materials and methods were solubilized in Triton X-100, and ultracentrifuged in a sucrose gradient. The fractions were collected and 1/20th of each fraction was used to identify the purity of the lipid raft fraction by immunoblotting with corresponding antibodies against CD48 and CD45 for the determination of raft and non-raft fractions, respectively. (B) Immunoblot with anti-flotillin-1 antibodies of each fraction of the sucrose gradient in control and CXCL12-stimulated primary human T cell, Jurkat cells and primary human T cells activated with immobilized anti-CD3 mAb and rhIL-2 (10 U/mL) for 7–10 days. (C) Raft and cytosolic components of control and CXCL12-treated T cells were pooled, equalized for protein content and then examined by immunoblotting with anti-flotillin-1 or -flotillin-2 antibody. (D) The relative density of the protein was measured by using ImageQuant software to determine the quantity of protein present in each said fractions. These data were assessed more than three times using different primary human T cell donors as well as Jurkat T cells (n ≥3).
Upon fraction pooling and protein quantitation, increased levels of flotillin-1 protein (typically ≥2.5-fold) redistributed to the lipid raft fractions upon CXCL12 treatment, compared to non-stimulated cells where no changes in flotillin-1 expression were noted (Fig. 1C, D). In contrast, flotillin-2 failed to demonstrate any redistribution to rafts in response to CXCL12 treatment (Fig. 1C, D). It should be noted that the baseline levels of raft- and non-raft-associated flotillin-1 varied with the activation state and the type of cells examined. However, in all cases using resting human T cells or Jurkat cells, CXCL12 treatment resulted in an increased association of flotillin-1 with raft fractions. Moreover, it should be noted that increasing the time of chemokine exposure to 15 min or greater did not significantly increase or alter the levels of flotillin-1 associated with lipid rafts compared to the Tcells treated for 5 min (data not shown).
Flotillin-1 redistribution was found to be CXCR4 specific, in that utilizing the CXCR4-specific blocking antibody, 12G5, inhibited the CXCL12-induced redistribution of flotillin-1 on T cells (Fig. 2). The CXCL12-treated human T cells demonstrated a 4.2-fold increase in flotillin-1 associated with rafts compared to the non-treated control cells, while the addition of the 12G5 blocking anti-CXCR4 antibody reduced flotillin-1 localization to approximately 1.8-fold above the non-treated control cells. These results support an active role for CXCR4 ligation in the recruitment of flotillin-1 to rafts in human T cells.
Figure 2.
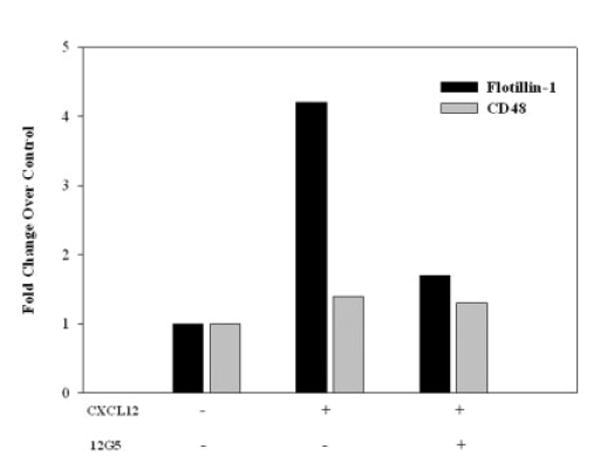
Recruitment of flotillin-1 to lipid rafts in response to CXCL12 is CXCR4 dependent. Raft and non-raft fractions were collected, pooled, equalized for protein content and immunoblotted for CD48, CD45 and flotillin-1 from primary human T cells treated with CXCL12 (100 ng/mL) or medium alone for 5 min in the presence or absence of the blocking anti-CXCR4 antibody, 12G5 (1 μg/mL). The graph represents the densitometric changes in flotillin-1 and CD48 protein localization in CXCL12-treated and CXCL12/12G5-treated human T cells compared to control non-treated T cells. No significant changes in CD48 association with the lipid raft fractions were observed in the presence or absence of CXCL12 or anti-CXCR4 antibody, while CXCL12-induced flotillin-1 association with raft fractions was inhibited by 12G5 treatment. While not shown, it should be noted that all of the CD45 protein was only observed in non-raft fractions, while CD48 protein was only found in lipid raft preparations.
Association of flotillin-1 with lipid rafts and signaling proteins after chemokine treatment
A number of studies have demonstrated an association or colocalization of several cell surface and signaling proteins with lipid rafts upon GPI-anchored protein, GM1, immunoglobulin receptor (IgGR) or TCR ligation [24]. Flotillins can colocalize with several lipid raft proteins including c-cbl and CAP in adipose tissue [12], GPI-anchored cell adhesion proteins in neurons [9, 16], and various components of the TCR and BCR signaling complex in human lymphocytes [4, 5, 13]. Similarly, we have observed an increased codistribution of flotillin-1 with CD48 (4-fold), LAT (12-fold) and CD11a (3.4-fold) in “total” cell lysates of CXCL12-treated primary human T cells (Fig. 3A) and Jurkat T cells (data not shown) when compared to control lysates. No significant difference in levels of flotillin-1 associated with Lck (typically 1–1.3-fold) was observed in either chemokine-or non-treated T cells, although flotillin did copre-cipitate with Lck, suggesting an interaction between these molecules independent of CXCL12 treatment. While it is possible that the use of NP40 in our immunoprecipitation procedure may influence raft molecules association with flotillin-1, the lack of any differences in Lck association with or without CXCL12 treatment suggests some specificity in the interactions between several of these raft proteins and flotillin-1 in response to this chemokine.
Figure 3.
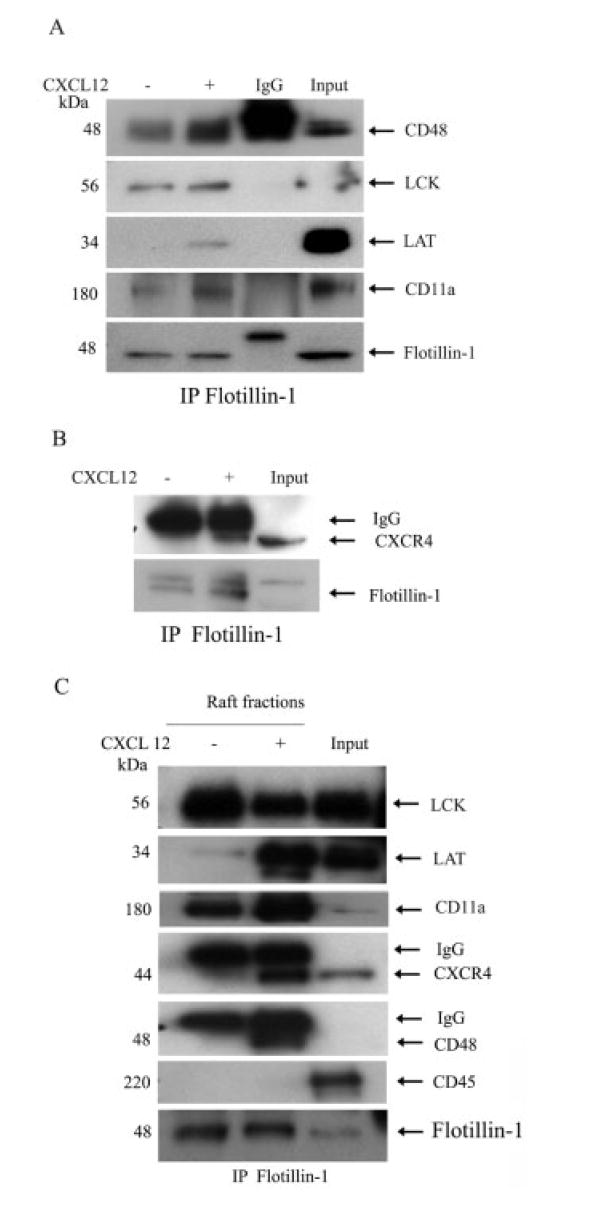
Association of flotillin-1 with various raft-associated proteins and CXCR4 after CXCL12 treatment. (A) Primary human T cells were cultured alone or in combination with CXCL12 (100 ng/mL) for 5 min, after which the total cell lysates were prepared and then immunoprecipitated with anti-flotillin-1 antibody. The immune pellets were then analyzed by immunoblotting with antibodies specific for CD48, Lck, LAT, and CD11a as described in Materials and methods. (B) These cell lysates were also immunoprecipitated with flotillin-1 and then examined for CXCR4 coprecipitation using Western blot analysis. (C) Immunoprecipitation of flotillin-1 was performed on the CXCL12-treated and untreated lipid raft fractions derived from primary T cells, after which Western blot analysis was performed on each protein. In all of the gels, the molecular weight of each protein is shown on the left side of each blot and arrows have been added to denote the band of interest. The fold changes of a given protein can be found in the text.
Moreover, these flotillin-protein interactions appear to occur within the lipid rafts, as similar blots using pooled raft preparations revealed significantly increased levels of interactions between flotillin-1 and LAT (22-fold), CD48 (12-fold), and CD11a (4-fold) in CXCL12-treated compared to vehicle control-treated T cells (Fig. 3C). Again, Lck failed to demonstrate any increased association with flotillin-1 in response to CXCL12 stimulation and, in fact, revealed a slightly diminished association with flotillin-1 upon chemokine treatment (again suggesting specificity with the flotillin-1 immunoprecipitation assays). In addition, flotillin-1 immunoprecipitation also demonstrated an increased association between flotillin-1 and CXCR4 in CXCL12-treated but not control cells in whole cell lysates (2.4-fold; Fig. 3B) and lipid raft preparations (4.5-fold; Fig. 3C). In addition, we found that Gαi2 is also immunoprecipitated with flotillin-1 post CXCL12 treatment of T cells (data not shown). Together, these results demonstrate that CXCL12 treatment induces an increased association between flotillin-1, several signaling and adhesion molecules and the chemokine receptor CXCR4.
Flotillin-1-specific small interfering RNA inhibits CXCR4 signaling and function
Based on the above results, we hypothesized that flotillin-1 may play an important role in the assembly of protein signaling complexes essential for CXCR4-mediated T cell signaling and function. To examine this question, we designed small interfering RNA (siRNA) specific for flotillin-1 to knock down flotillin-1 expression in both primary human T cells and T cell subsets as well as in Jurkat T cells. As noted in Materials and methods, the transfection efficacy of siRNA in human Tcells in the laboratory was between 70–80%, as gauged by examining the presence of the rhodamine-tagged siRNA by fluorescence microscopy 24 h post transfection. In all transfection experiments, we found a significant knockdown of flotillin-1 protein expression (typically ≥50% knockdown, with a range between 50–75% inhibition within the several T cell donors and Jurkat cells examined) compared to control scrambled siRNA-transfected resting T cells and Jurkat T cells (Fig. 4A, left panel), as assessed by Western blot analysis. No significant differences in β-actin expression were noted in any of the siRNA-transfected cells. Densitometric band intensity of the flotillin-1 is also shown in Fig. 4A (right panel).
Figure 4.
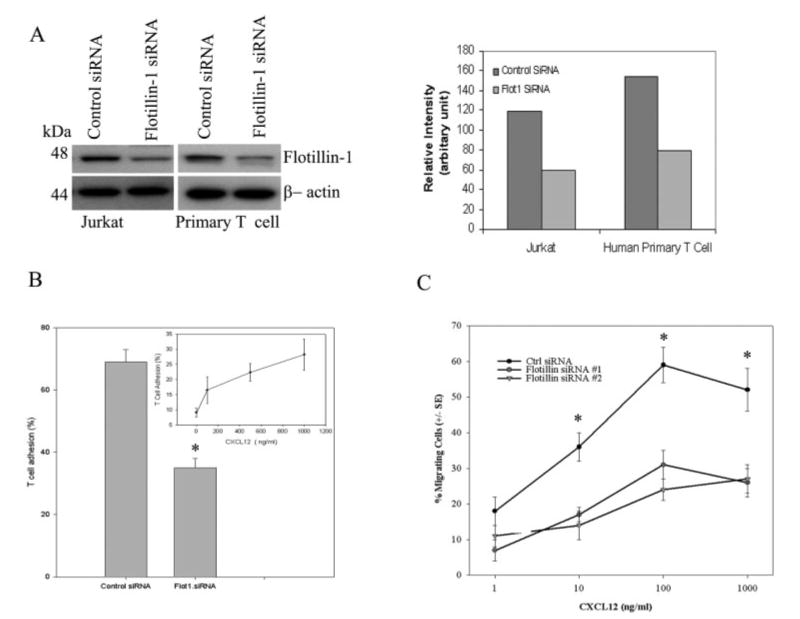
Down-regulation of flotillin-1 expression using iRNA technology inhibits CXCR4 signaling and function. (A) Jurkat and primary T cells were transfected with either flotillin-1-specific siRNA or control scrambled siRNA, after which the expression of flotillin-1 was assessed by immunoblotting with a flotillin-1-specific antibody. β-Actin was also examined as a loading control in these studies. The right panel shows the relative intensity of the flotillin-1 expression compared to control siRNA-treated T cells 48 h post transfection. (B) T cell adhesion to fibronectin-coated plates was examined using siRNA-transfected T cells as described in Materials and methods. The results are expressed in percentage adhesion of primary human T cells (means ± SEM of three independent experiments) in response to CXCL12 (1 μg/mL) in flotillin-1- and control siRNA-transfected cells compared to non-transfected control cells. The insert in (B) shows the dose-dependent increase in primary T cell adhesion to fibronectin in response to CXCL12. (C) Flotillin-1- and control scrambled siRNA-transfected primary T cells were labeled with Calcein AM dye and then tested for their ability to migrate in the presence or absence of CXCL12 as described in Materials and methods. The data are expressed as % migrating cells (± SEM) of triplicate determinations (n = 3). *p<0.05.
The significance of this flotillin-1 inhibition on CXCR4 activity was observed using both adhesion and migration assays. T cells transfected with the flotillin-1 siRNA demonstrated a significant reduction in both CXCL12-mediated T cell adhesion to immobilized fibronectin (Fig. 4B) and migration in a transwell chamber (Fig. 4C) as compared to control siRNA-transfected cells. In the majority of these studies, a greater than 50% inhibition of chemokine-induced adhesion or migration was observed. Moreover, we have also observed similar flotillin-1 knockdown effects on cell adhesion and migration using both flotillin-1 siRNA constructs in purified human CD4+ and CD8+ T cell subsets (data not shown). Also, it should be noted that primary human T cells demonstrated a dose-dependent adhesion to immobilized fibronectin in response to CXCL12, with the maximum adhesion observed at 1 μg/mL (Fig. 4B, insert). This data is shown to support the use of this optimal dose (1 μg/mL) in the siRNA adhesion studies in Fig. 4B.
Also, it should be noted that, using flow cytometric analysis, transfection of human T cells with either control or flotillin-1-speific siRNA failed to result in any diminished expression of cell surface CXCR4 or alterations in the internalization of this receptor in response to CXCL12 stimulation (data not shown). Thus, the effects of knocking down flotillin-1 expression on CXCR4-mediated adhesive or migratory function are not simply due to loss of CXCR4 expression or receptor internalization. Similarly, we also observed an inhibition of intracellular calcium mobilization in response to CXCL12 in flotillin-1 siRNA-transfected T cells as compared to non-treated control siRNA-treated cells (Fig. 5A). Although the chemokine-induced calcium response was not totally abrogated by flotillin-1 knockdown, the initiation of the calcium response to CXCL12 was delayed by as much as 20 s. Moreover, a 20–30% reduction in the maximal excitation ratio (peak) was also observed compared to control and control siRNA-transfected cells over multiple experiments. It should also be noted that upon examination of MAP kinase activation, we similarly noted a reduced capacity of flotillin-1 siRNA treated T cells to phosphorylate ERK at 2 and 5 min in response to CXCL12 treatment compared to control siRNA-transfected T cells (Supporting Information Fig. 1). This reduction failed to reach significance, but a trend of reduced ERK activation was noted. While the precise mechanism of this reduction in calcium and ERK phosphorylation in response to CXCL12 remains unclear, these data along with the migration and adhesion results suggest a potential role for flotillin-1 in optimizing signaling through CXCR4.
Figure 5.
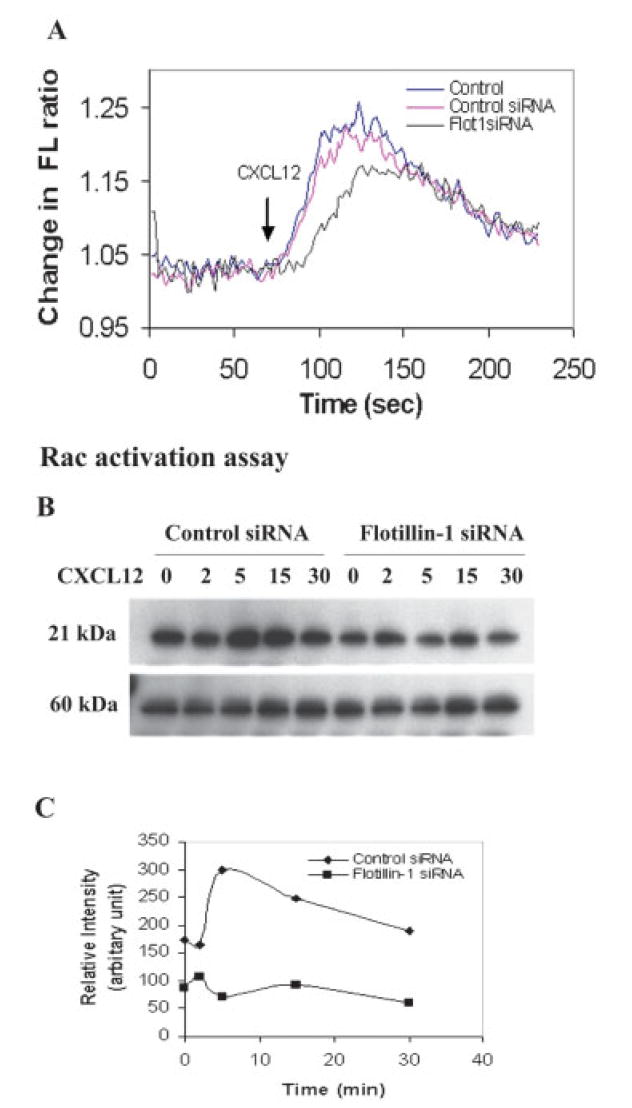
Inhibition of CXCL12-induced calcium mobilization and Rac activation in flotillin siRNA-transfected T cells. (A) Human primary T cells were examined 48 h post transfection with siRNA and then loaded with Fura-2 AM to evaluate [Ca2+]i mobilization in response to CXCL12 (100 ng/mL). The average fluorescence ratio for the first 60 s was standardized to a baseline value of 0, and the values are expressed as the change in the fluorescence (FL) ratio. (B) Jurkat T cells were transfected with a flotillin-1-specific siRNA or a control scrambled siRNA and then cultured for 48 h, after which the cells were treated with CXCL12 (100 ng/mL) for various times up to 30 min. At the designated times, the cells were lysed and incubated with a GST-tagged fragment of PAK PBD (p21 binding domain) protein beads (shown to bind Rac-GTP, but not Rac-GDP). Anti-Rac antibodies were utilized in the Western blot to demonstrate the extent of Rac activation at 2, 5, 15, and 30 min post CXCL12 treatment. PAK1 levels were also determined in all of these samples. This experiment was performed twice and demonstrated with similar results. (C) The densitometric intensities of the bands from (B) are shown here.
The actin cytoskeleton reorganization that is associated with cell spreading and migration is regulated by the Rho family of GTPases. Rho activation is shown to regulate the assembly of actin filaments, leading to the formation of stress fibers, and the Rho GTPase, Rac, has been shown to play an important role in the formation of lamellipodia at the leading edge [19, 25]. Given the effects of flotillin-1 knockdown on CXCL12-induced signaling and function, we sought to examine the effects of flotillin-1 expression on CXCL12-induced Rac activation. Control siRNA-transfected Jurkat T cells were found to demonstrate a rapid activation of Rac within 5–15 min after CXCL12 treatment (Fig. 5B, C), while little to no differences in the level of activated Rac were observed in the flotillin-1 siRNA-transfected T cells (Fig. 5C). Interestingly, even the baseline levels of activated Rac were diminished in the flotillin-1 siRNA-transfected T cells as compared to the controls. The relative intensity of activated Rac in each case was compared with total p21-activated kinase (PAK)1 levels, which did not significantly differ between samples.
Colocalization of flotillin-1, CXCR4 and lipid rafts upon CXCL12 stimulation
In order to further examine the role of flotillin-1 in the cellular redistribution of CXCR4 in T cells, Jurkat T cells transfected with flotillin-1 or control siRNA were cultured alone or stimulated with CXCL12 for 5 min, after which lipid raft fractions were prepared and then examined for CXCR4 redistribution to lipid rafts. Raft fractions 2 and 3 were examined here (as they demonstrate the majority of the raft components, including GM1 and CD48) and equal protein levels were examined for CXCR4 using Western blot analysis. The results shown in Fig. 6A demonstrate that transfection of human T cells with the flotillin-1 siRNA dramatically inhibited (>4-fold) the partitioning of CXCR4 to lipid rafts in response to CXCL12 stimulation when compared to control siRNA-transfected cells. In addition, untreated control siRNA-transfected T cells demonstrated a low but visible level of constitutive CXCR4 association, while flotillin-1-specific siRNA-transfected cells failed to demonstrate any CXCR4 association, suggesting a role for flotillin-1 in CXCR4 partitioning to rafts in human T cells.
Figure 6.
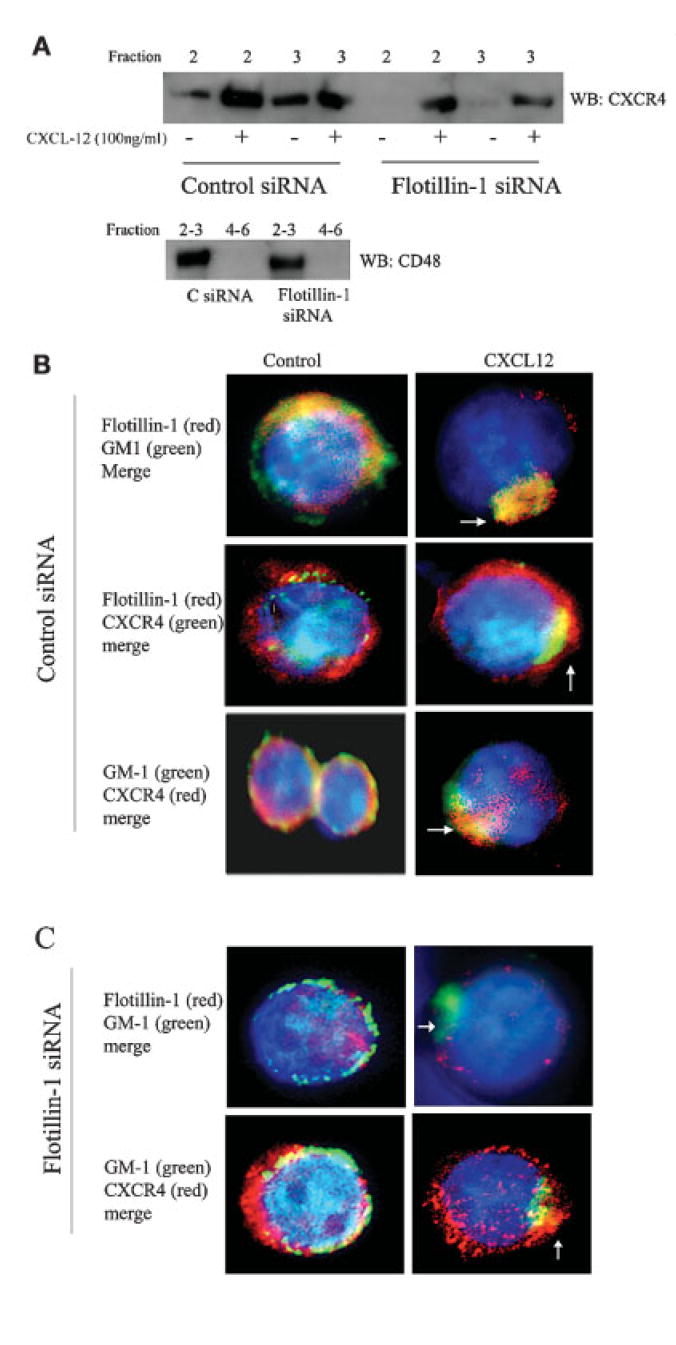
Inhibition of CXCR4 polarization and raft redistribution in flotillin-1 siRNA-transfected T cells. (A) Human T cells were transfected with control (upper blots) or flotillin-1-specific (lower blots) siRNA for 48 h and were then stimulated with and without CXCL12 for 5 min. After treatment, lipid raft isolation was performed as described in Materials and methods, and fractions 2 and 3 were examined for CXCR4 expression by Western blot analysis. A control blot examining CD48 levels is also shown, to demonstrate the integrity of the raft isolation process. (B, C) Jurkat cells were transfected with control (B) or flotillin-1 siRNA (C) and cultured for 48 h, after which the cells were stimulated in the presence or absence of CXCL12 for 5 min. The cells were subsequently stained with anti-flotillin-1 antibody (using either a rabbit polyclonal or mouse monoclonal antibody preparation) and anti-cholera toxin B antibody conjugated with Alexa Fluor 488 (green) to examine GM1 as well as with DAPI (blue) for nuclear staining (upper panel). The respective secondary antibodies were either conjugated with Alexa Fluor 488 (green) or Alexa Fluor 594 (red) for visualization. Yellow color indicates the overlap of red (Alexa Fluor 594) and green (Alexa Fluor 488). Magnification, × 63.
These data were supported through the use of high-resolution fluorescent microscopy. The results in Fig. 6B demonstrate that, in non-treated control siRNA T cells, flotillin-1 demonstrates more of a punctate distribution pattern within the cytosol and plasma membrane of the cells with some colocalization with GM1+ rafts (orange or yellow). This cell-wide pattern of colocalization was observed throughout the cell membrane, with little to no raft polarization being observed in these cells. Similarly, CXCR4 and flotillin-1, and CXCR4 and GM1, demonstrated both distinct and overlapping patterns of expression on the surface of non-stimulated T cells, although some colocalization was noted between CXCR4 and flotillin-1. Again, such colocalization was not uniform or polarized. Little to no flotillin-1, CXCR4 or GM1 polarization was observed in these control cell preparations. However, upon CXCL12 treatment, the majority of the T cells (greater than 75% of the 100 cells examined under high power) demonstrated a polarized “capped” phenotype with a greater colocalization between GM1-flotillin-1 and GM1-CXCR4. Similarly, significant colocalized staining of CXCR4 and flotillin-1 was noted in CXCL12-treated T cells, although some distinct staining patterns outside of rafts were also observed for these two molecules.
In flotillin-1 siRNA-transfected cells (Fig. 6C), only low levels of flotillin-1 staining was observed in control non-treated or CXCL12-treated T cells compared to the control siRNA-transfected T cells, further supporting the effectiveness of the flotillin-1 siRNA constructs utilized in our studies. Interestingly, similar to control siRNA-transfected cells, the loss of flotillin-1 did not alter the modest colocalization of CXCR4 with GM1-rich lipid rafts in these untreated populations. However, CXCR4 did not demonstrate the same degree of cellular polarization with GM1 in the majority of flotillin-1 siRNA-transfected cells after CXCL12 stimulation compared to the control siRNA-treated cells shown in Fig. 6B. While a low level of CXCR4 polarization was observed in these cells, the majority of CXCR4 was found outside of rafts in these cells, with a punctate distribution pattern around the cell. While only single cells are shown in the Fig. 6B and C panels, the phenotypes shown are the predominant (reproducibly greater than 75% of the cells examined under high power) phenotype observed for a given treatment in these microscopy studies. Thus, these results suggest that the loss of flotillin-1 may hinder CXCR4 polarization and possibly raft association in response to CXCL12 stimulation.
Discussion
Flotillin proteins were originally described as integral membrane-bound proteins within the caveolae-associated integral membrane protein [5, 26]. Caveolae are specialized lipid rafts that perform a number of signaling functions as approximately 50–100-nm “flask”- or “omega”-shaped invaginations of the plasma membrane. Caveolin proteins possess a scaffolding domain for which caveolins-1 and -3 appear to play a central role in many of the reported signal transduction pathways for caveolae [27-30]. While T cells do not express caveolins, they do express flotillin proteins, which are believed to serve as structural lipid raft components that assist in raft assembly similar to the role caveolins play in the scaffold development of caveolae [4, 31]. Whether flotillins interact with the cytoskeleton and serve as a signaling and protein scaffolds in immune cells is yet to be determined.
The flotillin proteins have been shown to be lipid raft-associated proteins in a number of cells types including T and B cells [4, 5, 13]. In several reports, cellular activation via antigen receptor or GPI-anchored protein cross-linking results in the relocalization of flotillin-1 to the sites of receptor engagement within lipid rafts [4, 5]. Nel and colleagues have demonstrated that Jurkat Tcells exhibit a constitutive association of flotillin-1 to the sites of receptor engagement within lipid rafts [4]. Upon stimulation with antibodies to the CD3 and CD28 markers, flotillin-1 was found to localize with the TCR caps. In contrast, we have found that only a portion of the cellular flotillin-1 is associated with lipid rafts in primary human T cells and Jurkat T cells and that the remainder of the cellular flotillin-1 is associated with the cell membrane. Upon chemokine treatment or TCR engagement, the specific partitioning of flotillin-1 to lipid rafts was observed, suggesting a potential role for this protein in chemokine signaling. This recruitment appears to be specific as prior blocking of the CXCR4 receptor with a blocking anti-CXCR4 antibody inhibits flotillin-1 partitioning to the lipid rafts.
It has recently been suggested that flotillin proteins may actually serve as adapter proteins, recruiting signaling molecules and complexes to lipid rafts upon receptor ligation. In adipocytes, flotillin-1 appears to be recruited to lipid rafts with a complex containing CAP and phospho-cbl upon ligation of the insulin receptor [12]. In addition, flotillin-1 and flotillin-2 are also coimmunoprecipitated with p59Fyn in Jurkat Tcells and PC12 cells [5, 30] and with neuroglobin in neuronal cells [32], while others have found that immune immobilization of rafts with anti-flotillin-1 results in the recruitment of Lck, LAT and the cytoskeletal protein vimentin [4, 5]. In this study, we have found that CXCR4 ligation results in the partitioning of flotillin-1, but not flotillin-2, to the lipid rafts within 5 min of treatment of human T cells. Moreover, we have also observed an association with flotillin-1 with several lipid raft components including LAT, Lck, CD48 and CD11a upon anti-flotillin-1 immunoprecipitation (Fig. 3). Lymphocyte function-associated antigen-1 (LFA-1; CD11a) is an important mediator of leukocyte migration and T cell activation. Our results indicate that LFA-1 contributes to the regulation of lymphocytic migration and adhesion activity via CD11a-mediated pathways, and suggest that flotillin-1 exerts its immunosuppressive effects in part via modification of lymphocytic functional activity. Upon CXCL12 treatment of T cells, an increased association of LAT, CD48, CD11a (Fig. 3) and Gαi2 (data not shown) with flotillin-1 in both total cell lysates and lipid raft preparations was observed, suggesting a possible role for these raft components in CXCR4 signaling and/or cell migration. While Lck was found to be associated with flotillin-1 in lipid rafts, this association did not appear to be influenced by CXCL12 treatment. Moreover, a potential role for flotillin-1 in CXCR4 signaling is further supported by the coprecipitation of flotillin-1 and CXCR4 in CXCL12-treated but not control T cell lysates and raft preparations. This association was quite significant and was not observed in the untreated cells, suggesting a possible physical association between flotillin-1 and CXCR4 proteins within rafts upon chemokine stimulation.
Similar to several other studies [4, 5, 16, 24], we have found through immunofluorescence analysis that flotillin-1 is associated with the cell membrane of human T cells and partitions to the lipid rafts upon cellular activation, TCR or Thy-1 cross-linking. We have similarly found that CXCL12 treatment alone mediates flotillin-1 partitioning to GM1+ lipid raft microdomains on the T cell surface (Fig. 6B, C). In contrast, resting T cells demonstrated only a small degree of flotillin-1 and GM1 colocalization, with a great deal of flotillin-1 being localized in other areas of the cell membrane and cytosol. More detailed studies are underway, examining the compartmentalization of flotillin-1 within human T cells. Interestingly, there was very little colocalization between flotillin-1 and CXCR4 in the majority (>75%) of resting T cells. However, upon CXCL12 treatment, a more polarized raft, flotillin-1 and CXCR4 distribution was observed. In the flotillin-1 siRNA-treated cells, significantly less CXCR4 polarization was observed with the GM1+ lipid rafts upon CXCL12 treatment. Despite this loss in flotillin-1 expression, CXCR4 still demonstrated a small degree of membrane polarization and raft colocalization upon CXCL12 treatment, suggesting that either flotillin-1 is not absolutely required for such polarization and/or that the small quantities of flotillin-1 still present in the siRNA-treated cells are sufficient to assist in raft-CXCR4 assembly. However, much more detailed studies are necessary to determine the requirement, if any, for flotillin-1 in CXCR4 redistribution and in raft assembly.
In summary, our results describe a functional role for flotillin-1 in CXCR4-mediated T cell migration, adhesion and signaling. Given that flotillin-1, but not flotillin-2, is specifically recruited to lipid rafts upon CXCL12 treatment, these results suggest a possible role of flotillin-1 as a scaffold protein facilitating chemokine receptor and protein redistribution to lipid rafts, which is required for efficient transduction of signals from the exterior to the interior of T cells. Flotillin-1 may actually play a role in cytoskeletal redistribution during chemokine-mediated signaling and migration. This is supported by the lack of Rac activation by CXCL12 in flotillin-1 siRNA-transfected T cells. Interestingly, CXCL12-mediated ERK phosphorylation was diminished, albeit not significantly, by the flotillin-1 but not control siRNA treatment, suggesting a possible role for flotillin-1 in several aspects of CXCR4 signaling (Supporting Information Fig. 1). Moreover, a recent study has demonstrated that flotillin-2-transfected melanoma cells demonstrate enhanced migration and proliferation in response to thrombin, and demonstrates increased expression of the protease-activated receptor-1 (PAR-1) [33]. Similar to our study, this report also demonstrates that transfected flotillin-2 binds to PAR-1, possibly influencing the activity of this receptor. More detailed studies are underway examining a role for flotillins in chemokine receptor partitioning and signaling in T cells and the role of lipid rafts in chemokine biology.
Materials and methods
T cell lines and culture
Primary human T cells were purified using T cell enrichment columns (R&D Systems, Minneapolis, MN) from peripheral blood mononuclear cells obtained from normal healthy human volunteers with informed consent. T cell purity was typically greater than 94% CD3+ T lymphocytes, with no monocyte or B cell contamination. Jurkat T cells (ATCC, Rockville, MD) were grown in cultured RPMI medium containing 10% FCS and penicillin and streptomycin.
Lipid raft isolation
Lipid raft and non-raft fractions were prepared as described [34-36]. Briefly, 1 × 108 to 2 × 108 freshly isolated primary human Tcells, Jurkat Tcells, and anti-CD3 mAb-activated Tcell lines (primary T cells stimulated on immobilized anti-CD3 mAb-coated plates for 5–7 days in the presence of 10 U/mL of rhIL-2) were cultured alone or stimulated with CXCL12 (100 ng/mL) for 5 min, after which the cells were lysed in 0.5 mL cold lysis buffer [36]. After 30 min on ice, the lysates were homogenized in a loosely fitted Dounce homogenizer and centrifuged at 5000 rpm for 3 min. The lysates were then separated using a sucrose gradient and centrifugation in an SW55Ti rotor (Beckman Instruments, Fullerton, CA) for 16 h at 4°C at 100 000 × g. Nuclei were removed before ultracentrifugation as described [36]. Briefly, T cells were treated with chemokine and washed with PBS and then lysed for 30 min at 4°C in 500 μL 1% Triton X-100 in TKM buffer (50 mM Tris-HCl pH 7.4, 25 mM KCl, 5 mM MgCl2, 1 mM EDTA). Lysates were centrifuged at 8000 × g for 10 min at 4°C to remove nuclei and debris. In certain experiments, total cell suspensions after detergent solubilization, without removing the nuclei and other insoluble components, were utilized in the raft isolation procedure. For equilibrium centrifugation, extracts were adjusted to 40% sucrose in TKM buffer and loaded into SW41 tubes. The extracts were overlaid with 6 mL of 38% sucrose-TKM, followed by 4.5 mL of 5% sucrose-TKM. Tubes were centrifuged at 100 000 × g for 18 h at 4°C. Eleven 1-mL fractions were collected from the bottom of the tube and stored at −20°C. Fractions were collected from the top to the bottom of the gradient, yielding approximately nine fractions. Of each fraction, 20 μL was routinely utilized to assess GM1, CD48 and CD45 levels by Western blot analysis. Fractions 1, 2 and 3 were typically GM1+CD48+CD45− and were thus designated as “lipid raft” fractions, while fractions 7, 8 and 9 were GM1−CD48−CD45+ and thus designated as “cytosolic-membrane” or “non-raft” fractions. The uniformity of lipid raft separation from the cell membrane and cytosolic fractions can be observed in Fig. 1A. In this figure, significant quantities of CD48 and GM1 (not shown) were found only in fractions 2 and 3, but not in the remaining fractions, while staining for the non-raft protein CD45 was predominantly found in fractions 8 and 9. This localization of CD48 within rafts and CD45 within the non-raft fractions was not influenced by CXCL12 treatment (Fig. 1A). These data were highly reproducible and performed for every single raft and non-raft preparation and support the integrity of the isolated fractions and their use in the subsequent experiments. Also, it should be noted that we have obtained similar flotillin-1 raft localization data in response to CXCL12 using the more classical isolation procedure where total cell suspensions in which the nuclei and other insoluble components were not removed prior to ultracentrifugation and fractionation, suggesting that these procedures yield comparable results (data not shown).
Western blot analysis
Protein fractions from pooled sucrose gradient lysates or from whole cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels, and electrophoretically transferred to polyvinylidene difluoride membranes [37]. Membranes were subsequently probed with mouse monoclonal antibody against flotillin-1 (BD Biosciences, San Diego, CA), LAT, Lck (Upstate Biotechnology, Charlottesville, VA), CD48, CD45 (Santa Cruz Biotechnology, Santa Cruz, CA), CD11a and cholera toxin B (Molecular Probes, Eugene, OR). After incubation followed by washing, the membranes were developed using peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG antibodies (Amersham Biosciences, Piscataway, NJ) and then developed using the ECL Western blotting analysis system (Amersham Biosciences).
Immunoprecipitation
Primary human T cell were incubated in the presence or absence of CXCL12 (100 ng/mL) for 5 min, after which the cell lysates were prepared using a buffer suitable for immunoprecipitation, containing 10 mM Tris-HCl pH 7.5, 150 mM NaCl, 10 mM MgCl2, 0.5% NP40, 5 mM EDTA, 1 mM sodium orthovanadate, leupeptin and pepstatin, and protein concentrations were determined using the Bradford reagent (Bio-Rad) as described by the manufacturer [38]. Of protein from the cell lysates, 250 μg was mixed with anti-flotillin-1 antibody and agarose G beads (Molecular Probes) and then incubated overnight with gentle rocking at 4°C. After incubation, the beads were washed three times with washing buffer and then resuspended in 10 μL of cell lysis buffer for 5 min at room temperature. An equal amount of 2× sample buffer was then added to each sample, after which each tube was boiled at 95°C for 5 min. These samples were subsequently examined by Western blot analysis.
Immunofluorescence
T cells were treated with CXCL12 (100 ng/mL) for 5 min, after which the cells were washed twice and then permitted to adhere to poly-l-lysine (1 mg/mL)-coated cover slips for 15 min at room temperature. The cover slips were washed gently with PBS and fixed with a 1% paraformaldehyde solution and then permeabilized using a 1% Triton X-100 solution for 30 min at 4°C. These cover slips were subsequently stained with anti-flotillin-1, anti-CXCR4 and anti-cholera toxin B antibody in 100 mL PBS with 0.5% FCS overnight for immunofluorescent microscopic analysis [39]. After incubation, the cover slips were washed twice and then incubated with secondary antibody conjugated to Alexa 488 or Alexa 594 for 1 h at room temperature. The cover slips were then washed twice and fixed to the slide by using Vectashield containing DAPI for nuclei staining. The cover slips were subsequently examined under oil immersion with a 100× objective on a Zeiss Axiovert S100 microscope (Carl Zeiss, Thornwood, NY).
Cell adhesion and chemotaxis assays
Human T cells or Jurkat T cells were resuspended in RPMI medium supplemented with 1% FCS and then labeled with Calcein AM (5 μM) for 30 min as described [39-41]. After labeling, the cells were washed twice and resuspended in medium (105 cells/mL/well) in the presence or absence of CXCL12 or PMA in 24-well flat-bottom plates previously coated with fibronectin (10 μg/mL; BD Biocoat, BD Biosciences). The plates were then incubated at 37°C in 5% CO2 for 30 min, after which the plates were gently washed and then analyzed on a Fluoroskan Ascent FL fluorescence plate reader (Thermo Lab Systems, Franklin, MA) with a 485 nm excitation and a 590 nm emission wavelength with up to three readings per well. The results were expressed as the mean percentage ± SE of bound T cells derived from triplicate wells with at least three separate experiments performed. In addition, Calcein AM-loaded Tcells were also examined for their ability to migrate in response to various concentrations of CXCL12 as previously described [17]. The results are expressed as % migrating cells (±SE).
Intracellular calcium mobilization
Measurement of calcium mobilization by chemokine stimulation was performed as previously described [17]. Fluorescence was monitored at λex1 = 340 nm, λex2 = 380 nm, and λem = 510 nm using an LS50B spectrophotometer (Perkin-Elmer, Wellesley, MA). The data are presented as the relative ratio of fluorescence excited at 340 and 380 nm. CXCL12 (PeproTech, Rocky Hill, NJ) was tested at a final concentration of 100 ng/mL.
siRNA transfections
In an effort to specifically target flotillin-1 expression, siRNA oligonucleotides specific for flotillin-1 mRNA were designed using the “siRNA Target Finder and Design Tool” by Dharmacon Research [42] with dTdT 3′-overhangs on each strand. siRNA were rhodamine tagged at the 3′ end. Two different sequences of oligonucleotides were designed for flotillin-1: 5′-GACAGGCAGAAAUUCUCAdTdT-3′ (forward) and 5′-UGAGAAUUUCUGCCUGUCCdTdT-3′ (reverse). Also, two control scrambled siRNA sequences were generated: 5′-UUCUCCGAACGUGUCACGUdTdT-3′ (forward) and 5′-ACACGUGACACGUU CGG AGAAdTdT-3′ (reverse). All of these oligonucleotides were synthesized by Qiagen (Valencia, CA). Human primary T cells or Jurkat T cells (2.0 × 106 to 3.0 × 106) were transfected with the above siRNA oligonucleotides using a Nucleofector Kit (AMAXA, Gaithersburg, MD). For several experiments, T cells were utilized 48 h post transfection and then treated in the presence or absence of CXCL12 and then utilized in adhesion, migration, calcium, Rac and Western blot analysis. The transfection efficacy on average was between 70–80%, as gauged by examining the presence of the rhodamine-tagged siRNA by fluorescence microscopy 24 h post transfection. It should be noted that rhodamine labeling of these oligomers may not be 100% efficient; thus it is possible that greater than the 70% rhodamine-positive cells are transfected.
Rac activation assay
The level of activated Rac-GTP was determined using an assay that measures the formation of Rac-GTP based on its specific binding to the GTPase-binding domain of PAK [43]. This assay was performed following the protocol described by the manufacturer (BK035; Cytoskeleton, Denver, CO). Briefly, T cells (107 cells/mL) were stimulated with CXCL12 (100 ng/mL) for the times indicated, after which the cell activation was stopped by washing the cells. Cells were subsequently lysed by the addition of ice-cold 2× lysis buffer provided by in the kit (50 mM Tris-HCl pH 7.5, 10 mM MgCl2, 200 mM NaCl, 2% NP40, 10% glycerol, 2 mM phenylmethylsulfonyl fluoride, 2 μg/mL leupeptin, and 2 μg/mL aprotinin). The cells were then vortexed and cell debris was clarified using low-speed centrifugation at 4°C. The guanine nucleotide loading of lysates and affinity precipitation using the GST-tagged PAK binding domain were carried out as described [43, 44]. The quantity of total protein utilized in these assays was determined using the BCA protein assay (Pierce, Rockford, IL).
Acknowledgments
We would like to thank Drs. Paritosh Ghosh and Michel Bernier for their helpful comments and careful review if this manuscript. In addition, we would like to thank Ms. Angela Feehley for her excellent editorial assistance on this manuscript. This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. The content of this publication does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Abbreviations
- CAP
cbl-associated protein
- PAK
p21-activated kinase
- siRNA
small interfering RNA
Footnotes
Supporting information for this article is available at http://www.wiley-vch.de/contents/jc_2040/2007/36680_s.pdf
References
- 1.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 2.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 3.Rodgers W, Farris D, Mishra S. Merging complexes: Properties of membrane raft assembly during lymphocyte signaling. Trends Immunol. 2005;26:97–103. doi: 10.1016/j.it.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter N, Laux I, Tu X, Whitelegge J, Zhu X, Effros R, Bickel P, Nel A. The flotillins are integral membrane proteins in lipid rafts that contain TCR-associated signaling components: Implications for T-cell activation. Clin Immunol. 2003;108:138–151. doi: 10.1016/s1521-6616(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 5.Stuermer CA, Plattner H, Gustafsson JA. The ‘lipid raft’ microdomain proteins Reggie-1 and Reggie-2 (flotillins) are scaffolds for protein interaction and signalling. Biochem Soc Symp. 2005:109–118. doi: 10.1042/bss0720109. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Reinherz EL. Dynamic recruitment of human CD2 into lipid rafts. J Biol Chem. 2001;276:18775–18785. doi: 10.1074/jbc.M009852200. [DOI] [PubMed] [Google Scholar]
- 7.Yashiro-Ohtani Y, Zhou X, Toyo-oka K, Tai X, Park C, Hamaoka T, Abe R, et al. Non-CD28 costimulatory molecules present in T cell rafts induce Tcell costimulation by enhancing the association of TCR with rafts. J Immunol. 2000;164:1251–1259. doi: 10.4049/jimmunol.164.3.1251. [DOI] [PubMed] [Google Scholar]
- 8.Salzer U, Prohaska R. Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood. 2001;97:1141–1143. doi: 10.1182/blood.v97.4.1141. [DOI] [PubMed] [Google Scholar]
- 9.Jacobowitz DM, Kallarakal AT. Flotillin-1 in the substantia nigra of the Parkinson brain and a predominant localization in catecholaminergic nerves in the rat brain. Neurotox Res. 2004;6:245–257. doi: 10.1007/BF03033435. [DOI] [PubMed] [Google Scholar]
- 10.Girardot N, Allinquant B, Duyckaerts C. Lipid rafts, flotillin-1 and Alzheimer disease. J Soc Biol. 2003;197:223–229. [PubMed] [Google Scholar]
- 11.Girardot N, Allinquant B, Langui D, Laquerriere A, Dubois B, Hauw JJ, Duyckaerts C. Accumulation of flotillin-1 in tangle-bearing neurones of Alzheimer’s disease. Neuropathol Appl Neurobiol. 2003;29:451–461. doi: 10.1046/j.1365-2990.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 12.Baumann CA, Ribon V, Kanzaki M, Thurmond DC, Mora S, Shigematsu S, Bickel PE, et al. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 13.Solomon S, Masilamani M, Rajendran L, Bastmeyer M, Stuermer CA, Illges H. The lipid raft microdomain-associated protein reggie-1/flotillin-2 is expressed in human B cells and localized at the plasma membrane and centrosome in PBMC. Immunobiology. 2002;205:108–119. doi: 10.1078/0171-2985-00114. [DOI] [PubMed] [Google Scholar]
- 14.Stuermer CA, Langhorst MF, Wiechers MF, Legler DF, Von Hanwehr SH, Guse AH, Plattner H. PrPc capping in T cells promotes its association with the lipid raft proteins Reggie-1 and Reggie-2 and leads to signal transduction. FASEB J. 2004;18:1731–1733. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- 15.Morrow IC, Rea S, Martin S, Prior IA, Prohaska R, Hancock JF, James DE, Parton RG. Flotillin-1/Reggie-2 traffics to surface raft domains via a novel Golgi-independent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. J Biol Chem. 2002;277:48834–48841. doi: 10.1074/jbc.M209082200. [DOI] [PubMed] [Google Scholar]
- 16.Lang DM, Lommel S, Jung M, Ankerhold R, Petrausch B, Laessing U, Wiechers MF, et al. Identification of Reggie-1 and Reggie-2 as plasma membrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol. 1998;37:502–523. doi: 10.1002/(sici)1097-4695(199812)37:4<502::aid-neu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DH, Taub D. CXCR4 function requires membrane cholesterol: Implications for HIV infection. J Immunol. 2002;168:4121–4126. doi: 10.4049/jimmunol.168.8.4121. [DOI] [PubMed] [Google Scholar]
- 18.Manes S, Martinez AC. Cholesterol domains regulate the actin cytoskeleton at the leading edge of moving cells. Trends Cell Biol. 2004;14:275–278. doi: 10.1016/j.tcb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Manes S, Lacalle RA, Gomez-Mouton C, del Real G, Mira E, Martinez AC. Membrane raft microdomains in chemokine receptor function. Semin Immunol. 2001;13:147–157. doi: 10.1006/smim.2000.0306. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen DH, Taub D. Cholesterol is essential for macrophage inflammatory protein 1 beta binding and conformational integrity of CC chemokine receptor 5. Blood. 2002;99:4298–4306. doi: 10.1182/blood-2001-11-0087. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen DH, Taub DD. Inhibition of chemokine receptor function by membrane cholesterol oxidation. Exp Cell Res. 2003;291:36–45. doi: 10.1016/s0014-4827(03)00345-8. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen DH, Espinoza JC, Taub DD. Cellular cholesterol enrichment impairs T cell activation and chemotaxis. Mech Ageing Dev. 2004;125:641–650. doi: 10.1016/j.mad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Jiao X, Zhang N, Xu X, Oppenheim JJ, Jin T. Ligand-induced partitioning of human CXCR1 chemokine receptors with lipid raft microenvironments facilitates G-protein-dependent signaling. Mol Cell Biol. 2005;25:5752–5762. doi: 10.1128/MCB.25.13.5752-5762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harder T. Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr Opin Immunol. 2004;16:353–359. doi: 10.1016/j.coi.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Del Pozo MA. Integrin signaling and lipid rafts. Cell Cycle. 2004;3:725–728. [PubMed] [Google Scholar]
- 26.Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- 27.Epand RM, Sayer BG, Epand RF. Caveolin scaffolding region and cholesterol-rich domains in membranes. J Mol Biol. 2005;345:339–350. doi: 10.1016/j.jmb.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 28.Navarro A, Anand-Apte B, Parat MO. A role for caveolae in cell migration. FASEB J. 2004;18:1801–1811. doi: 10.1096/fj.04-2516rev. [DOI] [PubMed] [Google Scholar]
- 29.Schulte T, Paschke KA, Laessing U, Lottspeich F, Stuermer CA. Reggie-1 and Reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development. 1997;124:577–587. doi: 10.1242/dev.124.2.577. [DOI] [PubMed] [Google Scholar]
- 30.Evans WET, Coyer RL, Sandusky MF, Van Fleet MJ, Moore JG, Nyquist SE. Characterization of membrane rafts isolated from rat Sertoli cell cultures: Caveolin and flotillin-1 content. J Androl. 2003;24:812–821. doi: 10.1002/j.1939-4640.2003.tb03132.x. [DOI] [PubMed] [Google Scholar]
- 31.Tu X, Huang A, Bae D, Slaughter N, Whitelegge J, Crother T, Bickel PE, Nel A. Proteome analysis of lipid rafts in Jurkat cells characterizes a raft subset that is involved in NF-kappaB activation. J Proteome Res. 2004;3:445–454. doi: 10.1021/pr0340779. [DOI] [PubMed] [Google Scholar]
- 32.Wakasugi K, Nakano T, Kitatsuji C, Morishima I. Human neuroglobin interacts with flotillin-1, a lipid raft microdomain-associated protein. Biochem Biophys Res Commun. 2004;318:453–460. doi: 10.1016/j.bbrc.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 33.Hazarika P, McCarty MF, Prieto VG, George S, Babu D, Koul D, Bar-Eli M, Duvic M. Up-regulation of flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Can Res. 2004;64:7361–7369. doi: 10.1158/0008-5472.CAN-04-0823. [DOI] [PubMed] [Google Scholar]
- 34.de Mello Coelho V, Nguyen D, Giri B, Bunbury A, Schaffer E, Taub DD. Quantitative differences in lipid raft components between murine CD4+ and CD8+ T cells. BMC Immunol. 2004;5:2–9. doi: 10.1186/1471-2172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Haller PD, Donohoe S, Goodlett DR, Aebersold R, Watts JD. Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics. 2001;1:1010–1021. doi: 10.1002/1615-9861(200108)1:8<1010::AID-PROT1010>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen DH, Giri B, Collins G, Taub DD. Dynamic reorganization of chemokine receptors, cholesterol, lipid rafts, and adhesion molecules to sites of CD4 engagement. Exp Cell Res. 2005;304:559–569. doi: 10.1016/j.yexcr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Cheng WH, von Kobbe C, Opresko PL, Fields KM, Ren J, Kufe D, Bohr VA. Werner syndrome protein phosphorylation by abl tyrosine kinase regulates its activity and distribution. Mol Cell Biol. 2003;23:6385–6395. doi: 10.1128/MCB.23.18.6385-6395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Kobbe C, Karmakar P, Dawut L, Opresko P, Zeng X, Brosh RM, Jr, Hickson ID, Bohr VA. Colocalization, physical, and functional interaction between Werner and Bloom syndrome proteins. J Biol Chem. 2002;277:22035–22044. doi: 10.1074/jbc.M200914200. [DOI] [PubMed] [Google Scholar]
- 39.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin-and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. A novel function for the Tec family tyrosine kinase Itk in activation of beta 1 integrins by the T-cell receptor. EMBO J. 2001;20:1232–1244. doi: 10.1093/emboj/20.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zanin-Zhorov A, Hershkoviz R, Hecht I, Cahalon L, Lider O. Fibronectin-associated Fas ligand rapidly induces opposing and time-dependent effects on the activation and apoptosis of T cells. J Immunol. 2003;171:5882–5889. doi: 10.4049/jimmunol.171.11.5882. [DOI] [PubMed] [Google Scholar]
- 42.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 44.Fujiwara H, Gu J, Sekiguchi K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp Cell Res. 2004;292:67–77. doi: 10.1016/j.yexcr.2003.08.010. [DOI] [PubMed] [Google Scholar]


