Summary
Objective
To determine the specificity and efficiency of the tamoxifen (TM)-induced Cre recombination in articular chondrocytes of adult Col2a1-CreERT2 transgenic mice.
Methods
Col2a1-CreERT2 transgenic mice were bred with Rosa26R reporter mice. 2-week-old Col2a1-CreERT2;R26R mice were administered TM for 5 days and were sacrificed 1 and 6 months after TM induction. X-Gal staining was performed.
Results
Efficient Cre recombination is achieved in adult articular chondrocytes 1 and 6 months after TM induction.
Conclusion
Our findings demonstrate that the Col2a1-CreERT2 transgenic mouse model is a valuable tool to target genes specifically expressed in articular chondrocytes in a temporally-controlled manner in adult mice.
Keywords: Articular chondrocyte, Tamoxifen, Cre-recombination
Introduction
Osteoarthritis (OA) is a degenerative joint disease that is common in aging and mainly occurs in both humans and animals. Alternations of gene expression in articular chondrocytes likely play an important role in the development of OA1-3. Articular chondrocytes are the only cell type in articular cartilage and they produce and maintain the extracellular matrix, which is responsible for providing the appropriate function to articular tissue4. Articular cartilage has minimal reparative potential and degradation of articular cartilage can have severe consequences. For this reason, envisioning strategies to maintain articular cartilage is an important objective in the arthritis field. Unfortunately, very little is known regarding the mechanisms involved in establishing and maintaining the articular chondrocyte phenotype.
In order to target chondrocyte-specific genes in a tissue-specific and inducible manner, we have recently generated a transgenic mouse model, Col2a1-CreERT2, in which expression of the Cre recombinase is driven by the chondrocyte-specific col2a1 promoter in a TM-inducible fashion. We have previously reported that chondrocyte-specific Cre-recombination was observed in growth plate chondrocytes of these mice postnatally5. In the present studies, we show that Cre-recombination is maintained in articular chondrocytes of adult transgenic mice.
Methods, results and discussion
Col2a1-CreERT2 transgenic mice were crossed with Rosa26 reporter mice in which expression of Escherichia coli β-galactosidase can be induced by Cre-mediated recombination6. Offspring were genotyped by PCR using Cre-specific primers and primers for detecting R26R alleles. The sequences of PCR primers for genotyping Col2a1-CreERT2 mice are: 5′-CCTGGAAAATGCTTCTGTCCGTTTGCC-3′ (forward primer) and 5′-GAGTTGATAGCTGGCTGGTGGCAGAG-3′ (reverse primer) and the size of the PCR product is 600-bp. The sequences of PCR primers for genotyping Rosa26R reporter mice are: R1295, 5′-GCGAAGAGTTTGTCCTCAACC-3′; R523, 5′-GGAGCGGGAGAAATGGATATG-3′ and R26F2, 5′- AAAGTCGCTCTGAGTTGTTAT-3′. The 600-bp PCR product was detected in wild-type mice and the 325-bp PCR product was detected in homozygous Rosa26R mice. In heterozygous Rosa26R mice, both 600 and 325-bp PCR products were detected.
To determine if the Col2a1-CreERT2 transgenic mice can be used to target genes expressed in articular chondrocytes in adult mice, they were bred with Rosa26 reporter mice to follow the time course of reporter expression. TM induction was performed in 2-week-old Col2a1-CreERT2;R26R transgenics (1 mg TM/mouse/day for 5 days) and mice were sacrificed 1 and 6 months after the last injection. The long bones were harvested and fixed in 0.2% glutaraldehyde, decalcified, and processed for frozen sectioning followed by X-Gal staining. Nuclear Fast Red staining was performed as a counter stain. The recombination efficiency was determined by counting the X-Gal-positive cells divided by the total number of cells on the articular surface (n=4). The results showed that TM induced efficient Cre-recombination in articular chondrocytes 1 and 6 months after TM induction (Fig. 1a,b). Eighty five percent and 82% Cre-recombination efficiency was achieved in articular cartilage area of these mice respectively (Fig. 1a,b) (n=4). It should be noted that some cells in the deep zone of the articular cartilage were negative for X-Gal staining; this may be due to the poor penetration of tamoxifen into these areas.
Fig. 1.
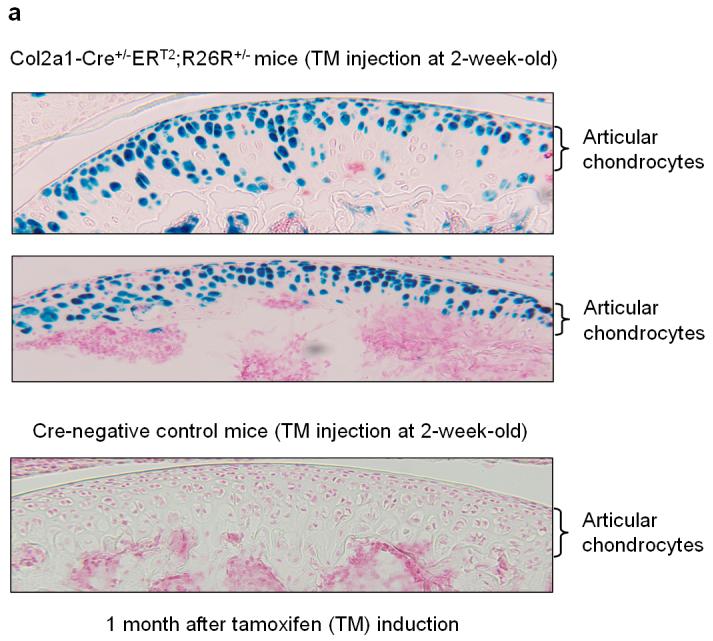
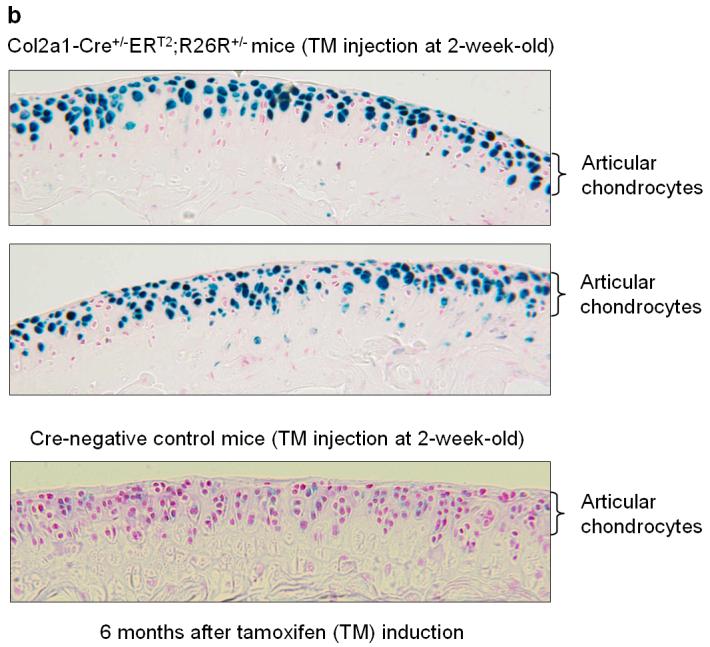
Histological analyses of the Col2a1-Cre+/-ERT2;R26R+/- mice. 2-week-old Col2a1-Cre+/-ERT2;R26R+/- double transgenic mice and Cre-negative control littermates were injected with TM (1 mg/mouse/day for 5 days). Mice were sacrificed 1 (a) and 6 months (b) after injections were completed and samples of long bones were fixed, decalcified and processed for frozen section preparation followed by X-Gal staining. Sections were then counterstained by Nuclear Fast Red. Articular chondrocytes of double transgenic mice that received TM showed X-Gal positive staining (a,b). Recombination efficiency was evaluated by counting the X-Gal-positive cells divided by total cell numbers in articular cartilage area from 3 non-consecutive histological sections of four transgenic mice (n=4). High Cre-recombination efficiency was achieved in articular chondrocytes of the transgenic mice 1 and 6 months after TM induction.
Overall, these results suggest that Col2a1-CreERT2 transgenic mice provide a strategy for causing tissue specific gene deletion in a temporal manner. Since the percentage of X-Gal-positive articular chondrocytes is similar at 1 and 6 months after TM induction, there is likely minimal turnover or cell renewal in articular cartilage, consistent with the limited reparative potential of this tissue.
Using the 3 kb Col2a1 promoter, Ovchinnikov et al.8 generated Col2a1-Cre transgenic mice which show efficient Cre-recombination in chondrocytes during mouse development. A similar approach was also reported by Nakamura et al.9 who generated Col2a1-CreERT transgenic mice using the same Col2a1 promoter construct as we used in our transgenic mice. Although efficient Cre-recombination was demonstrated in the embryonic and postnatal skeleton, it becomes undetectable in 12-week-old Col2a1-CreERT transgenic mice9. Several factors such as differences in transgene constructs, integration sites and copy numbers may contribute to these phenotypic distinctions.
Acknowledgements
We would like to thank Dr. Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Université Louis Pasteur de Strasbourg, Communauté Urbaine de Strasbourg, France) for providing us the pCreERT2 plasmid. This work was supported by grant R01 AR051189, R01 AR054465 and K02 AR052411 to Di Chen from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zemmyo M, Meharra EJ, Kuhn K, Creighton-Achermann L, Lotz M. Accelerated, aging-dependent development of osteoarthritis in alpha1 integrin-deficient mice. Arthritis Rheum. 2003;48:2873–80. doi: 10.1002/art.11246. [DOI] [PubMed] [Google Scholar]
- 2.Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM. Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 2003;48:3452–63. doi: 10.1002/art.11355. [DOI] [PubMed] [Google Scholar]
- 3.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–58. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 4.Kerin A, Patwari P, Kuettner K, Cole A, Grodzinsky A. Molecular basis of osteoarthritis: biomechanical aspects. Cell Mol Life Sci. 2002;59:27–35. doi: 10.1007/s00018-002-8402-1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Lichtler AC, Sheu T, Xie C, Zhang X, O’Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 7.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–6. [PubMed] [Google Scholar]
- 9.Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–12. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]


