We learn and remember better when new material can be related to what we already know. Professional athletes can remember details of particular plays that occurred in a long match. Experienced poker players can reconstruct the card distribution and betting sequence that occurred in previous hands. This is possible because these individuals have a rich background of relevant experience and therefore can organize new material into meaningful and orderly patterns. On page 76 of this issue, Tse et al. (1) make use of these ideas to explain their new findings in rats.
In his classic 1932 monograph on remembering (2), British psychologist Frederic Bartlett developed the concept of “schemas” to refer to preexisting knowledge structures into which newly acquired information can be incorporated. Although the schema concept is fundamental to the psychological science of human memory, it has been difficult to bring the concept to biology and especially to studies with experimental animals. Tse et al. show in rats how the schema concept is relevant to the phenomenon of memory consolidation. Memory consolidation refers to the gradual process of reorganization by which new memories become remote memories (3, 4). Initially, the learning of facts and events (declarative memory) depends on the hippocampus, a structure deep in the temporal lobe of the mammalian brain. As time passes after learning, the importance of the hippocampus gradually diminishes and a more permanent memory is established in distributed regions of the neocortex. This process typically takes a few years in humans and at least a month in rodents. According to one influential model (5), the process is slow because if changes were made rapidly, they would interfere with the preexisting framework of structured knowledge that has been built up from other experiences.
In the Tse et al. study, rats learned to associate six flavors with six places in a familiar testing arena. A rat was first cued with a particular flavor (a 0.5-g morsel of flavored food) in one of four start boxes and then could receive more of the same food by going to the correct location in the arena. Animals learned the six flavor-place associations gradually across 6 weeks of training; each flavor-place pair was presented once per session for training and three sessions were scheduled each week. Not surprisingly, animals with hippocampal lesions failed to learn the associations.
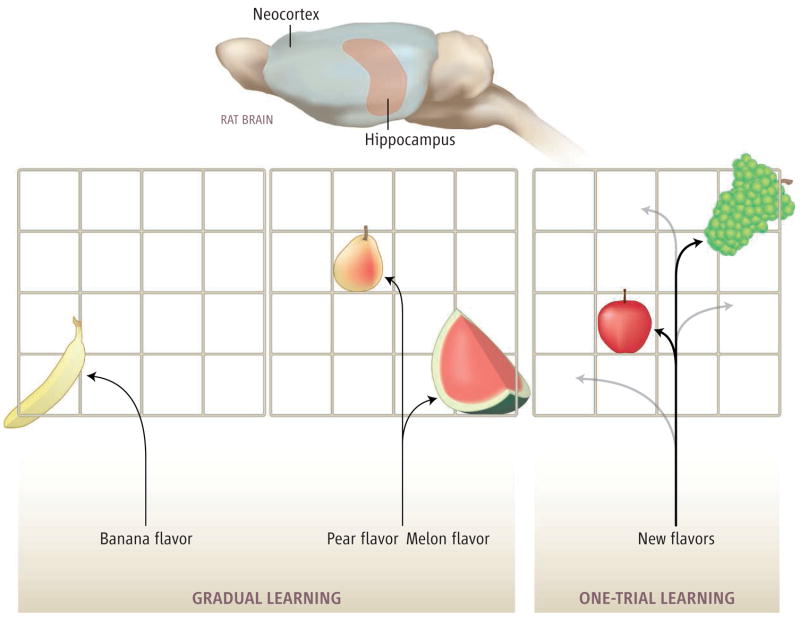
Evidence that flavor-place training afforded the development of a schema came from finding that animals were subsequently able to learn new flavor-place associations in a single trial and could remember the new associations for at least 2 weeks (see the figure). The extended training had helped because in a similar task in which rats were trained on a new flavor-place association each day (6), memory was only weakly established and persisted for less than a day. Tse et al. also tested other rats that learned six flavor-place associations in one arena (the consistent arena) and concurrently learned six different flavor-place associations in another arena but with the flavor-place combinations rearranged every two sessions (the inconsistent arena). In the consistent arena, animals could then learn new flavor-place associations in a single trial, as before. By contrast, in the inconsistent arena, animals failed at one-trial learning, presumably because they had not established a stable schema that could guide rapid learning.
Good schemas wanted.
When a rat learns associations between flavors and spatial locations, as studied by Tse et al. (1), the associations are initially learned as individual facts (left). With extended training, the animal develops an organized structure or schema for flavors and places (middle). This organized knowledge structure (bold lines) can then support rapid learning of new associations in a single trial and the rapid consolidation of information into the neocortex (right).
The most surprising finding by Tse et al., and what connected the schema concept to memory consolidation, was that removal of the entire hippocampus as early as 48 hours after the rapid learning of two new flavor-place associations fully spared memory of the associations (these animals had first been given extensive training on six other flavor-place associations, thus establishing a schema). It was not the case that memory of the new associations was never dependent on the hippocampus, nor that memory was somehow formed directly in the neocortex, because hippocampal lesions made 3 hours after learning abolished memory of the new associations. In short, the neocortex was able to incorporate new information rapidly. This is unexpectedly rapid for a process that, on the basis of as many as 20 studies in experimental animals, ordinarily takes at least a month (7).
It is tempting to suppose that memory consolidation proceeded rapidly because new information was fully compatible with what had already been learned—in other words, a good schema was available. If so, questions naturally arise about the minimum requirements for an effective schema. Perhaps continued training with two new flavor-place pairs every day would be sufficient (not just extended training with the same set of pairs, as was done by Tse et al.). Although new associations trained in this way ordinarily persist for less than a day (6), with extended experience they might persist much longer and also become rapidly independent of the hippocampus. And what might happen if animals simply explored the same arena day after day? Would merely having strong familiarity with a consistent environment support the rapid learning and rapid consolidation of new associations? Answers to these questions would help sharpen the notion of schema and clarify the conditions under which rapid memory consolidation occurs.
The study also casts fresh light on an issue of long-standing interest. As the authors point out, the fact that storage and recall of spatial memory can occur independently of the hippocampus runs counter to the proposal that the hippocampus forms and stores cognitive maps (8). Thus, the new findings, as well as other work (9), give no special emphasis to spatial cognition and suggest instead that the hippocampus is a general-purpose learner of new facts and events, both spatial and nonspatial.
The larger question concerns the nature of memory consolidation itself. Recent studies of brain metabolism and activity-related genes in mice describe the decreasing importance of the hippocampus as time passes after learning and the increasing importance of several cortical regions, including the prefrontal, temporal, and anterior cingulate cortex (4). The idea is not that memory is literally transferred from hippocampus to neocortex, but rather that the hippocampus guides gradual changes in the neocortex that increase the complexity, distribution, and interconnectivity of memory storage sites. This new study is the first to show that this process can occur rapidly.
References
- 1.Tse D, et al. Science. 2007;316:76. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett FC. Remembering. Cambridge Univ. Press; Cambridge: 1932. [Google Scholar]
- 3.Squire LR, Alvarez P. Curr Opin Neurobiol. 1995;5:169. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 4.Frankland PW, Bontempi B. Nat Rev Neurosci. 2005;6:119. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 5.McClelland JL, McNaughton BL, O’Reilly RC. Psychol Rev. 1995;102:419. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 6.Day M, Langston R, Morris RGM. Nature. 2003;424:205. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- 7.Squire LR, Clark RE, Bayley PJ. In: The Cognitive Neurosciences III. Gazzaniga M, editor. MIT Press; Cambridge, MA: 2004. [Google Scholar]
- 8.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford Univ. Press; Oxford: 1978. [Google Scholar]
- 9.Squire LR, Bayley PJ. Curr Opin Neurobiol. doi: 10.1016/j.conb.2007.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]