Abstract
G-protein-coupled receptors (GPCRs) respond to external stimuli by activating heterotrimeric G proteins inside the cell. There is increasing evidence that many GPCRs exist as dimers or higher oligomers, but the biochemical nature of such dimers and what roles they have, if any, in signal transduction remains unclear. We conducted a comprehensive study of dimerization of the 5HT2c serotonin receptor using disulphide-trapping experiments. We found a dimer interface between transmembrane (TM) helices IV and V that is markedly sensitive to the state of receptor activation. This dimer seems to be quasisymmetrical in interfacial geometry and asymmetrical in its association with its cognate Gα protein. We also found a second interface at TM I helices, which is insensitive to the state of activation.
Keywords: asymmetry, dimerization, GPCR, receptor, serotonin
Introduction
G-protein-coupled receptors (GPCRs) mediate intracellular responses to a wide variety of external stimuli (Pierce et al, 2002). These receptors have in common a seven-helical transmembrane (7TM) domain and the ability to activate heterotrimeric G proteins in response to ligand stimulation, presumably through a conserved mechanism (Luttrell, 2006).
Despite the biomedical importance of GPCRs, there is much uncertainty about how they function at a molecular level. Landmark crystal structures of bovine rhodopsin (Palczewski et al, 2000; Li et al, 2004; Salom et al, 2006) provide a powerful framework, but they also leave important questions unanswered. For example, the nature of rearrangements by which a receptor in the inactive state shifts on stimulation to one able to activate G proteins remains open for debate. Similarly, there seems to be no consensus on the nature and physiological relevance of GPCR oligomerization.
GPCRs were initially thought to exist and function only as monomers (reviewed by Chabre & le Maire, 2005). However, compelling results from the γ-aminobutyric acid type B (GABAB) receptor—a family C GPCR—first indicated that these receptors might function as dimers (Marshall et al, 1999). Since then, evidence for rhodopsin-like GPCRs (family A) being dimers or higher-order oligomers has increased quickly. This has come from various GPCRs in diverse experiments including fluorescence resonance energy transfer (FRET) and bioluminescence resonance energy transfer measurements, chemical crosslinking, co-immunoprecipitation, atomic force microscopy, neutron scattering and modelling (reviewed by Javitch, 2004; Park et al, 2004; Terrillon & Bouvier, 2004). Nevertheless, reports that strictly monomeric receptors are able to activate G proteins (Chabre & le Maire, 2005; White et al, 2007; Whorton et al, 2007) cast doubts on the dimer being the true or only functional unit.
Our studies focus on rat 5HT2c (Julius et al, 1988), the subtype 2c receptor for the neurotransmitter 5-hydroxytryptamine (5HT; serotonin). 5HT2 receptors couple to Gαq to activate the phospholipase C/β signalling pathway, and they have a rich pharmacology, including well-characterized agonists, partial agonists, antagonists and inverse agonists (Barnes & Sharp, 1999). Our ongoing efforts to determine the high-resolution structure of 5HT2c have led us to investigate the physiologically relevant state of this receptor. Dimeric associations of 5HT2c (Herrick-Davis et al, 2004) are postulated to have functional importance (Herrick-Davis et al, 2005), but the detergent-extracted receptor behaves as a monomer (F.M. & W.A.H., unpublished data). Thus, we sought to identify interfaces of self-association for 5HT2c on cells, both to investigate their roles in receptor function and to aid us in engineering physiologically meaningful receptor species for structural analysis.
We used a cysteine crosslinking approach (Guo et al, 2003, 2005; Klco et al, 2003; Kota et al, 2006) that was aimed at providing a systematic study of contacts between 5HT2c receptor molecules as expressed on the plasma membrane of living cells. Two distinct dimerization interfaces emerged: one at TM I, the other between TMs IV and V. The TM I interface does not vary with activation state, whereas the IV/V interface shows clear sensitivity to the character of ligands bound to 5HT2c. The IV/V interface seems to be asymmetric, and most noticeably so in the active state. It seems that activated 5HT2c is a conformational heterodimer.
Results
Design of cysteine mutants
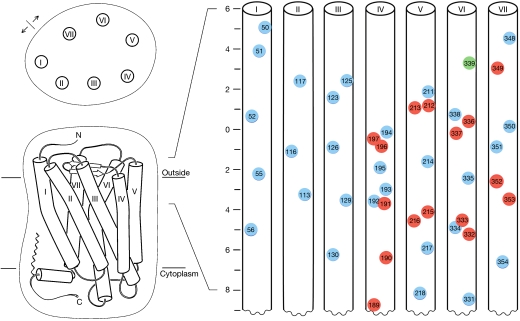
Cysteine replacement of an appropriately disposed pair of residues at an oligomeric interface is expected to generate a disulphide bridge. Therefore, we devised a disulphide-trapping plan that would be both comprehensive and also limited in scope for feasibility. We reasoned that residues near the extracellular surface would be susceptible to oxidation for disulphide formation and that surface-exposed residues would be the most likely interfacial contacts. Thus, by homology with rhodopsin (Palczewski et al, 2000; Li et al, 2004), we found 30 5HT2c sites with appropriate surface exposure within 8 Å of the putative external cell surface, including one naturally occupied by cysteine. This set, including 3–5 sites on each helix, was later supplemented by neighbours of the reactive residues to bring the total cysteine sites to 46 (Fig 1).
Figure 1.
Schematic plan for mutational design. A cartoon of the rhodopsin-based model of 5HT2c is viewed from above and within the membrane plane (left), and the indicated portion of the 7TM bundle is unwrapped and segments are viewed as vertical cylinders (right). Residues selected for mutation to cysteine are indicated in numbered circles centred at Cβ positions. Those meeting the criteria for surface exposure are circled in blue, except for a wild-type cysteine circled in green and those selected as neighbours of initial positive response that are circled in red. The scale reports z-displacement (Å) from the water–lipid interface. 5HT2c, subtype 2c receptor for 5-hydroxytryptamine; TM, transmembrane.
Mapping of disulphide accessible dimer interfaces
We cloned all of the mutants into a vector for expression in mammalian cells (Mancia et al, 2004), and tested each one individually (45 transfections) by using transient transfection into human embryonic kidney 293 (HEK293) cells and as pairs by cotransfection. Cell-surface expression was verified by immunocytochemistry (supplementary Fig S1 online). Pairs were chosen so that each single mutant would reside in a different helix and with expected heights along the helices within 5 Å from each other. Pairs were tested from all contiguous helices, as well as from TMs I/III, I/VI, II/IV, II/VII, III/V, IV/VI, IV/VII and V/VII. A total of 323 different pairs were cotransfected. To maximize the likelihood of disulphide bond formation, for these initial rounds of experiments, transfected cells were exposed to the hyperoxidizing environment of Cu-phenanthroline (CuP) before collection. Dimer formation was assessed by western blot analysis of detergent-solubilized and deglycosylated (supplementary Fig S2 online) cellular lysates or of enriched preparations following immunoprecipitation with 5HT2c antibodies (Mancia et al, 2007; supplementary Fig S3 online). These experiments led to the identification of 30 possible candidates.
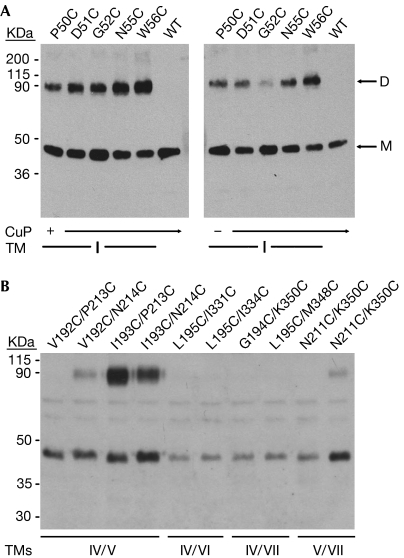
The definition of dimer interfaces was refined under a more stringent protocol. The 30 transfections that showed interactions following CuP-mediated oxidation were repeated using only natural air oxidation, keeping all other experimental procedures the same. Transfection of individual mutants revealed only a single interface at TM I, most prominently marked by N55C and W56C (Fig 2A). Cotransfected pairs of mutants showed a clear interface between TMs IV and V (Fig 2B). The residues involved were carefully mapped along these helices (data not shown), and the crucial pairs responsible for this interaction were identified as Ile 193 on TM IV matched with Pro 213 and Asn 214 on TM V (Fig 2B). Crosslinked yields were close to 50% as expected from the trapping of AB ‘heterodimers' away from non-crosslinkable AA (e.g. I193C) and BB (e.g. P213C) ‘homodimers', in which each dimer species was equally probable. These ratios remained unchanged for IV/V pairs even when forced by CuP oxidation, whereas for the TM I mutants higher CuP concentrations could force complete dimer capture (data not shown). The air-oxidized TM I and IV/V crosslinkings seem to reflect authentic dimerization, as captured dimer fractions are only weakly sensitive to 100-fold dilution of transfected DNA (supplementary Fig S4 online; supplementary Table S1 online).
Figure 2.
Identification of dimer interfaces. (A) Dimerization along TM I. Western blots compare transfected 5HT2c mutants as oxidized by CuP (left) and by air (right). WT is wild-type 5HT2c. Monomer (M) and dimer (D) bands are indicated by arrows. (B) Dimerization at the TM IV/V interface. Pairs that showed dimerization under CuP-promoted oxidation were re-screened using air oxidation. Western blots for ten of these are shown, highlighting the IV/V interaction. 5HT2c, subtype 2c receptor for 5-hydroxytryptamine; CuP, Cu-phenanthroline; TM, transmembrane.
Ligand sensitivity at dimer interfaces
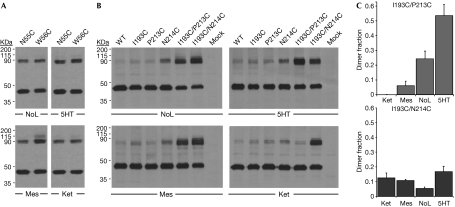
To assess the ligand sensitivity of the two interfaces, cells transfected with either the single mutants from TM I or the pairs of mutants from TMs IV and V were cultured in the presence of 10 μM agonist (serotonin), antagonist (mesulergine), inverse agonist (ketanserin) or no ligand. Dialysed serum was used to minimize the amount of naturally present serotonin. The TM I interface seemed insensitive to the activation state of 5HT2c (Fig 3A); crosslinkings of N55C and W56C mutants were the same for cells cultured with the different ligands and then subjected to various means for cysteine crosslinking (supplementary Fig S5 online; supplementary Table S2 online). By contrast, the TM IV/V interface showed marked ligand sensitivity (Fig 3B). In particular, crosslinking for the I193C/P213C pair of mutant variants seemed to follow the activation state of the receptor. The pair shows no crosslinking for the inactive state of inverse-agonist-treated cells, but the amount of dimer increases progressively through an antagonist-bound state and the absence of ligand, and on to a fully active state with serotonin (Fig 3C, supplementary Table S2 online). Both IV/V disulphides form in the absence of ligand, but only the I193C/N214C bridge is consistent with the inactive, ketanserin state, whereas the I193C/P213C bridge prevails for the active, serotonin state. These data are indicative of a substantial conformational change at the TM IV/V interface during 5HT2c receptor activation.
Figure 3.
Ligand sensitivity at dimer interfaces. Cells were incubated with no ligand, serotonin, mesulergine or ketanserin. (A) TM I. Western blots compare transfected 5HT2c mutants N55C and W56C from cells maintained in the four ligand environments. (B) TM IV/V. Western blots compare 5HT2c mutants transfected individually and as pairs (I193C/P213C and I193C/N214C), with wild-type (WT) and empty vector (Mock) controls. (C) Quantification from replicated TM IV/V experiments. 5HT, serotonin; 5HT2c, subtype 2c receptor for 5-hydroxytryptamine; Ket, ketanserin; Mes, mesulergine; NoL, no ligand; TM, transmembrane.
Evidence for dimer asymmetry
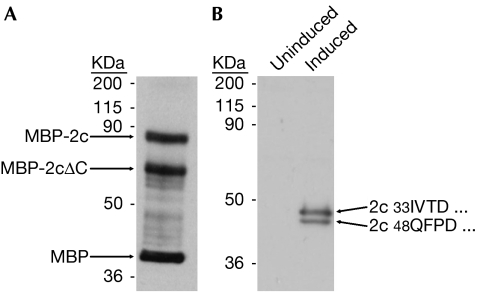
Our earliest bacterial expressions had already provided evidence of asymmetry in 5HT2c dimers as half of the protein from membranes was cleaved (Fig 4A). More recently, we found that receptors expressed in glycosylation-deficient HEK293 cells (Mancia et al, 2007) were also half cleaved, removing 15 amino-terminal residues and a glycosylation site (Fig 4B). We consistently observed approximately 50:50 cleavage patterns, indicating in each case that just one protomer of a dimer was susceptible to proteolysis. Receptors from each source bind to ligands and conformation-sensitive antibodies.
Figure 4.
Half-of-the-sites proteolysis of 5HT2c in membranes. (A) Receptor expressed in bacteria as a maltose-binding protein (MBP) fusion and probed with anti-MBP. Length of the MBP-2cΔC half is consistent with loop V–VI cleavage. (B) Western blot of receptor induced to express in human embryonic kidney 293 GntI− cells. Amino-terminal sequencing identified products of signal-peptide (33IVTD…) and adventitious (48QFPD…) proteolysis. 5HT2c, subtype 2c receptor for 5-hydroxytryptamine.
Although a IV/V dimer interface between rhodopsin-like receptors could be twofold symmetrical as viewed into the membrane (Fig 1), it might also be quasisymmetrical. Then, the environment of helix IV on one protomer interacting with V′ on the other would differ from that of V with IV′. Asymmetry could arise because of protomers having distinct conformations or because of rigid-body rotations. To investigate the symmetry of the TM IV/V dimer interface, we generated two double-modified receptors: one bearing I193C-P213C mutations and another I193C-N214C mutations. We transfected these double mutants using our standard protocol and compared them with the cotransfected single mutants. We expected that an intrinsically symmetrical TM IV/V interface would be enforced in such double mutants, and that a distinctly asymmetrical dimer would form a disulphide linkage with whichever alternative cysteine pair was in contact. Either way, the dimer:monomer ratio should then increase in our crosslinking gels. However, what we should expect from a quasisymmetric interface that partly occluded the alternative site was unclear.
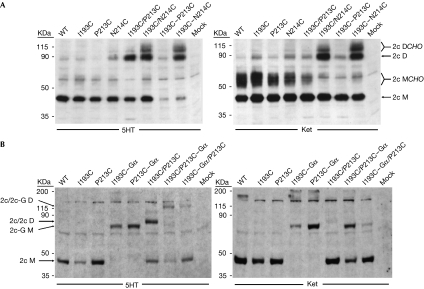
Intriguingly, the experiment showed a rather complex picture (Fig 5A). When cells were maintained in the presence of serotonin, dimers prevailed for the I193C–N214C pairing, whereas expression levels were greatly reduced for the I193C–P213C pairing. Analogous double-serine mutations were fully tolerated (supplementary Fig S6 online). For inverse-agonist-treated cells, the crosslinking pattern did not vary between the cotransfected single mutants and the transfected double mutants. We interpreted the agonist-state results as indicative of a quasisymmetrical receptor in which both 193/214 crosslinks can form comfortably, but where formation of dual 193/213 crosslinks destabilizes the receptor. The contrasting ketanserin results are somewhat puzzling, but perhaps the dimer is more labile in the inactive state than when activated. Re-equilibration of cotransfected species might then lead to the same crosslinking results as from the double mutants.
Figure 5.
Conformational asymmetry in the 5HT2c dimer. (A) Two singles compared with one double. Western blots compare transfectants from cells maintained in serotonin (5HT) and ketanserin (Ket), in which transfectants include single mutants, pairs of single mutants (I193C/P213C, I193C/N214C) and receptors with two mutations (I193C–P213C, I193C–N214C). Species of 5HT2c receptor (2c) are identified as monomer (M) or dimer (D), with or without glycosylation (CHO). (B) 5HT2c–Gαq fusions. Western blots compare alternative carboxy-terminal fusions of Gαq to 5HT2c mutants as transfected into cells, alone or in cotransfections, and maintained either with 5HT or Ket. Controls are labelled as in Fig 3B. Receptor species are labelled according to the absence (2c) or presence (2c-G) of a Gαq fusion and whether monomeric (M) or dimeric (D). 5HT2c, subtype 2c receptor for 5-hydroxytryptamine.
As the double-cysteine experiments showed the greatest sensitivity for the I193C/P213C pairing in serotonin (Fig 5A), we studied activation using carboxy-terminal fusions of the cognate Gαq protein to these receptors (Milligan et al, 2004). We cotransfected mutant receptors having Gα fused alternatively to one or the other cysteine mutant in the pair. We expected that the location of the G protein should not matter for a symmetrical homodimer, but that one alternative might be preferred for a conformationally asymmetrical dimer. We observed marked differences for the alternatives (Fig 5B, supplementary Fig S7 online). I193C/P213C–Gαq forms well and, in 5HT, it favours dimerization when compared with I193C/P213C, whereas productive expression of I193C–Gαq is much reduced in the I193C–Gαq/P213C pairing. Both Gαq fusions express robustly when alone (Fig 5B). Expression patterns also differed for the alternative 193/213 fusions in ketanserin (Fig 5B, right), but there was much less asymmetry for the 193/214 pair (supplementary Fig S7 online). The results are consistent with Gα stabilization of the activated receptor in an apparently asymmetrical dimer.
Discussion
Our experimental design aimed to provide a systematical and comprehensive survey of potential sites of intermolecular contact for 5HT2c expressed in mammalian cells. We tested 46 cysteine residues, distributed along all seven TM segments (Fig 1), in more than 350 individual or pairwise experiments covering all possible combinations. Although several candidate contacts were detected with CuP-promoted oxidation, only a few survived in natural air oxidation, which selects for proximal sites appropriately oriented for disulphide formation. Two independent interfaces emerged from these studies: one along TM I, and the other between TMs IV and V (Fig 2).
Our data indicate that 5HT2c exists as a dimer on the plasma membrane of living cells. This is not surprising given other data on GPCR dimerization (Javitch, 2004) and FRET evidence for 5HT2c being a dimer (Herrick-Davis et al, 2004). Moreover, our finding of an interface between IV and V is roughly compatible with crosslinking, mostly CuP- or HgCl2-promoted, of the dopamine receptor D2R (Guo et al, 2003) and of rhodopsin (Kota et al, 2006), as well as with models of rhodopsin dimers (Fotiadis et al, 2004). In 5HT2c, this interface is clearly sensitive to activation state (Fig 3B), as was also observed for D2R (Guo et al, 2005). Conformational rearrangements are such that Ile 193 of TM IV and Pro 213 of TM V come closer together on serotonin activation from the basal state and move out of crosslinking range on inactivation by the inverse agonist ketanserin. By contrast, crosslinking between I193C and N214C is comparable in all these states.
From crosslinking observations on double mutants (Fig 5A), we conclude that the IV/V interface is only quasisymmetrical, that is, symmetrical enough to allow dual I193C–N214C disulphide bonds, but asymmetrical enough not to tolerate dual I193C–P213C bonds. Thus, at least in the active state, it seems that 5HT2c is not a homodimer, but a conformational heterodimer. This hypothesis is strengthened by data on receptor–Gα fusions; receptor stability and the extent of dimerization differs according to whether Gα is fused to the receptor mutated on TM IV or to the one on TM V (Fig 5B). Moreover, 5HT2c in membranes is susceptible to half-of-the-sites proteolysis (Fig 4). These data are in line with emerging hypotheses of a distinct role for each protomer in the GPCR dimer during activation (Damian et al, 2006; Vilardaga et al, 2008).
The TM I interface is insensitive to activation state (Fig 3A) and presumably symmetrical. However, as TMs IV and V are opposite from TM I, existence of both IV/V and I/I interfaces suggests that higher-order oligomers should form. Oligomeric assemblages of rhodopsin in rod outer segments, seen by atomic force microscopy, have been modelled in just this manner (Fotiadis et al, 2004), and such clustering has also been indicated for C5a following disulphide-trapping experiments (Klco et al, 2003). Postsynaptic clustering of 5HT2c and other neurotransmitter GPCRs is also plausible; however, we have yet to see proof of such oligomerization. Cotransfection of one receptor bearing both N55C (TM I) and I193C (TM IV), with another having both N55C and P213C (TM V), gave no evidence of higher-order oligomers under our experimental conditions, which was possibly inconclusive owing to low abundances of diverse large species (supplementary Fig S8 online). Thus, the physiological role of TM I-mediated dimerization is still unclear.
Speculation
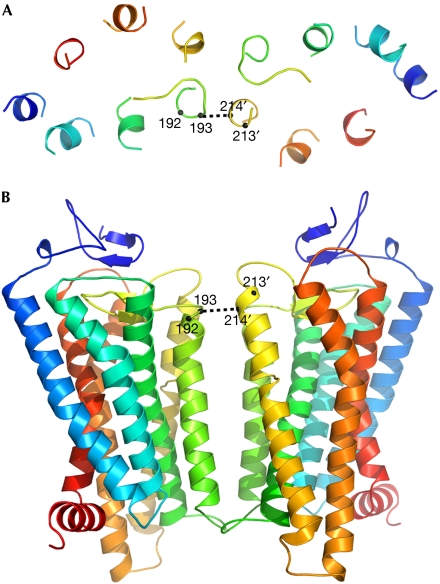
Our structural image of the ligand-sensitive 5HT2c dimer is based on homology with rhodopsin. Keeping the monomer conformation intact, we built a dimer that has a slightly asymmetrical IV/V interface (Fig 6) to be consistent with our observations. As 11-cis retinal rhodopsin is in the inactivated state, this image is probably akin to 5HT2c with the inverse agonist ketanserin. Pro 213 (TM V) is not accessible to Ile 193 (TM IV) in this conformation, and its observed accessibility in the presence of the agonist serotonin requires a conformational change. Details of this state remain to be seen, but we expect a conformational heterodimer that is asymmetrical with respect to its interaction with Gα. This molecular snapshot of membrane-bound 5HT2c dimers in action might also capture other GPCRs.
Figure 6.
Ribbon diagram of a quasisymmetrical 5HT2c dimer. The model is based on rhodopsin (1GZM) and viewed from (A) above and (B) within the plane of the membrane. Selected Cα atoms are identified. Cα-Cα distances are 5.4 Å for 193–214′ and 8.2 Å for 193′–214. 5HT2c, subtype 2c receptor for 5-hydroxytryptamine.
Methods
Selection of sites for cysteine mutagenesis. Sites in 5HT2c were chosen so that the homologous residue in the rhodopsin structure (Protein Data Bank code 1GZM) would be exposed on the receptor surface, to make cysteine crosslinking feasible, and to be near the cell surface to facilitate oxidation for disulphide formation. Residues with more than 40% surface area exposed and lying within the region from approximately 6 Å outside to approximately 8 Å below the extracellular surface were selected for modification to cysteine in 5HT2c (Fig 1). Other sites were selected later on the basis of positive responses of their neighbours.
Generation of cysteine mutants and of Gα fusions. Mutations were introduced into rat 5HT2c complementary DNA following standard protocols. Receptor–Gα fusions were generated by in-frame cloning of a GGGS linker segment in place of the 5HT2c stop codon and ahead of codon Thr 2 of human Gαq. The resulting DNA constructs were cloned into pFM1.2 (Mancia et al, 2004) for expression in mammalian cells.
Cell culture and transfections. HEK293 cells were maintained as described previously (Mancia et al, 2007). Transfections were performed in a six-well format with 2 μg total of DNA, using Lipofectamine and Plus Reagent (Invitrogen, Carlsbad, CA, USA). Dialysed serum was used in the cell growth medium for experiments involving 5HT2c ligands. Ligands were added to the transfected cells at 10 μM concentration and maintained until collection, 48 h later.
Cysteine crosslinking. For air-promoted oxidation, cells were washed in PBS and plated with PBS-based cell dissociation solution (Enzyme Free; Chemicon, Billerica, MA, USA). For CuP-promoted oxidation, cells were washed with PBS, and incubated for 30 min at ∼22°C in PBS with 200 μM CuSO4 and 640 μM 1,10-phenanthroline (Sigma, St Louis, MO, USA). The reaction was quenched by washing the cells with PBS and harvesting them with Enzyme Free containing 10 μM N-ethylmaleimide. Ligands, when required, were present at all times.
Immunoprecipitation and immunoblotting. Cell pellets were solubilized in dodecylmaltoside/cholesteryl-hemisuccinate, cleared of insoluble material, and deglycosylated with PNGaseF (New England Biolabs, Ipswich, MA, USA). Immunoprecipitations were performed as described previously using 5HT2c monoclonal antibody 5H8 (Mancia et al, 2007). Nonreducing gels were loaded with 20 μg of crude lysate or the immunoprecipitate from one well of the six-well dish. Blots were probed with a monoclonal antibody against an epitope tag for crude lysates and those on immunoprecipitated samples were probed with 2HT2c polyclonal antibodies.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Material
Acknowledgments
We thank R. Axel for discussions, Q. Fan for help with modelling and illustrations, and Y. Sun for excellent technical assistance. This work was supported in part by National Institutes of Health Grant GM68671.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152 [DOI] [PubMed] [Google Scholar]
- Chabre M, le Maire M (2005) Monomeric G-protein-coupled receptor as a functional unit. Biochemistry 44: 9395–9403 [DOI] [PubMed] [Google Scholar]
- Damian M, Martin A, Mesnier D, Pin JP, Baneres JL (2006) Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J 25: 5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K (2004) The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett 564: 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shi L, Javitch JA (2003) The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem 278: 4385–4388 [DOI] [PubMed] [Google Scholar]
- Guo W, Shi L, Filizola M, Weinstein H, Javitch JA (2005) Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci USA 102: 17495–17500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Mazurkiewicz JE (2004) Biochemical and biophysical characterization of serotonin 5-HT2C receptor homodimers on the plasma membrane of living cells. Biochemistry 43: 13963–13971 [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Harrigan TJ, Mazurkiewicz JE (2005) Inhibition of serotonin 5-hydroxytryptamine2c receptor function through heterodimerization: receptor dimers bind two molecules of ligand and one G-protein. J Biol Chem 280: 40144–40151 [DOI] [PubMed] [Google Scholar]
- Javitch JA (2004) The ants go marching two by two: oligomeric structure of G-protein-coupled receptors. Mol Pharmacol 66: 1077–1082 [DOI] [PubMed] [Google Scholar]
- Julius D, MacDermott AB, Axel R, Jessell TM (1988) Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science 241: 558–564 [DOI] [PubMed] [Google Scholar]
- Klco JM, Lassere TB, Baranski TJ (2003) C5a receptor oligomerization. I. Disulfide trapping reveals oligomers and potential contact surfaces in a G protein-coupled receptor. J Biol Chem 278: 35345–35353 [DOI] [PubMed] [Google Scholar]
- Kota P, Reeves PJ, Rajbhandary UL, Khorana HG (2006) Opsin is present as dimers in COS1 cells: identification of amino acids at the dimeric interface. Proc Natl Acad Sci USA 103: 3054–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Edwards PC, Burghammer M, Villa C, Schertler GF (2004) Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol 343: 1409–1438 [DOI] [PubMed] [Google Scholar]
- Luttrell LM (2006) Transmembrane signaling by G protein-coupled receptors. Methods Mol Biol 332: 3–49 [DOI] [PubMed] [Google Scholar]
- Mancia F, Patel SD, Rajala MW, Scherer PE, Nemes A, Schieren I, Hendrickson WA, Shapiro L (2004) Optimization of protein production in mammalian cells with a coexpressed fluorescent marker. Structure 12: 1355–1360 [DOI] [PubMed] [Google Scholar]
- Mancia F, Brenner-Morton S, Siegel R, Assur Z, Sun Y, Schieren I, Mendelsohn M, Axel R, Hendrickson WA (2007) Production and characterization of monoclonal antibodies sensitive to conformation in the 5HT2c serotonin receptor. Proc Natl Acad Sci USA 104: 4303–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B (1999) GABAB receptors—the first 7TM heterodimers. Trends Pharmacol Sci 20: 396–399 [DOI] [PubMed] [Google Scholar]
- Milligan G, Feng GJ, Ward RJ, Sartania N, Ramsay D, McLean AJ, Carrillo JJ (2004) G protein-coupled receptor fusion proteins in drug discovery. Curr Pharm Des 10: 1989–2001 [DOI] [PubMed] [Google Scholar]
- Palczewski K et al. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289: 739–745 [DOI] [PubMed] [Google Scholar]
- Park PS, Filipek S, Wells JW, Palczewski K (2004) Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry 43: 15643–15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K (2006) Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA 103: 16123–16128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep 5: 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ (2008) Conformational cross-talk between α2A-adrenergic and μ-opioid receptors controls cell signaling. Nat Chem Biol 4: 126–131 [DOI] [PubMed] [Google Scholar]
- White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, Northrup JK, Grisshammer R (2007) Dimerization of the class A G-protein coupled neurotensin receptor NTS1 alters G-protein activation. Proc Natl Acad Sci USA 104: 12199–12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA 104: 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Material