Abstract
Introduction
The metabolic syndrome and insulin resistance represent growing concerns related to atypical antipsychotic (AAP) use as their incidence in the schizophrenia population is two to four fold higher than the general population. Reduced methylenetetrahydrofolate reductase (MTHFR) activity, resulting in aberrant folate metabolism and hyperhomocysteinemia, has been linked to cardiovascular disease and is unstudied in relation to AAP associated metabolic complications.
Purpose
To examine the relationship between MTHFR, metabolic syndrome, and insulin resistance in schizophrenia subjects receiving AAPs for ≥ 12 months.
Methods
Fifty-eight subjects were included in this cross-sectional analysis and screened for the metabolic syndrome, insulin resistance and MTHFR 677C/T and 1298 A/C genotype.
Results
Overall, 23 subjects (40%) met metabolic syndrome criteria. There were no differences in age, gender, race, or AAP exposure between genotype groups. For the 677 T allele carriers, 53% met metabolic syndrome criteria, compared to 23% in the CC genotype group, giving an OR = 3.7, (95% CI = 1.24 – 12.66, p = 0.02). Thus, for T allele subjects, the risk was almost four times greater, despite similar antipsychotic exposure. Both waist circumference and MTHFR genotype significantly predicted insulin resistance (F = 8.35, df = 5, 51, p < 0.0001), with these two terms interacting (F = 8.6, df = 2, p = 0.0006) suggesting TT subjects are at greater risk for insulin resistance with increasing central adiposity, which is independent of age, gender, BMI, or metabolic syndrome diagnosis.
Conclusion
Results should be taken cautiously due to the small sample size, but suggest the MTHFR 677C/T variant may predispose patients to AAP metabolic complications.
Keywords: Pharmacogenomics, Folate, Antipsychotics, Schizophrenia, MTFHR, Metabolic Syndrome
1. Introduction
The metabolic syndrome has been associated with significant cardiovascular mortality (Maggi, S et al. 2006; Onat, A et al. 2006), and the growing association between atypical antipsychotic (AAPs) use and this syndrome represents a particular problem for psychiatric populations treated with these medications. Although several definitions for the metabolic syndrome have been cited, the National Cholesterol Education Program Adult Treatment Protocol (NCEP-ATP III) is most often used and requires at least three of the following: abdominal obesity, elevated triglycerides, low HDL, elevated blood pressure, or elevated fasting glucose (Lee, TH et al. 2001). AAPs, primarily used for the treatment of schizophrenia, have been associated with significant metabolic complications, including hyperlipidemia (Gaulin, BD et al. 1999; Osser, DN et al. 1999; Spivak, B et al. 1999; Henderson, DC et al. 2000; Huang, TL et al. 2005), insulin resistance and diabetes mellitus (Henderson, DC et al. 2000; Gianfrancesco, F et al. 2003; Ollendorf, DA et al. 2004; Carlson, C et al. 2005; Henderson, DC et al. 2005; Lambert, BL et al. 2005; Sernyak, MJ et al. 2005; Guo, JJ et al. 2006), obesity (Henderson, DC et al. 2000; Volavka, J et al. 2002; Simpson, GM 2005; Zipursky, RB et al. 2005), and hyperhomocystenemia (Levine, J et al. 2002; Applebaum, J et al. 2004; Muntjewerff, J-W et al. 2005; Muntjewerff, JW et al. 2005). These metabolic complications result in a two-to-four-fold increase in the rate of metabolic syndrome in patients with schizophrenia (Kato, MM et al. 2004; McEvoy, JP et al. 2005; Nasrallah, HA 2006). The presence of the metabolic syndrome itself is associated with an increased risk for coronary heart disease, CVD, and diabetes mellitus (Wilson, PW et al. 2005), and patients with schizophrenia treated with AAPs are at greater risk for vascular disease and have a three fold increase in sudden cardiac death compared to the general population (Saari, KM et al. 2005).
Because APPs such as clozapine and olanzapine may provide superior clinical benefits (Lieberman, JA et al. 2005; McEvoy, JP et al. 2006) there is a pressing need to identify patients at risk for the metabolic syndrome and the associated complications. Among the risk factors associated with the development of CVD, insulin resistance, and diabetes mellitus in the general population are the 677C/T and 1298A/C genetic variants of methylenetetrahydrofolate reductase (MTHFR) which are involved in folate and homocysteine metabolism (Klerk, M et al. 2002; Lewis, SJ et al. 2005). These polymorphisms have been associated with up to a 70% reduction in folate acid metabolism, hyperhomocysteinemia, and a greater risk for CVD (Klerk, M et al. 2002; Matthews, RG 2002; Gueant-Rodriguez, RM et al. 2005). The relationship between these variants and risk for the metabolic syndrome or insulin resistance in the schizophrenia population receiving AAPs has not been previously investigated, as to our knowledge; our research group is the first to report an association.
2. Methods and Materials
2.1 Subjects
Subjects were recruited through the University of Iowa Department of Psychiatry. All met DSM-IV criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder, were between the ages of 18–90, and had been receiving treatment with an antipsychotic for at least 12 months. Subjects were excluded if they had a history of thyroid problems or other medical condition that may affect body weight (i.e. cancer), were unable to provide informed consent, or unwilling to participate. Subjects with a history of substance abuse were not excluded from this investigation. All subjects gave written informed consent to the protocol approved by the University of Iowa Human Subjects Institutional Review Board. The consent process was ongoing and the subjects were reminded throughout study that their participation was voluntary.
2.2 Clinical Measures
Once informed consent was obtained, subjects were seen in the morning at the University of Iowa General Clinical Research Center (GCRC) after fasting for at least 8 hours. Upon arrival to the GCRC, the subjects’ vitals were obtained as well as height, weight, and waist and hip circumference. Blood was then drawn and assayed for insulin, glucose, lipids (total cholesterol, triglycerides, high density lipids, low density lipids), hemoglobin A1c, homocysteine, leptin, and thyroid stimulating hormone. The laboratory analysis was completed by the University of Iowa Hospital and Clinics Clinical Pathology Laboratories and the GCRC Laboratory. A single blood sample was also drawn for the genetic analysis.
Subjects underwent a detailed medical and prescription history along with assessments of psychopathology using the Brief Psychiatric Rating Scale (BPRS) and Scale for Assessment of Negative Symptoms (SANS). Subjects were assessed for smoking status and history to determine pack year history. Each subject’s physical activity was also determined for the month previous to the study visit and the frequency and duration of any physical activity was documented.
The National Cholesterol Education Program Adult Treatment Protocol (NCEP ATP-III) criteria was used for the metabolic syndrome diagnosis as follows: abdominal obesity (waist circumference > 40 inches in males or 35 inches in females), elevated triglycerides (≥ 150 mg/dL), low HDL (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (≥ 130/85 or on antihypertensive medication), or elevated fasting glucose (≥ 110 mg/dL or on medication for diabetes) (Lee, TH et al. 2001). Insulin Resistance was measured using the homeostasis model assessment insulin resistance (HOMA-IR) value (http://www.dtu.ox.ac.uk/).
2.3 Determination of MTHFR 677C/T and 1298A/C Variants
Genomic DNA was isolated from whole blood with the salt precipitation method. Genotyping was done with Pyrosequencing™ Technology. Polymerase Chain Reaction (PCR) and pyrosequencing primers were designed using Pyrosequencing single nucleotide polymorphism (SNP) Primer Design Version 1.01 software (http://www.biotage.com). Pyrosequencing was performed and analyzed as previously described using a PSQ MD instrument and software (Pyrosequencing AB, Uppsala, Sweden).
The MTHFR 677C/T and 1298A/C variants previously described (Frosst, P et al. 1995; van der Put, NM et al. 1998) were analyzed for their relationship to metabolic syndrome and HOMA-IR value in subjects with schizophrenia receiving antipsychotic treatment for at least 12 months. The sequence was accessed from GENBANK (NM 005957). Forty five PCR cycles were done for reactions in a 30 ul volume with 1.5 mM Mg2+ and 10 pmol of each primer according to the following specifications: 94°C for 30 sec, 56°C for 30 seconds and, 72°C × 30 seconds. A 168 bp (677C/T) and 85bp (1298A/C) PCR products were visualized by electrophoresis on 1.5% agarose gels stained with ethidium bromide prior to Pyrosequencing. Table 1, list the primers used for genotyping of the 677C/T and 1298A/C variants.
Table 1.
Primers used for genotyping MTHFR 677C/T and 1298A/C variants
| MTHFR 677C/T Variant | |
| Forward Primer | 5′-Biotin/ACTGTCATCCCTATTGGCAGGTTA-3′ |
| Reverse Primer | 5′-TCGGTGCATGCCTTCACAA-3′ |
| Pyrosequencing Primer | 5′-AGGAGCTGACCAGTGA-3′ |
| MTHFR 1298A/C Variant | |
| Forward Primer | 5′-GGAGCTGCTGAAGATGTGG-3′ |
| Reverse Primer | 5′-Biotin/TGGTTCTCCCGAGAGGTAAAG-3′ |
| Pyrosequencing Primer | 5′-AGGAGCTGACCAGTGA-3′ |
2.4 Data Analysis
Differences in mean values for the primarily outcomes and socio-demographic variables between genotype and allelic groups were determined by the use of one-way analysis of variance (ANOVA) for normally distributed variables (BMI, age, SANS scores, BPRS scores, smoking pack year history, minutes of physical activity). Previous analyses of the MTFHR SNPs have shown a relationship between cardiovascular disease risk and presence of the 677T allele or heterozygosity for the 677C/T and 1298A/C genotypes and cardiovascular risk (Klerk, M et al. 2002; Lewis, SJ et al. 2005). Therefore, allelic group comparisons in demographic and outcome variables were also done in persons carrying at least one T allele for the 677 variant, as well as for 677 and 1298 heterozygotes. Chi squared analysis was used to compare dichotomous variables (e.g. gender and metabolic syndrome diagnosis) by genotype groups. A linear regression was conducted using HOMA-IR as the dependant variable. The independent variables included the MTFHR genotypes, age, gender, metabolic syndrome diagnosis, waist circumference, and interactions. A p-value less than 0.05 was considered statistically significant.
3. Results
A total of fifty-nine subjects were recruited for this study, with one opting to not provide a DNA sample which resulted in a final sample size of fifty-eight (males = 38 (66%), females = 20 (34%)). There were no differences in age, gender, race, smoking, or psychopathology between the genotype groups. Table 2 provides details regarding the baseline characteristics of this population by 677 genotype group.
Table 2.
Genotype Groups Characteristics
| MTHFR genotype | p-value | ||
|---|---|---|---|
| CC (n = 26) | T allele carriers (n = 32) | ||
| Age (± s.d.) in years
[Age Range in years] |
36.7 ± 10.5
[23 – 57] |
36.2 ± 9.6
[20 – 52] |
0.85 |
| Gender F/M (% male) | 11 female/15
male (58%) |
9 female/23
male (72%) |
0.26 |
| Race (% Caucasian) | 81% | 96% | 0.10 |
| BMI (± s.d.) | 30.83 ± 9.47 | 30.16 ± 5.51 | 0.73 |
| % receiving AAPs | 92% | 84% | 0.20 |
| Cigarette pack year history (± s.d.) | 13.07± 17.68 | 11.69 ± 17.07 | 0.77 |
| Minutes of physical activity per week (± s.d.) | 82.8 ± 114.97 | 217.44 ± 324.81 | 0.05 |
| % meeting metabolic syndrome criteria | 23% | 53% | 0.02 |
| Fasting blood glucose in mg/dl (± s.d.) | 113.42 ± 14.7 | 113.65 ± 29.89 | 0.97 |
| Fasting total cholesterol in mg/dl (± s.d.) | 171.08 ± 42.41 | 186.55 ± 40.87 | 0.17 |
| Fasting triglycerides in mg/dl | 119.17 ± 62.13 | 164.45 ± 98.77 | 0.06 |
| Fasting HDL in mg/dl | 52.46 ± 21.11 | 48.03 ± 12.12 | 0.33 |
| Blood pressure in mmHg (± s.d.) | 123/72 ± 14/9 | 119/73 ± 15/12 | 0.39 |
| Waist Circumference in cm (± s.d.) | 103.9 ±20.7 | 105.88 ±13.15 | 0.66 |
| HOMA-IR (± s.d.) | 3.79 ± 3.37 | 5.44 ± 6.41 | 0.24 |
| Brief Psychiatric Rating Scale (BPRS) Score (± s.d.) | 39.35 ± 8.95 | 38.91 ± 11.82 | 0.88 |
| Scale for Assessment of Negative Symptoms (SANS) Score (± s.d.) | 13.31 ± 7.45 | 11.31 ± 7.78 | 0.33 |
At the time of screening, subjects were receiving a variety of antipsychotic medications with 42 on monotherapy (n = 11 clozapine, n = 8 olanzapine, n = 8 risperidone, n = 6 quetiapine, n = 4 haloperidol, n = 3 aripiprazole, and n = 2 ziprasidone) and 16 on polytherapy (n = 6 two AAPs, n = 6 one AAP and one typical antipsychotic, n = 2 ziprasidone and one typical antipsychotic, and n = 2 aripiprazole and one AAP). Overall, 81% (47/58) were currently receiving an AAP associated with DM and weight gain, although all subjects had significant past AAP exposure.
The distribution of 677 and 1298 genotypes were as follows CC (44%), CT (47%), TT (9%), AA (47%), AC (46%), CC (7%), both of which were in Hardy Weinberg equilibrium (677: χ2 = 0.296, p = 0.58, 1298: χ2 = 0.04, p = 0.46). We found high degree of linkage between these two SNPS, which has been reported previously (D′ = 1) (Martin, YN et al. 2006).
Twenty-three subjects met criteria for metabolic syndrome, giving a prevalence rate of 40%, which is similar to national estimates of 30–50% in schizophrenia (McEvoy, JP et al. 2005; Nasrallah, HA 2006). For the 677 T allele carriers (male = 23, female = 9) the incidence of metabolic syndrome was 53%, compared to the CC genotype allele carriers (male = 15, female = 11), where 23% of these subjects met metabolic syndrome criteria (OR = 3.7, 95% CI = 1.24 – 12.66, p = 0.02). Thus, for the T allele subjects the risk of developing metabolic syndrome while receiving APs was 3.6 times greater than those with the CC genotype, despite similar AP exposure. Addition of the 1298 genotypes to the analysis did not significantly add to the model and so this data is not presented.
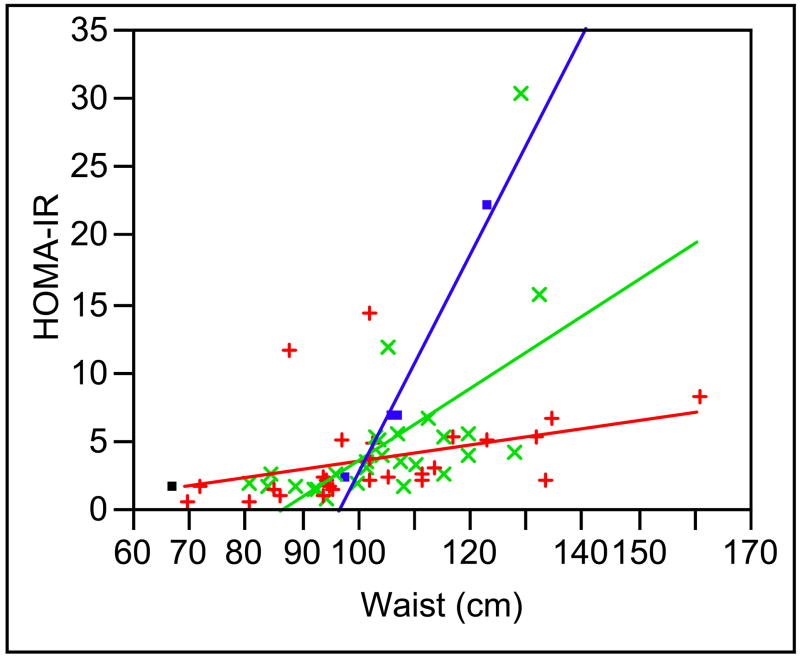
In looking at this data further we also examined the relationship between the homeostasis model assessment insulin resistance (HOMA-IR) value, waist circumference, and the MTHFR genotype (Figure 1). Both waist circumference and MTHFR genotype were significant factors associated with the HOMA-IR values (F = 8.35, df = 5, 51, p < 0.0001) with an interaction between these two terms which was statically significant (F = 8.6, df = 2, p = 0.0006) suggesting that subjects with a 677 TT genotype are at the greatest risk for increased insulin resistance with increasing waist circumference compared to TC and CC genotype subjects. This was independent of age, gender, BMI, or metabolic syndrome diagnosis. Overall the power of this model to detect differences based on genotype was 0.72. The effect size for the TT genotype/waist interaction variable is 19. The addition of the 1298 genotypes to this analysis did not contribute significantly to the model. We also examined plasma homocysteine in a subset of subjects (n = 46) and found an interesting trend. For the 677TT group the mean homocysteine concentration was 10.2 ± 4.9, where the CT and CC groups were lower (CT 9.1 ± 2.8, CC 9.9 ± 3.5). Although these are not statistically different (p = 0.67), the TT group’s values are higher, suggesting a greater risk for CVD, which accompanies the increased risk of metabolic syndrome and insulin resistance in this group.
Figure 1. Relationship Between MTHFR 677C/T Genotype, HOMA-IR and Waist Circumference.
Graphical representation of relationship between HOMA-IR (Y-axis) waist circumference in centimeters (X-Axis) and, MTHFR 677C/T genotype (TT = ●, TC = x, CC = +).
4. Discussion
This investigation is the first to examine the relationship between the MTHFR 677C/T and 1298A/C variants and metabolic syndrome and insulin resistance risk in a schizophrenia population receiving atypical antipsychotics. Recent research has highlighted the importance of genes as well as environmental/nutritional factors in the body’s ability to regulate genome machinery (Ames, BN 2001). This has lead to the field of epigenetics, whose focus is on how diet and nutrition affect DNA functioning or DNA binding proteins without altering the nucleotide sequence of DNA (Oommen, AM et al. 2005; Reik, W 2007).
Mechanistically, MTHFR plays an important role in folate metabolism and DNA maintenance. Folic acid is a water soluble B-vitamin involved in the synthesis, repair, and methylation of DNA (Friso, S et al. 2005), whose effective utilization is dependant on adequate daily intake as well as genetic variants altering metabolism (Friso, S et al. 2005). Methylenetetrahydrofolate reductase (MTHFR) is the enzyme responsible for the formation of methyltetrahydrofolate (5-methyl THF) from dietary folic acid, which allows conversion of homocysteine to methionine and adenosyl methionine which is the universal methyl donor for DNA and protein synthesis. Lack of this universal donor may result in DNA hypomethylation, leading to alterations in gene silencing. Functionally, the MTHFR variant is very important, particularly in the field of obstetrics as presence of the variant allele is a risk factor for the development of neural tube defects in an unborn child (Gueant, JL et al. 2003). Additionally, previous reports have linked MTHFR variants to cardiovascular risk as well as to schizophrenia risk, but have not been examined in the context of metabolic complications seen with antipsychotic use (Klerk, M et al. 2002; Gueant, JL et al. 2003; Lewis, SJ et al. 2005; Muntjewerff, JW et al. 2005; Kempisty, B et al. 2006; Lee, YS et al. 2006; Zintzaras, E 2006). Additionally, a recent report showed the MTHFR T variant to be associated with the occurrence of negative symptoms in schizophrenia (Roffman, JL et al. 2007).
In general, the metabolism of both folate and homocysteine are undeniably linked and dependent on MTHFR. Thus, reduction in folate due to either low dietary intake, genetic predisposition, or both, may result in hyperhomocysteinemia, which has been linked to cardiovascular disease in the general population (Danesh, J et al. 1998). Folate administration prevents homocysteine oxidative stress and consequential endothelial damage (Setola, E et al. 2004) as hyperhomocysteinemia (Moat, SJ et al. 2004) directly damage endothelial cells (Wall, RT et al. 1980) and impair the release of nitrous oxide (Stamler, JS et al. 1993), reducing endothelial dependent vasodilatation (Tawakol, A et al. 1997). Prolonged increases in plasma homocysteine of ≥1 umol/L result in a 5% increased CVD risk.
From our data it appears that the MTHFR 677T allele is associated with a 3.6 fold greater risk for developing AAP associated metabolic syndrome, and the TT genotype may place individuals at greater risk for insulin resistance with greater central adiposity. Higher levels of insulin resistance pose an elevated danger for developing diabetes mellitus, metabolic syndrome, and endothelial dysfunction, which are significant risk factors for the development of CVD. As stated previously, the relationship between MTHFR and cardiovascular disease is not unique to patients with schizophrenia, but within this investigation the risk associated with the T allele was much higher than that expected in the general population. In normal controls, previous meta-analyses have found that patients with the MTHFR 677TT genotype have approximately a 16% increase in CVD compared to the CC genotype which is potentially related to hyperhomocysteinemia, and poor dietary folate intake (Klerk, M et al. 2002; Lewis, SJ et al. 2005). Previous reports have shown that on average, homocysteine concentrations in individuals with a MTHFR 677TT genotype are 2.5 umol/L higher than CC patients (Brattstrom, L et al. 1998; Danesh, J et al. 1998; Ueland, PM et al. 2000). Thus, it may be that addition of medications, such as the atypical antipsychotics, associated with weight gain, insulin resistance, diabetes mellitus, and hyperlipidemia may be the “trigger” that results in a greater risk for metabolic syndrome in the schizophrenia population. Possibly most importantly, in looking at this group of subjects, this was a fairly young group with an age range of 20–57 years, and an overall mean age of 36 years,. The occurrence of these potentially fatal conditions at such an early age, place patients at risk for developing even more cardiovascular complications, leading to greater physical and health related impairments. Not surprisingly, when the individual components of the metabolic syndrome are compared between the genotype groups very little differences were seen (Table 2). Given that presence of a metabolic syndrome diagnosis places individuals at greater risk for CVD than each of its individual components (Wilson, PW et al. 2005), better characterization of the unexplored relationship between folate pharmacogenetics, metabolic syndrome, insulin resistance, endothelial function, dietary folate intake, and AAPs in schizophrenia may help to identify risk factors for these serious complications, and aid in developing preventative measures.
5. Study Limitations
Due to the small number of subjects included in this analysis and the cross-sectional nature of this study, our data has several limitations. First, in looking at our current analysis, it appears that the majority of the relationships found with metabolic syndrome and insulin resistance is being driven by the small number of subjects with the 677TT genotype. Thus, future investigations need to expand the number of subjects included. Additionally, for many of these subjects this was the first time they had undergone screening for the metabolic syndrome, which is similar to other reports in the literature concerning the monitoring for these metabolic consequences in this population (McEvoy, JP et al. 2005). Thus we cannot determine if these subjects developed the metabolic syndrome before or after starting AAP treatment. Secondly, we did not have dietary information available concerning folate intake, which may also limit our data analysis, as the effect of the MTFHR T allele has been show to be more exaggerated in the face of poor dietary folate intake. Lastly, the subjects included in this study were not excluded due to substance abuse and were receiving a variety of antipsychotics. The decision to include all subjects was done in an effort to gain an understanding of metabolic syndrome risk in the “real world”, but unfortunately does introduce variability in the data.
Regardless, this investigation represents an important first step in delineating the relationship between MTHFR and cardiovascular risk in schizophrenia subjects treated with AAPs. Thus, it is imperative that we continue our investigation, expanding our sample size and including variants associated with other enzymes involved in folate and homocysteine metabolism resulting in reduced metabolic activity to further elucidate their role in the development, prevention, and treatment of AAP associated metabolic complications. Lastly, understanding the role of supplemental folate in the attenuation of these metabolic complications may help to improve the treatment of our patients and needs to be further investigated as we look for ways to reduced overall cardiovascular risk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ames BN. DNA Damage from Micronutrient Deficiencies Is Likely to Be a Major Cause of Cancer. Mutat Res. 2001;475(1–2):7–20. doi: 10.1016/s0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J. Homocysteine Levels in Newly Admitted Schizophrenic Patients. J Psychiatr Res. 2004;38(4):413–416. doi: 10.1016/j.jpsychires.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Brattstrom L, Wilcken DE, Ohrvik J, Brudin L. Common Methylenetetrahydrofolate Reductase Gene Mutation Leads to Hyperhomocysteinemia but Not to Vascular Disease: The Result of a Meta-Analysis. Circulation. 1998;98(23):2520–2526. doi: 10.1161/01.cir.98.23.2520. [DOI] [PubMed] [Google Scholar]
- Carlson C, Hornbuckle K, Delisle F, Kryzhanovskaya L, Breier A, Cavazzoni P. Diabetes Mellitus and Antipsychotic Treatment in the United Kingdom. Eur Neuropsychopharmacol. 2005 doi: 10.1016/j.euroneuro.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Danesh J, Lewington S. Plasma Homocysteine and Coronary Heart Disease: Systematic Review of Published Epidemiological Studies. J Cardiovasc Risk. 1998;5(4):229–232. [PubMed] [Google Scholar]
- Friso S, Choi SW. Gene-Nutrient Interactions in One-Carbon Metabolism. Curr Drug Metab. 2005;6(1):37–46. doi: 10.2174/1389200052997339. [DOI] [PubMed] [Google Scholar]
- Frosst P, Blom HJ, Milos R, et al. A Candidate Genetic Risk Factor for Vascular Disease: A Common Mutation in Methylenetetrahydrofolate Reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- Gaulin BD, Markowitz JS, Caley CF, Nesbitt LA, Dufresne RL. Clozapine-Associated Elevation in Serum Triglycerides. Am J Psychiatry. 1999;156(8):1270–1272. doi: 10.1176/ajp.156.8.1270. [DOI] [PubMed] [Google Scholar]
- Gianfrancesco F, White R, Wang RH, Nasrallah HA. Antipsychotic-Induced Type 2 Diabetes: Evidence from a Large Health Plan Database. J Clin Psychopharmacol. 2003;23(4):328–335. doi: 10.1097/01.jcp.0000085404.08426.3a. [DOI] [PubMed] [Google Scholar]
- Gueant-Rodriguez RM, Juilliere Y, Candito M, et al. Association of Mtrra66g Polymorphism (but Not of Mthfr C677t and A 1298c, Mtra2756g, Tcn C776g) with Homocysteine and Coronary Artery Disease in the French Population. Thromb Haemost. 2005;94(3):510–515. doi: 10.1160/TH05-04-0262. [DOI] [PubMed] [Google Scholar]
- Gueant JL, Gueant-Rodriguez RM, Anello G, et al. Genetic Determinants of Folate and Vitamin B12 Metabolism: A Common Pathway in Neural Tube Defect and Down Syndrome? Clin Chem Lab Med. 2003;41(11):1473–1477. doi: 10.1515/CCLM.2003.226. [DOI] [PubMed] [Google Scholar]
- Guo JJ, Keck PE, Jr, Corey-Lisle PK, et al. Risk of Diabetes Mellitus Associated with Atypical Antipsychotic Use among Patients with Bipolar Disorder: A Retrospective, Population-Based, Case-Control Study. J Clin Psychiatry. 2006;67(7):1055–1061. doi: 10.4088/jcp.v67n0707. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Cagliero E, Gray C, et al. Clozapine, Diabetes Mellitus, Weight Gain, and Lipid Abnormalities: A Five-Year Naturalistic Study. Am J Psychiatry. 2000;157(6):975–981. doi: 10.1176/appi.ajp.157.6.975. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Cagliero E, Copeland PM, et al. Glucose Metabolism in Patients with Schizophrenia Treated with Atypical Antipsychotic Agents: A Frequently Sampled Intravenous Glucose Tolerance Test and Minimal Model Analysis. Arch Gen Psychiatry. 2005;62(1):19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- Huang TL, Chen JF. Serum Lipid Profiles and Schizophrenia: Effects of Conventional or Atypical Antipsychotic Drugs in Taiwan. Schizophr Res. 2005;80(1):55–59. doi: 10.1016/j.schres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kato MM, Currier MB, Gomez CM, Hall L, Gonzalez-Blanco M. Prevalence of Metabolic Syndrome in Hispanic and Non-Hispanic Patients with Schizophrenia. Prim Care Companion J Clin Psychiatry. 2004;6(2):74–77. doi: 10.4088/pcc.v06n0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempisty B, Mostowska A, Gorska I, et al. Association of 677c>T Polymorphism of Methylenetetrahydrofolate Reductase (Mthfr) Gene with Bipolar Disorder and Schizophrenia. Neuroscience Letters. 2006;400(3):267. doi: 10.1016/j.neulet.2006.02.055. [DOI] [PubMed] [Google Scholar]
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. Mthfr 677c-->T Polymorphism and Risk of Coronary Heart Disease: A Meta-Analysis. Jama. 2002;288(16):2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- Lambert BL, Chou CH, Chang KY, Tafesse E, Carson W. Antipsychotic Exposure and Type 2 Diabetes among Patients with Schizophrenia: A Matched Case-Control Study of California Medicaid Claims. Pharmacoepidemiol Drug Saf. 2005;14(6):417–425. doi: 10.1002/pds.1092. [DOI] [PubMed] [Google Scholar]
- Lee TH, Cleeman JI, Grundy SM, et al. Executive Summary of the Third Report of the National Cholesterol Education Program (Ncep) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel Iii) Jama. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Lee YS, Han DH, Jeon CM, et al. Serum Homocysteine, Folate Level and Methylenetetrahydrofolate Reductase 677, 1298 Gene Polymorphism in Korean Schizophrenic Patients. Neuroreport. 2006;17(7):743–746. doi: 10.1097/01.wnr.0000215777.99473.52. [DOI] [PubMed] [Google Scholar]
- Levine J, Stahl Z, Sela BA, Gavendo S, Ruderman V, Belmaker RH. Elevated Homocysteine Levels in Young Male Patients with Schizophrenia. Am J Psychiatry. 2002;159(10):1790–1792. doi: 10.1176/appi.ajp.159.10.1790. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Ebrahim S, Davey Smith G. Meta-Analysis of Mthfr 677c->T Polymorphism and Coronary Heart Disease: Does Totality of Evidence Support Causal Role for Homocysteine and Preventive Potential of Folate? Bmj. 2005;331(7524):1053. doi: 10.1136/bmj.38611.658947.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Maggi S, Noale M, Gallina P, et al. Metabolic Syndrome, Diabetes, and Cardiovascular Disease in an Elderly Caucasian Cohort: The Italian Longitudinal Study on Aging. J Gerontol A Biol Sci Med Sci. 2006;61(5):505–510. doi: 10.1093/gerona/61.5.505. [DOI] [PubMed] [Google Scholar]
- Martin YN, Salavaggione OE, Eckloff BW, Wieben ED, Schaid DJ, Weinshilboum RM. Human Methylenetetrahydrofolate Reductase Pharmacogenomics: Gene Resequencing and Functional Genomics. Pharmacogenet Genomics. 2006;16(4):265–277. doi: 10.1097/01.fpc.0000194423.20393.08. [DOI] [PubMed] [Google Scholar]
- Matthews RG. Methylenetetrahydrofolate Reductase: A Common Human Polymorphism and Its Biochemical Implications. Chem Rec. 2002;2(1):4–12. doi: 10.1002/tcr.10006. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the Metabolic Syndrome in Patients with Schizophrenia: Baseline Results from the Clinical Antipsychotic Trials of Intervention Effectiveness (Catie) Schizophrenia Trial and Comparison with National Estimates from Nhanes Iii. Schizophr Res. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of Clozapine Versus Olanzapine, Quetiapine, and Risperidone in Patients with Chronic Schizophrenia Who Did Not Respond to Prior Atypical Antipsychotic Treatment. Am J Psychiatry. 2006;163(4):600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Moat SJ, Lang D, McDowell IF, et al. Folate, Homocysteine, Endothelial Function and Cardiovascular Disease. J Nutr Biochem. 2004;15(2):64–79. doi: 10.1016/j.jnutbio.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Muntjewerff J-W, Blom HJ. Aberrant Folate Status in Schizophrenic Patients: What Is the Evidence? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(7):1133. doi: 10.1016/j.pnpbp.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, Methylenetetrahydrofolate Reductase and Risk of Schizophrenia: A Meta-Analysis. Mol Psychiatry. 2005;11(2):143. doi: 10.1038/sj.mp.4001746. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA. Metabolic Findings from the Catie Trial and Their Relation to Tolerability. CNS Spectr. 2006;11(7 Suppl 7):32–39. doi: 10.1017/s1092852900026663. [DOI] [PubMed] [Google Scholar]
- Ollendorf DA, Joyce AT, Rucker M. Rate of New-Onset Diabetes among Patients Treated with Atypical or Conventional Antipsychotic Medications for Schizophrenia. MedGenMed. 2004;6(1):5. [PMC free article] [PubMed] [Google Scholar]
- Onat A, Hergenc G, Uyarel H, Can G, Ozhan H. Prevalence, Incidence, Predictors and Outcome of Type 2 Diabetes in Turkey. Anadolu Kardiyol Derg. 2006;6(4):314–321. [PubMed] [Google Scholar]
- Oommen AM, Griffin JB, Sarath G, Zempleni J. Roles for Nutrients in Epigenetic Events. J Nutr Biochem. 2005;16(2):74–77. doi: 10.1016/j.jnutbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Osser DN, Najarian DM, Dufresne RL. Olanzapine Increases Weight and Serum Triglyceride Levels. J Clin Psychiatry. 1999;60(11):767–770. doi: 10.4088/jcp.v60n1109. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and Flexibility of Epigenetic Gene Regulation in Mammalian Development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Purcell S, et al. Contribution of Methylenetetrahydrofolate Reductase (Mthfr) Polymorphisms to Negative Symptoms in Schizophrenia. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Saari KM, Lindeman SM, Viilo KM, et al. A 4-Fold Risk of Metabolic Syndrome in Patients with Schizophrenia: The Northern Finland 1966 Birth Cohort Study. J Clin Psychiatry. 2005;66(5):559–563. doi: 10.4088/jcp.v66n0503. [DOI] [PubMed] [Google Scholar]
- Sernyak MJ, Gulanski B, Rosenheck R. Undiagnosed Hyperglycemia in Patients Treated with Atypical Antipsychotics. J Clin Psychiatry. 2005;66(11):1463–1467. doi: 10.4088/jcp.v66n1117. [DOI] [PubMed] [Google Scholar]
- Setola E, Monti LD, Galluccio E, et al. Insulin Resistance and Endothelial Function Are Improved after Folate and Vitamin B12 Therapy in Patients with Metabolic Syndrome: Relationship between Homocysteine Levels and Hyperinsulinemia. Eur J Endocrinol. 2004;151(4):483–489. doi: 10.1530/eje.0.1510483. [DOI] [PubMed] [Google Scholar]
- Simpson GM. Atypical Antipsychotics and the Burden of Disease. Am J Manag Care. 2005;11(8 Suppl):S235–241. [PubMed] [Google Scholar]
- Spivak B, Lamschtein C, Talmon Y, et al. The Impact of Clozapine Treatment on Serum Lipids in Chronic Schizophrenic Patients. Clin Neuropharmacol. 1999;22(2):98–101. doi: 10.1097/00002826-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Osborne JA, Jaraki O, et al. Adverse Vascular Effects of Homocysteine Are Modulated by Endothelium-Derived Relaxing Factor and Related Oxides of Nitrogen. J Clin Invest. 1993;91(1):308–318. doi: 10.1172/JCI116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocyst(E)Inemia Is Associated with Impaired Endothelium-Dependent Vasodilation in Humans. Circulation. 1997;95(5):1119–1121. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- Ueland PM, Refsum H, Beresford SA, Vollset SE. The Controversy over Homocysteine and Cardiovascular Risk. Am J Clin Nutr. 2000;72(2):324–332. doi: 10.1093/ajcn/72.2.324. [DOI] [PubMed] [Google Scholar]
- van der Put NM, Gabreels F, Stevens EM, et al. A Second Common Mutation in the Methylenetetrahydrofolate Reductase Gene: An Additional Risk Factor for Neural-Tube Defects? Am J Hum Genet. 1998;62(5):1044–1051. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Sheitman B, et al. Clozapine, Olanzapine, Risperidone, and Haloperidol in the Treatment of Patients with Chronic Schizophrenia and Schizoaffective Disorder. Am J Psychiatry. 2002;159(2):255–262. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]
- Wall RT, Harlan JM, Harker LA, Striker GE. Homocysteine-Induced Endothelial Cell Injury in Vitro: A Model for the Study of Vascular Injury. Thromb Res. 1980;18(1–2):113–121. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Nam BH, Pencina M, D’Agostino RB, Sr, Benjamin EJ, O’Donnell CJ. C-Reactive Protein and Risk of Cardiovascular Disease in Men and Women from the Framingham Heart Study. Arch Intern Med. 2005;165(21):2473–2478. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- Zintzaras E. C677t and A1298c Methylenetetrahydrofolate Reductase Gene Polymorphisms in Schizophrenia, Bipolar Disorder and Depression: A Meta-Analysis of Genetic Association Studies. Psychiatr Genet. 2006;16(3):105–115. doi: 10.1097/01.ypg.0000199444.77291.e2. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Gu H, Green AI, et al. Course and Predictors of Weight Gain in People with First-Episode Psychosis Treated with Olanzapine or Haloperidol. Br J Psychiatry. 2005;187:537–543. doi: 10.1192/bjp.187.6.537. [DOI] [PubMed] [Google Scholar]