1. Introduction
Norepinephrine (NE) appears to mediate a heightened state of arousal and attention to changes in the environment and to species-specific stimuli. In response to a novel stimulus, NE neurons in the locus coeruleus (LoC) react as a group with increased activity [56]. NE has been implicated in the control of behavior and reproduction [13,16]. The LoC may affect reproductive behavior directly through its effects on arousal and attention e.g., [47] or indirectly through its modulation of hypothalamic-pituitary-gonadal function [38,52,66]. Research also suggests that NE plays a role in some aspects of learning, e.g. discrimination [31], olfactory learning [18,19,20,21,62], and temporal learning [1,36].
Central NE-producing cell bodies form cell groups that are located in the brainstem. The LoC is the largest of these with the remaining cell groups forming the lateral tegmental system (LT). For the most part, the target areas of the LoC and LT do not overlap. The LoC sends highly collateralized noradrenergic fibers to the cortex/telencephalon, the cerebellum, and some brain-stem sensory nuclei [56]. In both rats and pigeons, LoC projections are the primary source of NE projections to the cortex/telencephalon [40,43,44]. In general, projections of the LT are restricted to thalamic and hypothalamic brain regions [24].
The noradrenergic neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) is widely used to lower brain NE to investigate the functions of the central noradrenergic system [7,11,12,55,64]. When administered systemically in rats, DSP-4 crosses the blood-brain barrier and causes rapid, profound, long-term decreases in NE and markers of noradrenergic function in the cortex but leaves hypothalamic NE function relatively unaltered [39,58]. These differential effects on NE function in the cortex and hypothalamus presumably result from DSP-4's high selectivity for noradrenergic axon terminals that originate in the LoC [33,34,37,39,57,58].
Singing in zebra finches (Taenopygia guttata) is controlled by a series of hormone-sensitive interconnected brain nuclei that are located primarily in the telencephalon and are known collectively as the vocal control system (VCS) [26,51]. Only male zebra finches sing. Barclay and Harding [9,10] found high levels of NE in the male VCS. Hormone treatments, which reinstated high levels of courtship singing in castrates significantly increased levels and turnover of NE in vocal control nuclei (VCN) on the motor pathway controlling the muscles of the syrinx, the avian vocal organ. Data suggest that NE may also be involved in song learning. When we quantified NE function across development in male zebra finches, NE levels and turnover in brain areas controlling singing showed striking peaks during the sensory acquisition phase of song learning [35]. During sensory acquisition, juvenile males attend to and memorize the songs of adult tutors [15]. The increase in NE function was not seen outside auditory and vocal control nuclei, suggesting that heightened NE function in these areas might be related to song learning.
To determine whether NE played a significant role in controlling singing behavior in adults, we used DSP-4 in an attempt to lower NE levels in the telencephalic vocal control nuclei but leave NE levels in the hypothalamus relatively unaffected. When DSP-4 was administered systemically, it produced significant deficits in courtship singing [11]. While brain NE levels were not significantly depleted by DSP-4 treatment, the nonsignificant decreases in NE levels in three VCN on the motor pathway (interfacial nucleus of the nidopallium - NIf, robust nucleus of the arcopallium - RA, and dorsomedial portion of the intercollicular nucleus - DM) were positively correlated with decreased frequencies of singing. Since this systemic DSP-4 treatment did not significantly lower telencephalic NE levels, in a second study we administered DSP-4 centrally via the third ventricle in an attempt to cause greater depletions [12]. Central administration of DSP-4 also caused significant deficits in courtship singing and, in addition, significantly depleted NE levels in three VCN (Area X, lateral magnocellular nucleus of the anterior nidopallium - lMAN, and NIf). However, NE levels in two hypothalamic nuclei, the magnocellular portion of the paraventricular nucleus (PVN) and the preoptic area (POA) were also significantly lowered. In both studies [11,12], the effects on male sexual behavior appeared to be mediated by decreased attentiveness to females. Males took a longer time before beginning to court, but once they began, their behavior appeared normal.
The current study had two goals. We wanted to determine if we could significantly decrease the central NE innervation if birds were given two systemic injections of DSP-4, because a single systemic dose failed to significantly deplete central NE levels in our previous study [11]. We also wanted to verify that the hypothalamic NE innervation would be spared as it was previously when we administered DSP-4 systemically. To make our results more comparable to work done in rats and other species e.g., [3,26,65], we quantified the effects of DSP-4 treatment in male zebra finch brains using standard immunocytochemical procedures to visualize dopamine- -hydroxylase (DBH)-containing neurons. DBH is the enzyme that catalyzes the conversion of dopamine to NE, and as such is a good marker of noradrenergic cells.
2. Materials and Methods
2.1. Subjects
Adult zebra finches were obtained from Canary Bird Farm (Englishtown, NJ). Males and females were treated with ivermectin to reduce parasite infestations and housed in separate aviaries until needed. Bird rooms were kept on a 14:10 h light:dark cycle with the temperature controlled (24 ± 2°C) and the humidity kept over 50% to maintain optimal breeding conditions. Birds were fed a vitamin-supplemented (8 in 1, Pet Products) commercial finch seed mix, grit, water and cuttlebone ad libitum, supplemented with fresh greens and oranges. During the experiment, males were housed in individual cages (56 cm)3 and stimulus females were group housed until needed. All animal care, experimental procedures, and euthanasia were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of Hunter College of the City University of New York
2.2. DSP-4 Injections
The two drugs used in this study were zimelidine dihydrochloride, a serotonergic re-uptake blocker, and DSP-4 (generous gifts of Trevor Archer, Astra Pharmaceuticals, Ltd.). Males were randomly assigned to either experimental or control groups. All males received 200 μg, i.p. zimelidine dihydrochloride to protect serotonergic neurons [58]. After 30 minutes, experimental males (n=8) received 500 μg DSP-4 i.p. and controls (n=8) received saline vehicle alone i.p. Administration of DSP-4 occurred within 10 minutes of drug preparation to prevent the formation of the non-toxic aziridinium derivative [42]. These doses were based on the typical rat dosages which are expressed as mg/kg, e.g., 50mg/kg DSP-4 [45,67]. Since the average weight of our finches was 10g, our doses were close to the typical rat dosage. This is the dose we have used with finches in our two previous studies. To maximize noradrenergic depletion, drug treatments were repeated ten days later. One hour after the second drug administration, each male was housed with a stimulus female. Our hypothesis was that the two groups would interact differently with the females and this would maximize differences in the availability of DBH between groups. We expected the control males to court the females more frequently, increasing their hormone levels. Increased gonadal steroid levels should stimulate their noradrenergic neurons and, given our prior results, increase NE levels [9,10]. We hypothesized that a treatment that increased NE levels should also increase DBH-ir. Ten days later, males were anesthetized, perfused, and their brains processed for immunocytochemistry and cresyl violet staining.
2.3. Perfusion
Males were mildly anesthetized with a combination of xylazine:ketamine (5 μg each/g body weight) in 0.5 ml saline injected into the pectoral muscle. Metofane (Pitman-Moore) was administered as necessary to keep birds deeply anesthetized during the procedure. Males were transcardially perfused with 0.2 M phosphate buffer (PB) followed by 4% paraformaldehyde in 0.1 M PB. Brains were quickly removed and post-fixed in 4% paraformaldehyde for 2 hours. The post-fixing solution was replaced with cryoprotectant (300 g sucrose, 10 g polyvinyl-pyrrolidone, 30 ml ethylene glycol, 2 g sodium azide, volume adjusted to 1000 ml with 0.1 M PB) and stored at 5°C. The brains stayed in the cryoprotectant until they sank [14,22].
2.4. Immunocytochemistry
Brains were mounted onto frozen cryostat chucks with double-distilled water. Frozen brains were equilibrated in an American Optical Cryo-cut II microtome or an IEC Minotome Plus at −15°C for an hour, and then sliced into 40-micron-thick coronal sections. Three series of sections were collected into glass vials containing cryoprotectant. Sections were either stored at −70°C until needed or stored at 5°C for immediate immunocytochemical processing. One vial was used for immunocytochemistry. Another vial containing unprocessed tissue was rinsed, sorted, mounted onto gelatin-coated slides and stained with cresyl violet. This set of slides served as a cytoarchitectural reference for the DBH-immunolabeled slides.
Sections were processed to optimize DBH staining in finch brains. Unless otherwise stated, all rinses were performed three times for five minutes each in Buffer A (0.05 M tris-buffered saline) while sections were gently agitated. To control for variations in staining intensity, tissues from experimental and control birds were always processed in the same assay. Free-floating sections were treated according to Bamshad et al. [8] with the following modifications. After pretreatment with 0.1% NaBH4 for 10 minutes in Tris-NaCl, sections were incubated in the following solutions: 1) 1% H2O2 in Tris-NaCl for 10 minutes; 2) Tris-Triton rinse for 10 minutes; 3) anti-DBH serum (Eugene Tech International, Inc; New York, NY; DBH TE103), 1:500 in Tris-Triton for 24 hrs at 5°C; 4) Tris-Triton rinse. The next steps followed the directions of the Vectastain ABC Elite kit (Vector Labs, Burlingame, CA); 5) incubation with biotinylated anti-rabbit IgG at a dilution of 1:600 in Tris-Triton for 45 minutes at room temperature; 6) two Tris-Triton rinses, then two Tris-NaCl rinses; 7) incubation with ABC reagent diluted in Tris-NaCl for 40 minutes; 8) rinsed and treated with DAB peroxidase substrate (SigmaFast 3,3'-diaminobenzidine tablet sets) in Tris-NaCl for 5 minutes. After the final rinse with Tris-NaCl, sections were sorted and mounted onto gelatin-coated slides. Slides were dehydrated in a graded alcohol series, cleared with xylene and coverslipped with Permount.
Adrenergic neurons also contain DBH; however in rats and birds, adrenergic neurons are restricted to the medulla [50,60]. Since tissues from the medulla were not included in this study, all DBH-positive cells and are presumably noradrenergic.
2.4.1. Specificity Check
There were two checks for the specificity of DBH-antiserum in finch brains. In the positive control, the antiserum was preadsorbed with an excess of DBH (Sigma) and was used in an assay. In the negative control, incubation with the anti-DBH serum was omitted from the assay. Both specificity checks resulted in DBH-immunonegative tissue.
2.5. Image Processing
Sections were examined using a Nikon Optiphot microscope. Images were captured by a Dage MTI CCD72 video camera interfaced via a Perceptics Pixel pipeline framegrabber to a MAC2 FX computer. Quantification of the density of DBH-ir cell bodies and/or fibers in a given brain area was performed using NIH Image version 1.55. Images were sharpened to focus the digitized image. The density slice feature was used for thresholding and adjusted so that only immunopositive cell bodies and/or fibers were quantified. For all measurements, all images which contained a given brain area were captured and measured and the mean per coronal section was then calculated. For each brain area, two calculations were performed. The first measured the area of DBH-ir within the outlined brain area. The brain area of interest in each image was outlined with the freehand selection tool. This outline was saved also for later calculation of percent area labeled. The atlas of Stokes et al. [61] was used as a reference, but areas are designated using the updated avian nomenclature [53]. The program was calibrated by measuring lines of known length so that Image could convert the area of labeled pixels in the area of interest into square microns.
The second calculation divided the area of DBH-ir in the defined regions by the size of the brain area measured to determine the percentage of each brain area that was labeled (i.e. brain area with thresholding enabled divided by the same brain area without thresholding enabled). Percent area calculations corrected for the possibility that DSP-4 treatment altered the size of the nucleus in addition to affecting the area of DBH innervation. This measurement also allowed meaningful comparisons between brain areas of different sizes.
2.6. Figure Preparation
Sections were examined using a Nikon Eclipse E400 microscope and digitized using SPOT version 3.5.5 for Windows (Diagnostic Instruments, Inc.). Images were autobalanced using PhotoShop 6.0 and labels added in CorelDraw 9.0
2.7. Data Analyses
DBH-ir cells were counted on a Nikon Optiphot-2 microscope (10× power) equipped with a reticle. These data were collected from all sections that contained DBH-ir cells. Since only one third of brain sections were processed for DBH, the resulting cell counts were multiplied by three to estimate total DBH-ir cells.
All data were analyzed by unpaired Student's t-tests (GraphPad Prism version 4.03 for Windows, GraphPad Software, San Diego, California, USA, www.graphpad.com). If the variances of the two groups differed significantly, the data were square-root or log transformed prior to analysis. All statistical tests were one-tailed, because we predicted DSP-4 would decrease DBH labeling.
The LoC was divided into dorsal and ventral sections because these regions may project to different targets [63], and we wanted to determine if DSP-4 had differential effects within the LoC. Previous research in rats [63] based this division on the appearance of the DBH-labeled neurons. In finches, DBH-labeled cells were categorized on the basis of their position relative to the fasciculus longitudinalis medialis (FLM). At the level of the fourth ventricle, cells located above FLM were classified as dorsal LoC, and cells located lateral to FLM were classified as ventral LoC. Any DBH-ir cells below FLM were classified as ventral subcoeruleus (SCv).
3. Results
3.1. Noradrenergic cells
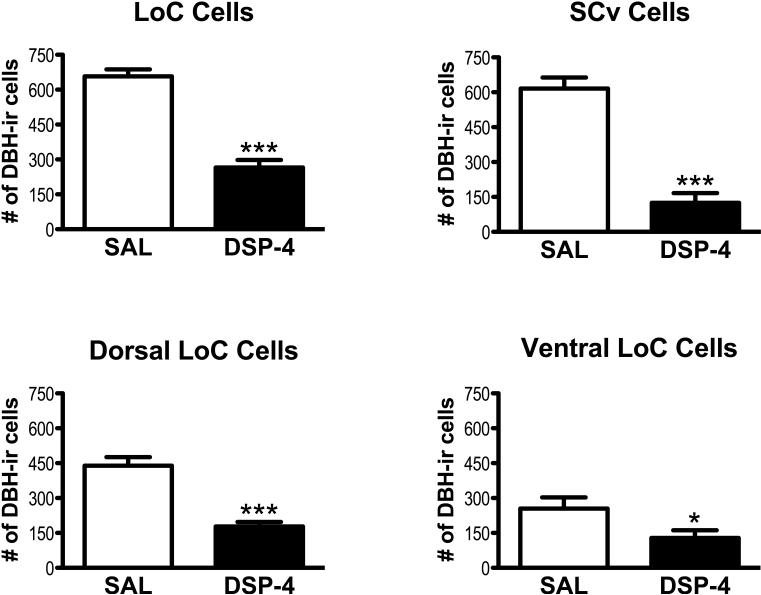
In control birds, the mean estimated total DBH-ir cell number in the LoC was 667 ± 30 (± SEM) and in the SCv was 616 ± 47. In the LoC, DSP-4 treatment significantly decreased the total number of DBH-ir cells compared to saline controls [t(14)=8.993), p=0.0001; see Fig. 1, 3A]. A significant loss of DBH-ir cells occurred in both dorsal LoC [t(14)=6.221 p=0.0001] and in ventral LoC [t(14)=2.169, p=0.0239, see Fig. 1]. DBH-ir cells in the SCv cell groups were also significantly decreased in the drug-treated group [t(14)=7.765, p=0.0001; see Fig. 1]. The reductions in cell numbers were quite striking. DSP-4 treatment decreased DBH-ir cells in the LoC by 60%, the dorsal LoC by 60%, the ventral LoC by 58%, and the SCv by 80%. DBH-ir cells in the LoC and SCv were arranged differently. Cells in the dorsal LoC were usually tightly clustered while cells in ventral LoC were diffuse, and cells in SCv were even more diffuse. In terms of their morphology, LoC cells were either round or angular while those in the SCv were predominately elongated.
Figure 1.
Mean (± SEM) estimated DBH-ir cells in locus coeruleus (LoC), ventral subcoeruleus (SCv), dorsal LoC, and ventral LoC in saline-treated (SAL) and DSP-4-treated males. Significant decrease in cell numbers compared to saline group, **p<0.01, ***p<0.0001.
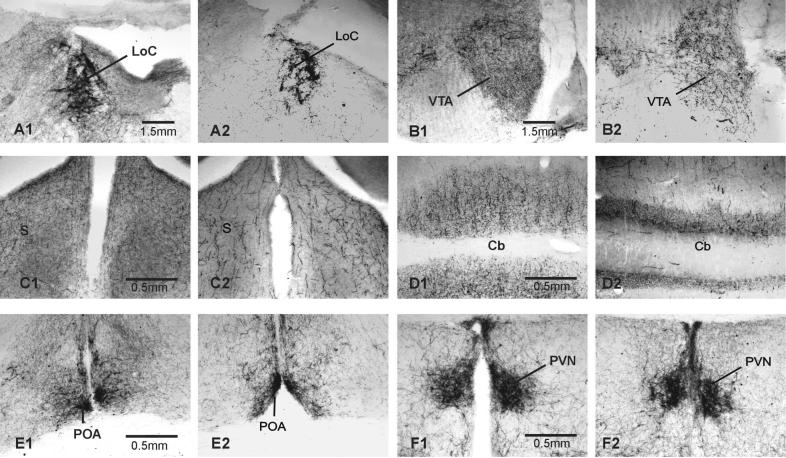
Figure 3.
Photomicrographs of DBH ir in areas outside of the vocal control system in male zebra finch brains. In each pair, the brain on the left is from a saline treated male, that on the right from a DSP-4 treated male. A) LoC, B) VTA, C) S, D) Cb, E) POA, F) PVN. See text for abbreviations.
In finches, there were clear differences in the intensity of DBH labeling of cell bodies within the LoC and SCv of the saline-treated and drug-treated groups. In controls, some cells were intensely stained while others were very lightly stained. In the drug-treated group, no lightly-stained cells were detected in the LoC, and the DBH-ir cells that remained were not tightly clustered.
3.2. DBH-ir labeling across brain areas
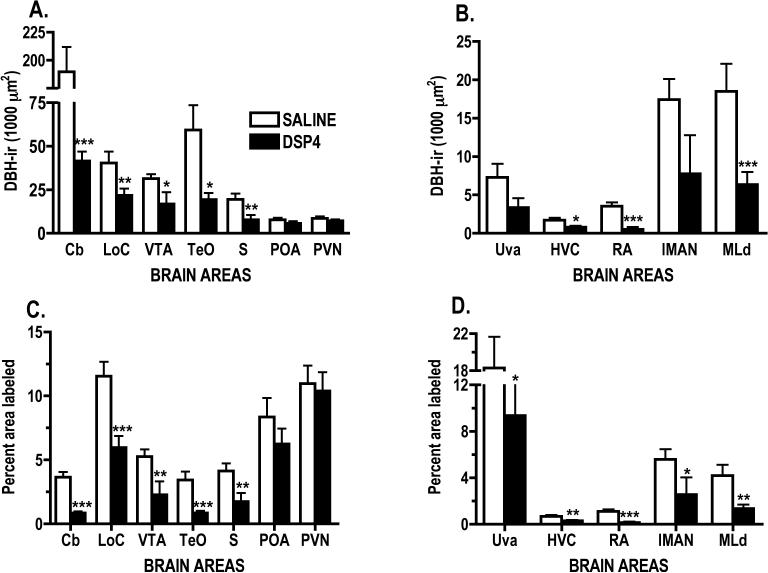
Drug treatment significantly reduced the areas of DBH-ir cells and fibers in the LoC [t(12)=2.593, p=0.0118], and DBH-ir fibers in cerebellum (Cb) [t(12)=7.76, p<0.0001], ventral tegmental area (VTA) [t(11)=2.373, p=0.0185], optic tectum (TeO) [t(12)=2.638, p= 0.0108] and septum (S) [t(11)=2.566, p= 0.0131; see Figs. 2A & 3A-D]. DSP-4 treatment did not significantly affect the mean area covered by DBH-ir fibers in the two hypothalamic nuclei, PVN and POA (Fig. 2A & 3E-F).
Figure 2.
Mean area (± SEM) covered by DBH-ir labeling per coronal section of A. areas outside the vocal control system and B. vocal control and auditory nuclei. Mean (± SEM) percent of each brain area covered by DBH-ir labeling per coronal section in C. areas outside the vocal control system and D. in vocal control and auditory nuclei. Note broken Y axes in A and D. Scale is not linear in A. Significant change compared to saline-treated males, *p<0.05, **p<0.01, ***p<0.001. See text for abbreviations.
In this study, as in our previous research, POA was defined as the area under the septopalliomesencephalic tract (TSM). Other authors have included a much larger area, including this area and its posterior extension located above PVN and directly under the anterior commissure as the medial preoptic nucleus based on aromatase labeling [6]. In comparison to Mello et al. [48], POA as defined here included only the anterior extent of POM, i.e. the area under TSM.
DSP-4 treatment significantly decreased DBH-ir labeling and the mean area covered by DBH-ir fibers in two of the four VCN examined, {high vocal center (HVC) [t(12)=2.64, p=0.0108], robust nucleus of the arcopallium (RA) [t(11)=4.375, p=0.0006], and one auditory nucleus (AN) - dorsal part of the lateral mesencephalic nucleus (MLd) [t(9)=3.27, p<0.005]; see Fig. 2B & Fig. 4C-4E, 5A-B}. Data from two other VCN just missed being significantly affected by DSP-4 treatment {nucleus uvaeformis [t(11)=1.773, p=0.0520] and lateral magnocellular nucleus of the anterior nidopallium (lMAN) [t(9)=1.783, p=0.0541]; see Fig. 2B & Fig. 4A-4B]}.
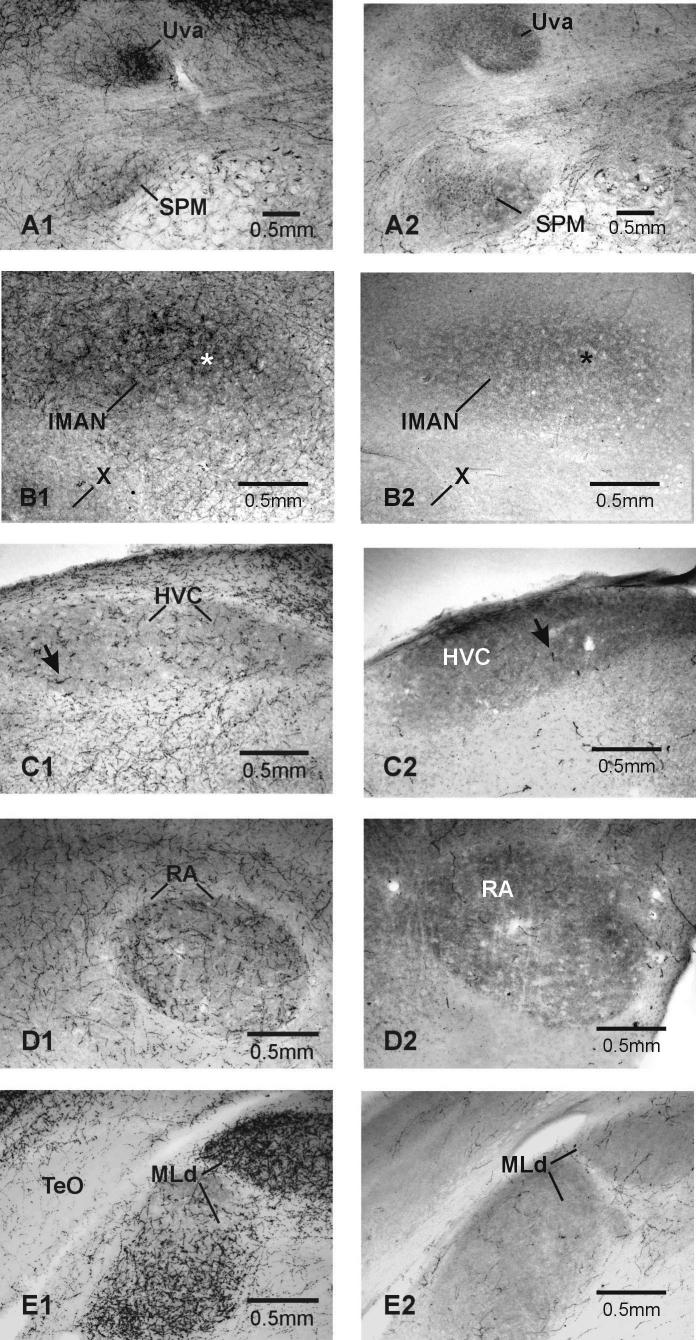
Figure 4.
Photomicrographs of DBH-ir labeling in vocal control and auditory nuclei in male zebra finch brains. In each pair, the brain on the left is from a saline-treated male, that on the right from a DSP-4-treated male. A) Uva, B) lMAN & X, C) HVC, D) RA, E) TeO & MLd. See text for abbreviations. Note that in the saline-treated controls, the DBH-ir labeling inside the vocal control nuclei is denser than that in surrounding tissue, except in Area X. There are two issues here. First, there are more aggregations of dense punctate labeling and fibers within the vocal control nuclei compared to surrounding tissue. Second, the background of the VCN is darker than that of the surrounding tissue. This is caused by smaller, lighter puncta of DBH ir, making the vocal control areas in the DSP-4-treated birds darker than the surrounding tissue. DSP-4 treatment significantly lowered, but did not eliminate DBH-ir. The VCN in DSP-4-treated birds stand out because they still have more DBH-ir than the surround. For the most part, tissue from DSP-4-treated birds lacks the dense fibers and puncta seen in controls. This can be seen in higher magnification in Figure 5. The arrows in HVC and the asterisks in lMAN in this Figure correspond to those in these areas in Figure 5.
Figure 5.
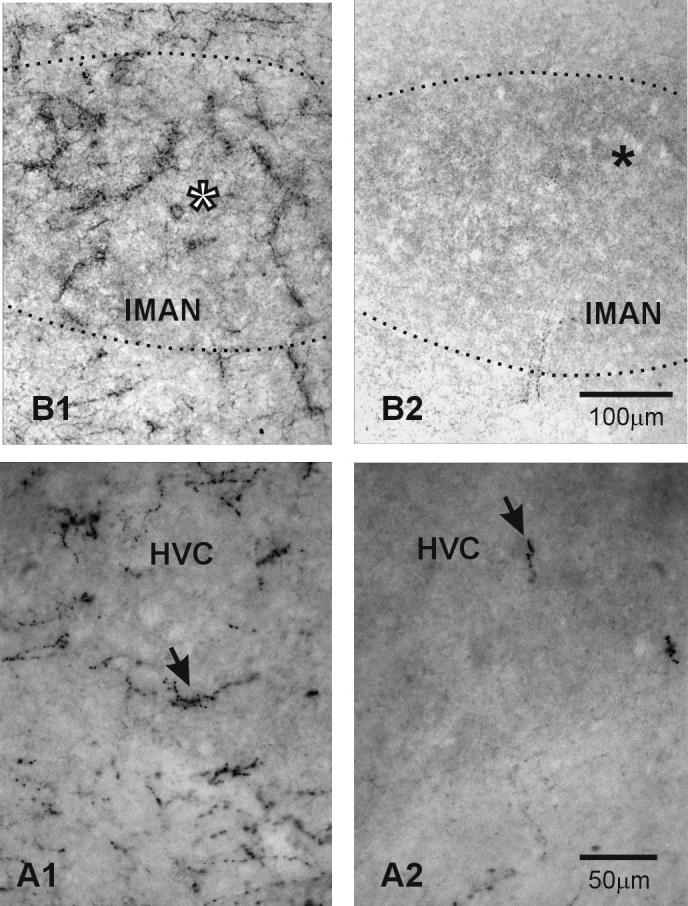
Higher magnification of DBH-ir labeling in the sections of HVC and lMAN shown in Figure 4. In each pair, the brain on the left is from a saline-treated male, that on the right from a DSP-4-treated male. The arrows in HVC and the asterisks in lMAN in this Figure correspond to those in these areas in Figure 4. Note how the pattern and density of DBH-ir labeling over lMAN in the control bird is different from that outside the nucleus. This is particularly clear in comparison to the area below lMAN. If you refer back to Figure 4, you can see that the arrow in HVC in the control bird points to an area of dense labeling on the ventral border of the nucleus. It is clear at higher magnification that no labeling below the arrow matches the aggregations of high density DBH-ir labeling seen within HVC.
3.3. Percent of each brain area labeled by DBH
DSP-4 treatment significantly decreased the percent area of DBH-ir labeling in the LoC [t(12) = 3.925, p=0.001], Cb [t(12) = 7.509, p<0.0001], VTA [t(11) = 2.745, p=0.0095], TeO [t(12) = 4.328, p=0.0005], S [t(11) = 2.636, p=0.0116], see Fig. 2C. The percent area covered by DBH-ir fibers was not significantly affected by drug treatment in the two hypothalamic nuclei examined; PVN and POA (Figure 2C). In the VCN and AN, DSP-4 treatment significantly decreased the percent area of DBH-ir labeling in all nuclei: Uva [t(11)=2.005, p=0.0351], HVC [t(12) = 2.757, p=0.0087], RA [t(11)=4.289, p=0.0006], lMAN [t(9) = 1.847, p=0.0489], MLd [t(9) = 3.115, p=0.0062], see Fig. 2D].
4. Discussion
4.1. Typical effects of DSP-4 treatment
The actions of DSP-4 have been most thoroughly documented in rats. In this species, DSP-4 treatment causes a rapid, dose-dependent reduction in NE levels and NE-ir which is maximal by 8−16 hours after treatment [3,26]. NE depletion is most pronounced in brain areas innervated by the LoC, including the hippocampus, cortex, and olfactory bulb. NE levels in these areas are extremely low following treatment with 50mg DSP-4/kg body weight, the dose of DSP-4 commonly used in rats. Brain areas like the hypothalamus and basal forebrain that are innervated by the LT are much less affected. [3,26]. The neural distribution of DBH-ir fibers is affected in a similar fashion following DSP-4 treatment, except that significant decreases in DBH-ir are not seen until four days after treatment with profound decrements by day 5 [26]. In rats, NE cell bodies in the LoC are not affected until several months after treatment [26,30]. Typically, there is recovery of NE function over time, with surviving LoC neurons showing strong regenerative responses [30].
4.2. Effects of DSP-4 treatment on noradrenergic cells and projections in finches
NE levels In finch brains are approximately ten times higher than those found in comparable brain areas in rats [9]. This may account for the inability of a single systemic treatment with the standard rat dose of DSP-4 to significantly deplete telencephalic NE levels [11]. Our current results document that giving birds two systemic injections of DSP-4 spaced 10 days apart is much more effective in decreasing noradrenergic function. This study also confirms, that when administered systemically, DSP-4 does not significantly affect noradrenergic function in the hypothalamus.
The double DSP-4 treatment profoundly decreased the number of DBH-ir cell bodies in finches-by 60% in the LoC and 80% in the SCv. This dramatic cell loss occurred within twenty days of the first drug treatment. While control birds had both lightly- and intensely-labeled cells in the LoC and SCv, the DBH-ir cells remaining in DSP-4-treated males were all darkly stained, suggesting that cells showing low levels of DBH-ir are more vulnerable to the neurotoxin.
As might be expected from the fact that DSP-4-treated males had fewer DBH-ir cell bodies, they also had fewer DBH-ir fibers in most brain areas compared to the saline-treated controls. The differences in DBH fiber staining between the two groups were obvious and in some brain regions could be seen without the aid of a microscope. In general, the distribution of DBH-ir fibers in the saline-treated finches formed a clear network of fibers, though the intensity of staining varied from lightly to densely stained. DSP-4-treated males did not have this fibrous network, and their remaining DBH-ir fibers appeared turgid and fragmented.
Drug treatment reduced DBH-ir fibers in two of the four VCN (HVC and RA) and significantly reduced the percentage tissue labeled in all four VCN (HVC, RA, Uva, and lMAN). The VTA has a strong noradrenergic innervation [41], and DSP-4 treatment significantly reduced fiber staining in this area. NE is noted for its modulation of sensory inputs [23], and so it was not surprising that DSP-4 treatment significantly decreased DBH labeling in the primary visual (TeO) and auditory processing areas (MLd). Noradrenergic modulation of these brain areas may be related to birds' attentiveness to conspecifics. DSP-4 treatment has been shown to decrease the responsiveness of male finches to females placed in their home cages [11,12] and the responsiveness of female canaries to the songs of conspecific males [2]. DSP-4 treatment also decreased DBH ir in the Cb and S. In rats, both areas are innervated by and their function modulated by the LoC [24]. However, fiber labeling in the two hypothalamic nuclei, PVN and POA, was not significantly affected by drug treatment.
4.2. Most VCN receive stronger noradrenergic innervation than surrounding tissue
In control birds, DBH labeling clearly delineated all VCN examined from surrounding tissue except for Area X. In most birds, there was a denser network of darkly-stained DBH-ir inside MLd, Uva, HVC, RA and lMAN than there was in the surrounding tissue. This striking labeling of the vocal control nuclei was not observed by Mello et al., except in DM [48]. Four methodological differences might account for discrepancies in DBH labeling between the two studies. First, the concentration of antibody used in our study was double that used by Mello et al. [48]. However, if the staining differences were attributable only to the stronger antibody concentration, then one would expect an overall increase in staining intensity across all brain areas, which wasn't the case. Second, Mello et al. performed their assay on slide-mounted tissue, not free-floating sections. The free-floating sections used in our study provided two surfaces and increased the opportunity for the antibody to bind to available antigens. Third, Mello et al. used sagittal sections to examine many brain areas. Our study used only coronal sections. The orientation of fibers (cross-sections versus tracts) may also have contributed to the observed differences in both fiber density and innervation. Finally, all males in our study were housed with females to maximize neurochemical differences between treatment groups. These males were given the opportunity to court and mate with females for ten days prior to sacrifice. Since our prior studies suggested that high levels of singing behavior were correlated with high levels of NE in the motor pathway controlling singing [11], we expected that housing with females would increase levels of DBH in the saline-treated males, maximizing differences between treatment groups.
Although we previously found that levels of NE in NIf were higher than those in two other telencephalic vocal control nuclei, HVC and RA [10,11], NIf was not strongly labeled by DBH-ir in these brains, so we did not quantify labeling in this vocal control nucleus.
4.3. Mismatch between DBH-ir labeling and other noradrenergic markers in Area X
Despite the fact that we consistently found moderate to high levels of NE in Area X in previous studies [9,10,11,12,35], like Mello et al., [48] we found few if any DBH-ir fibers in Area X and the surrounding medial striatum. Area X is very large and highly visible making it easy to accurately microdissect. Although DBH labeling was very limited or absent within and around Area X, significant levels of α2-adrenergic receptors were found in Area X in both zebra finches [54] and starlings [5]. A recent study [17] documented the existence of projections from both the LoC and SCv to Area X in finches. The mismatch between the existence of these projections, high NE levels, and significant levels of α2 adrenergic receptors yet the lack of significant levels of DBH-ir fibers remains to be explained.
4.4. Comparison of DSP-4's effects across species
In saline-treated finches, the estimate of total LoC DBH-ir cell numbers (667 ± 30, mean ± SEM) were much lower than the 1300 reported in Japanese quail and in chickens [4,49] and the 2900 reported in albino rats [63]. Our estimates were based on counting every DBH-ir cell found in every third section through the LoC. While counting cells in every section is clearly preferable, these data should provide a reasonable estimate of LoC cell number. Interestingly, even though finches appear to have fewer noradrenergic neurons than rats, their NE levels are approximately ten times higher in comparable brain areas [9].
In birds and mammals, the telencephalon receives NE projections primarily from the LoC [40,43,44]. Because DSP-4's neurotoxic effects occur first at the axon terminals, then work their way back to the soma, researchers have argued that the decrease in the number of labeled-cell bodies suggests complete retrograde destruction of many noradrenergic neurons e.g., [29]. The DSP-4-induced loss of DBH-ir cell bodies in zebra finches was incredibly fast compared to the effects of DSP-4 on the LoC in rats in which it took up to six months after drug treatment to achieve a comparable magnitude of DBH-ir cell loss [30]. One possible explanation for the rapid loss of DBH-ir cells in this study compared to that seen in rats was that these finches were treated twice with the standard dose typically used in rat studies. In goldfish, this dose of DSP-4 significantly decreased DBH-ir fibers but had no effect on DBH-labeled cell bodies [64]. DSP-4's effect on noradrenergic neurons in rats was long lasting, but in goldfish the central noradrenergic innervation was restored 40 days after drug treatment. If the neurotoxin only affects axons and does not destroy cell bodies, then rapid axonal regeneration appears likely.
In rats, morphologically distinct cell populations have been identified within the LoC [32,63] that appeared to have different efferent targets [44]. Loughlin et al. [44] found that dorsal LoC cells sent their projections to the cortex, hippocampus and hypothalamus, while ventral LoC cells sent their projections to the cerebellum. To determine if there were regional differences in DSP-4's effects on LoC cells in finches, it was divided into dorsal and ventral regions, as was previously described by Swanson [63] in rats. In this study, all cells were classified based on their locations. This was the most reliable and objective way to categorize DBH-ir cells, especially between those in ventral LoC and SCv. There were fewer DBH-ir cells in the ventral than in the dorsal LoC. However, in terms of percent reduction, DBH-ir cells in both areas were decreased by 57−60% in DSP-4-treated males.
4.5. Variation in DSP-4's effects-Species, strains, methodological issues
When administered systemically in our previous studies, DSP-4 lowered noradrenergic function in telencephalic nuclei only [11], but when administered directly into the third ventricle, it significantly depleted NE in both telencephalic and hypothalamic nuclei [12]. The pattern of DSP-4-induced destruction of DBH-ir fibers in finches in the current study was very similar to that observed in Sprague-Dawley rats treated systemically with DSP-4 [26,27,28,29]. In this strain of rats, DSP-4 treatment depleted NE levels in cortical areas like the olfactory bulb, hippocampus and neocortex, but the hypothalamus was not significantly affected [42]. However, in Long-Evans rats, the same dose of DSP-4 did not significantly affect DBH-ir fibers anywhere in the brain [59]. Fornai et al. [25] also reported strain, species, and depletion pattern differences in the effects of DSP-4 administration without zimelidine pretreatment. In Japanese quail, systemic treatment of DSP-4 without zimelidine pretreatment significantly lowered NE levels in the hypothalamus [7]. Therefore, depending upon the route of administration, the number of drug treatments, and the use of zimelidine pretreatment, DSP-4 can target different populations of noradrenergic neurons.
In Sprague-Dawley rats, DSP-4's differential effects on noradrenergic fibers in the cortex versus the hypothalamus suggested that some neurons were more susceptible to the drug's neurotoxic effects. Moreover, DSP-4 treatment in rats did not destroy descending projections from the LT to the ventral horn of the spinal cord [46]. Since LoC projections were destroyed but the LT system was spared, it was suggested that the primary noradrenergic innervation of the hypothalamus originated from the LT system [42]. However, Loughlin et al., [44] found that some LoC neurons in rats projected to the hypothalamus. Both Zaczek et al., [67] and Fritschy & Grzanna [29] reported that cortical noradrenergic transport sites had a higher affinity for DSP-4 than hypothalamic sites. In rats, noradrenergic axons originating from the LoC were thinner than those from the LT system [27,50,67]. The structural and functional differences observed between LoC and LT projection neurons in rats may account for the differential effects of DSP-4 on central noradrenergic neurons in this species. However, this reported structural difference in the noradrenergic axons in different regions in rat brains was not observed in zebra finch brains.
In comparison to its effects in other species, DSP-4 treatment in finches significantly decreased DBH-ir cells in the SCv, which is part of the LT system. However, because the noradrenergic innervation in the hypothalamus was not significantly affected, this may indicate that in finches, the major source of NE to the hypothalamus originates from other NE-cell groups of the LT system. As mentioned previously, the SCv has recently been shown to project to at least one vocal control nucleus, Area X [17].
4.6. Conclusions
In conclusion, repeated DSP-4 treatment in male zebra finches caused striking reductions in the number of DBH-ir cell bodies within 20 days of the first treatment, a 60% reduction in the LoC and 80% in the SCv. In finches, unlike rats, DSP-4 destroyed a large proportion of cells in the SCv rather than affecting primarily the LoC. However, drug treatment in finches as in Sprague Dawley rats significantly decreased the noradrenergic innervation in both telencephalic and mesencephalic nuclei but left noradrenergic innervation in hypothalamic areas relatively unaffected. This study also found differential DBH labeling of several VCN compared to surrounding brain areas. This treatment paradigm, administering two systemic DSP-4 injections 10 days apart, should be a valuable tool for studying the importance of NE in modulating the vocal control system and singing behavior.
Acknowledgements
This research was supported by a fellowship and research funds from NIH Minority Biomedical Research Support Grant GM 08176, a MAGNET Dissertation Year Fellowship from the Graduate School, CUNY, a Mina S. Rees Dissertation Fellowship from the Graduate School, CUNY, a Robert L. Thompson Award and research grants from the Biopsychology and Behavioral Neuroscience Doctoral Program to SAW, NIH Grant MH 49431 to CFH and NIH Grant GM 60654 to Hunter College, and Research Centers in Minority Institutions Award RR 03037 from the NCRR, NIH, which supports the infrastructure of the Biopsychology and Behavioral Neuroscience Doctoral Program. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR/NIH. Dr. Sharon R. Barclay (deceased) made helpful suggestions for optimizing the immunocytochemistry. The authors thank Sandra Ann Rowe, Yelena Sorokina, Diane G. Coombs for assistance with the research, and Dr. Sarah Durand for assistance in preparing the photomicrographs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- 1.Al-Zahrani SSA, Al-Ruwaitea ASA, Ho M- Y, Bradshaw CM, Szabadi E. Destruction of central noradrenergic neurons with DSP4 impairs the acquistion of temporal discrimination but does not affect memory for duraton in a delayed conditional discrimination task. Psychopharm. 1997;130:166–73. doi: 10.1007/s002130050225. [DOI] [PubMed] [Google Scholar]
- 2.Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res. 2002;133:221–35. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 3.Archer T, Jonsson G, Ross SB. A parametric study of the effects of the noradrenaline neurotoxin DSP4 on avoidance acquisition and noradrenaline neurones in the CNS of the rat. Brit J Pharmacol. 1984;82:249–57. doi: 10.1111/j.1476-5381.1984.tb16465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailhache T, Balthazart J. The catecholaminergic system of the quail brain: Immunocytochemical studies of dopamine--hydroxylase and tyrosine hydroxylase. J Comp Neurol. 1993;329:230–56. doi: 10.1002/cne.903290206. [DOI] [PubMed] [Google Scholar]
- 5.Ball GF, Castro JM, Bernard DJ. Sex differences in the volume of avian song control nuclei: Comparative studies and the issue of brain nucleus delineation. Psychoneuroendocrinol. 1994;19:485–504. doi: 10.1016/0306-4530(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 6.Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): Implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31:128–48. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Balthazart J, Libioulle JM, Sante P. Stimulatory effects of the noradrenergic neurotoxin DSP4 on sexual behavior in male quail. Behav Proc. 1988;17:27–44. doi: 10.1016/0376-6357(88)90048-4. [DOI] [PubMed] [Google Scholar]
- 8.Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993;5:247–55. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 9.Barclay SR, Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: Steroid effects on brain monoamines. Brain Res. 1988;459:333–43. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- 10.Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–62. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- 11.Barclay SR, Harding CF, Waterman SA. Correlations between catecholamine levels and sexual behavior in male zebra finches. Pharmacol Biochem Behav. 1992;41:195–201. doi: 10.1016/0091-3057(92)90082-q. [DOI] [PubMed] [Google Scholar]
- 12.Barclay SR, Harding CF, Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol Biochem Behav. 1996;53:213–20. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 13.Baum MJ. Neuroendocrinology of sexual behavior in the male. In: Becker JB, Breedlove SM, Crews D, editors. Masachusetts: MIT Press; Behavioral Endocrinology: 1992. pp. 97–130. [Google Scholar]
- 14.Beltz BS, Burd GD. Immunocytochemical Techniques. Blackwell Scientific; Cambridge,MA: 1989. [Google Scholar]
- 15.Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. J Neurobiol. 1997;33:495–500. [PubMed] [Google Scholar]
- 16.Carter CS. Neuroendocrinology of sexual behavior in the female. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral Endocrinology. MIT Press; Massachusetts: 1992. pp. 71–95. [Google Scholar]
- 17.Castelino CB, Diekamp B, Ball GF. Noradrenergic projections to the song control nucleus area × of the medial striatum in male zebra finches (Taeniopygia guttata) J Comp Neurol. 2007;502:544–62. doi: 10.1002/cne.21337. [DOI] [PubMed] [Google Scholar]
- 18.Cornwell CA, Chang JW, Cole B, Fukada Y, Gianulli T, Rathbone EA, McFarlane H, McGaugh JL. DSP-4 treatment influences olfactory preferences of developing rats. Brain Res. 1996;711:26–33. doi: 10.1016/0006-8993(95)01327-x. [DOI] [PubMed] [Google Scholar]
- 19.Cornwell-Jones CA. DSP4, a noradrenergic neurotoxin, impairs male rats' attraction to conspecific odors. Behav Neural Biol. 1988;50:1–15. doi: 10.1016/s0163-1047(88)90720-0. [DOI] [PubMed] [Google Scholar]
- 20.Cornwell-Jones CA, Decker MW, Gianulli T, Wright EL, McGaugh JL. Norepinephrine depletion reduces the effects of social and olfactory experience. Brain Res Bull. 1990b;25:643–9. doi: 10.1016/0361-9230(90)90038-2. [DOI] [PubMed] [Google Scholar]
- 21.Cornwell-Jones C, Palfai T, Young T, Desai J, Krasenbaum D, Morrison J. Impaired hoarding and olfactory learning in DSP-4-treated rats and control cagemates. Pharmacol Biochem Behav. 1990;36:707–11. doi: 10.1016/0091-3057(90)90064-o. [DOI] [PubMed] [Google Scholar]
- 22.Cote SL, Ribeiro-DaSilva A, Cuello AC. Current protocols for light microscopy immunocytochemistry. In: Cuello AC, editor. Immunohistochemistry II. John Wiley and Sons Ltd.; New York: 1993. [Google Scholar]
- 23.Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 2006;26:9860–72. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillenz M. Noradrenergic Neurons. Cambridge University Press; New York: 1990. p. 238. [Google Scholar]
- 25.Fornai F, Bassi L, Torracca MT, Alessandri MG, Scalori V, Corsini GU. Region- and neurotransmitter-dependent species and strain differences in DSP-4-induced monoamine depletion in rodents. Neurodegeneration. 1996;5:241–9. doi: 10.1006/neur.1996.0032. [DOI] [PubMed] [Google Scholar]
- 26.Fritschy JM, Geffard M, Grzanna R. The response of noradrenergic axons to systemically administered DSP-4 in the rat: An immunohistochamical study using antibodies to noradrenaline and dopamine-β-hydroxylase. J Chem Neuroanat. 1990;3:309–21. [PubMed] [Google Scholar]
- 27.Fritschy JM, Grzanna R. Immunohistochemical analysis of the neurotoxic effects of DSP-4 identifies two populations of noradrenergic axon terminals. Neurosci. 1989;30:181–97. doi: 10.1016/0306-4522(89)90364-3. [DOI] [PubMed] [Google Scholar]
- 28.Fritschy J- M, Grzanna R. Experimentally-induced neuron loss in the locus coeruleus of rats. Exp Neurol. 1991;111:123–7. doi: 10.1016/0014-4886(91)90058-k. [DOI] [PubMed] [Google Scholar]
- 29.Fritschy J- M, Grzanna R. Selective effects of DSP-4 on locus coeruleus axons: Are there pharmacologically different types of noradrenergic axons in the central nervous system? Prog Brain Res. 1991;88:257–68. doi: 10.1016/s0079-6123(08)63815-7. [DOI] [PubMed] [Google Scholar]
- 30.Fritschy J- M, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: Response to neurotoxin-induced cell death in the adult rat brain. J Comp Neurol. 1992;321:421–41. doi: 10.1002/cne.903210309. [DOI] [PubMed] [Google Scholar]
- 31.Griffin MG, Taylor GT. Norepinephrine modulation of social memory: Evidence for a time-dependent functional recovery of behavior. Behav Neurosci. 1995;109:466–73. doi: 10.1037//0735-7044.109.3.466. [DOI] [PubMed] [Google Scholar]
- 32.Grzanna R, Molliver ME. The locus coeruleus in the rat: An immunohistochemical delineation. Neurosci. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- 33.Hallman H, Jonsson G. Pharmacological modifications of the neurotoxic action of the noradrenaline neurotoxin DSP4 on central noradrenaline neurons. Eur J Pharmacol. 1984;103:269–78. doi: 10.1016/0014-2999(84)90487-4. [DOI] [PubMed] [Google Scholar]
- 34.Hallman H, Sundstrom E, Jonsson G. Effects of the noradrenaline neurotoxin DSP4 on monoamine neurons and their transmitter turnover in rat CNS. J Neural Transm. 1984;60:89–102. doi: 10.1007/BF01245027. [DOI] [PubMed] [Google Scholar]
- 35.Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol. 1998;34:329–46. [PubMed] [Google Scholar]
- 36.Ho MY, Velazquez Martinez DN, Lopez Cabrera M, Al-Zahrani SS, Bradshaw CM, Szabadi E. Retarded acquisition of a temporal discrimination following destruction of noradrenergic neurones by systemic treatment with DSP4. Psychopharm. 1995;118:332–7. doi: 10.1007/BF02245963. [DOI] [PubMed] [Google Scholar]
- 37.Jaim-Etcheverry G, Zieher LM. DSP-4: a novel compound with neurotoxic effects on noradrenergic neurons of adult and developing rats. Brain Res. 1980;188:513–23. doi: 10.1016/0006-8993(80)90049-9. [DOI] [PubMed] [Google Scholar]
- 38.Jennes L, Beckman WC, Stumpf WE, Grzanna R. Anatomical relationships of serotinergic and noradrenalinergic projections with the GnRH system in septum and hypothalamus. Exp Brain Res. 1982;46:331–8. doi: 10.1007/BF00238628. [DOI] [PubMed] [Google Scholar]
- 39.Jonsson G, Hallman H, Ponzio F, Ross S. DSP4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine) - A useful denervation tool for central and peripheral noradrenaline neurons. Eur J Pharmacol. 1981;72:173–88. doi: 10.1016/0014-2999(81)90272-7. [DOI] [PubMed] [Google Scholar]
- 40.Kitt CA, Brauth SE. Telencephalic projections from midbrain and isthmal cell groups in the pigeon. I. Locus coeruleus and subcoeruleus. J Comp Neurol. 1986;247:69–91. doi: 10.1002/cne.902470105. [DOI] [PubMed] [Google Scholar]
- 41.Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse. 2004;52:233–44. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- 42.Lookingland KJ, Chapin DS, McKay DW, Moore KE. Comparative effects of the neurotoxin N-chloroethyl-N-ethyl-2-bromobenzylamine hydrochloride (DSP4) and 6-hydroxydopamine on hypothalamic noradrenergic, dopaminergic and 5-hydroxytryptaminergic neurons in the male rat. Brain Res. 1986;365:228–34. doi: 10.1016/0006-8993(86)91633-1. [DOI] [PubMed] [Google Scholar]
- 43.Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: Topographic organization of cells of origin demonstrated by three-dimension reconstruction. Neurosci. 1986a;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 44.Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus: Morphologic subpopulations have different efferent targets. Neurosci. 1986b;18:307–19. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- 45.Lupo C, Beani C, Cervo R, Lodi L, Dessifulgheri F. Steroid hormones and reproductive history of grey partridge (Perdix perdix). BollZool. 1990;57:247–52. [Google Scholar]
- 46.Lyons WE, Fritschy J- M, Grzanna R. The noradrenergic neurotoxin DSP-4 eliminates the coeruleospinal projection but spares projections of the A5 and A7 groups to the ventral horn of the rat spinal cord. J Neurosci. 1989;9:1481–9. doi: 10.1523/JNEUROSCI.09-05-01481.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntosh TK, Barfield RJ. Brain monoaminergic control of male reproductive behavior. III. Norepinephrine and the post-ejaculatory refractory period. Behav Brain Res. 1984;12:275–81. doi: 10.1016/0166-4328(84)90153-0. [DOI] [PubMed] [Google Scholar]
- 48.Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol. 1998;400:207–28. [PubMed] [Google Scholar]
- 49.Moons L, D'Hondt E, Pijcke K, Vandesande F. Noradrenergic system in the chicken brain: Immunocytochemical study with antibodies to noradrenaline and dopamine--hydroxylase. J Comp Neurol. 1995;360:331–48. doi: 10.1002/cne.903600210. [DOI] [PubMed] [Google Scholar]
- 50.Moore RY, Card JP. Noradrenaline-containing neuron systems. In: Bjorklund A, Hokfelt T, editors. Classical Transmitters in the CNS. Elsevier; New York: 1984. pp. 123–56. [Google Scholar]
- 51.Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–57. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez VD, Feder HH, Sawyer CH. The Role of Brain Catecholamines in the regulation of LH secretion: A critical inquiry. In: Martini L, Ganong WR, editors. Frontiers in Neuroendocrinology. Raven Press; New York: 1984. pp. 27–84. [Google Scholar]
- 53.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Guturkun O, Lee DW, Mello C V, Powers A, White SA, Hough G, Kubikova L, Smulders T V, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riters L V, Ball GF. Sex differences in the densities of alpha 2-adrenergic receptors in the song control system, but not the medial preoptic nucleus in zebra finches. J Chem Neuroanat. 2002;23:269–77. doi: 10.1016/s0891-0618(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 55.Rodman HR, Karten HJ. Laminar distribution and sources of catecholaminergic input to the optic tectum of the pigeon (Columba livia). J Comp Neurol. 1995;359:424–42. doi: 10.1002/cne.903590306. [DOI] [PubMed] [Google Scholar]
- 56.Role LW, Kelly JP. The brain stem: Cranial nerve nuclei and the monoaminergic systems. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of Neural Science. Elsevier; New York: 1991. pp. 683–99. [Google Scholar]
- 57.Ross SB. Long-term effects of N-2-chloroethyl-N-ethyl-2-bromobenzylamine hydrochloride on noradrenergic neurons in the rat brain and heart. Brit J Pharmacol. 1976;58:521–7. doi: 10.1111/j.1476-5381.1976.tb08619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ross SB, Renyi A. On the long-lasting inhibitory effect of N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP4) on the active uptake of noradrenaline. J Pharm Pharmacol. 1976;28:458–9. doi: 10.1111/j.2042-7158.1976.tb04659.x. [DOI] [PubMed] [Google Scholar]
- 59.Schuerger RJ, Balaban CD. N-(2-Chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) has differential efficacy for causing central noradrenergic lesions in two different rat strains: comparison between Long-Evans and Sprague-Dawley rats. J Neurosci Methods. 1995;58:95–101. doi: 10.1016/0165-0270(94)00163-b. [DOI] [PubMed] [Google Scholar]
- 60.Steeves JD, Taccagna CA, Bell KA, Vincent SR. Distribution of phenylethanolamine-N-methyltransferase (PNMT)-immunoreactive neurons in the avian brain. Neurosci Lett. 1987;76:7–12. doi: 10.1016/0304-3940(87)90183-2. [DOI] [PubMed] [Google Scholar]
- 61.Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156:337–74. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Res Bull. 1994;35:467–72. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 63.Swanson LW. The locus coeruleus: A cytoarchitectonic, golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- 64.Villani L, Guarnieri T, Facchinetti F, Virgil M, Poli A. Neurotoxic effects of DSP-4 on the noradrenergic system of the goldfish brain. Brain Behav Evol. 1996;47:219–24. doi: 10.1159/000113242. [DOI] [PubMed] [Google Scholar]
- 65.Villani L, Guarnieri T, Facchinetti F, Virgili M, Poli A. Neurotoxic effects of DSP-4 on the noradrenergic system of the goldfish brain. Brain Behav Evol. 1996;47:219–24. doi: 10.1159/000113242. [DOI] [PubMed] [Google Scholar]
- 66.Yang SP, Voogt JL. Mating-activated brainstem catecholaminergic neurons in the female rat. Brain Res. 2001;894:159–66. doi: 10.1016/s0006-8993(01)01990-4. [DOI] [PubMed] [Google Scholar]
- 67.Zaczek R, Fritschy JM, Culp S, Desouza EB, Grzanna R. Differential effects of DSP-4 on noradrenaline axons in cerebral cortex and hypothalamus may reflect heterogeneity of noradrenaline uptake sites. Brain Res. 1990;522:308–14. doi: 10.1016/0006-8993(90)91474-u. [DOI] [PubMed] [Google Scholar]