Abstract
The results of blood or marrow transplantation in patients with chemorefractory aggressive lymphoma, i.e., those not responding to conventional-dose chemotherapy at the time of transplant, have been poor. The relapse rate has been high after autologous bone marrow transplant while allogeneic transplantation has been associated with excessive transplant-related toxicity. Administration of cyclosporine after autologous transplantation can induce an autoreactive syndrome that resembles graft-versus-host disease. This syndrome termed autologous graft-versus-host disease has clear antitumor activity in animal models that can be enhanced by the addition of cytokines such as γ-interferon and interleukin-2. A randomized, prospective study was conducted to evaluate the antitumor effect of autologous graft-versus-host disease induced with cyclosporine, and augmented by the administration of γ-interferon and interleukin-2 in patients with chemorefractory Hodgkin’s and aggressive non-Hodgkin’s lymphomas. Fifty-one patients were randomized, 24 to the autologous graft-versus-host disease induction arm, and 27 to the non-induction arm after autologous transplant using mobilized peripheral blood stem cell grafts. There were no differences in treatment-related mortality, overall and event-free survival between both groups; however, in the induction arm, graft-versus-host disease developed only in 4 patients. The administration of oral cyclosporine followed by interleukin-2 and γ-interferon is generally not well tolerated and does not appear to be an effective method to induce autologous graft-versus-host disease in patients receiving autologous peripheral blood stem cell grafts.
Keywords: Autologous graft-versus-host disease, Graft-versus-host disease, Lymphoma, Hodgkin lymphoma, Autologous stem cell transplant
Introduction
The use of high dose chemotherapy or chemo-radiotherapy with autologous stem cell rescue is a common strategy to treat patients with relapsed or refractory lymphoma. However, it is clear that this approach does not cure all patients especially those transplanted with chemorefractory disease, i.e., not responding to conventional-dose chemotherapy at the time of transplant (1–4). This has triggered an interest in evaluating the graft-versus-tumor (GVT) effect of allogeneic bone marrow transplantation (BMT) in these diseases in an attempt to exploit the immune response against the neoplastic cells (1;3;5–7). However, many patients will lack an HLA matched donor, and transplant-related toxicity has been high in chemoresistant lymphoma patients. Therefore, immunotherapy utilizing autologous graft-versus-host disease (GVHD) induction is an attractive approach for these high risk patients.
The administration of cyclosporine after an autologous peripheral blood stem cell or BMT can induce an autoimmune clinical syndrome that closely resembles acute GVHD in up to 80% of treated patients(8–11). This syndrome, autologous GVHD, is a mild, self-limited disease that generally involves only the skin. Histologic changes in the skin during autologous GVHD are identical to those of allogeneic GVHD. In rodent models, autologous GVHD induced by treatment with cyclosporine is mediated by autoreactive lymphocytes directed against class II histocompatibility (HLA-DR or Ia) antigens(12). These autoreactive lymphocytes also lyse MHC class II positive tumor cells in vivo. Tumor cell lysis was increased with gamma interferon (γ-IFN) and interleukin-2 (IL-2)(13;14). The mechanism of this effect appears to be different for the two cytokines: γ-IFN increases class II expression on the tumor thereby enhancing tumor cell recognition(13) while IL-2 augments the effector mechanisms(14–16). Since most hematopoietic malignancies express MHC class II antigens, autologous GVHD could potentially produce a clinical immunologic anti-tumor effect without significantly increasing post-transplant toxicity. Preliminary clinical studies also suggested that autologous GVHD might improve disease-free survival(17;18). To determine if this approach provides an anti-tumor benefit, a randomized prospective clinical trial was conducted comparing induction of autologous GVHD to standard therapy in patients with chemotherapy resistant aggressive lymphomas.
Patients, Materials and Methods
Study Group
All patients receiving autologous transplants for chemoresistant Hodgkin lymphoma (HL) or aggressive non- Hodgkin lymphoma (NHL) were eligible for inclusion in this study. Low-grade lymphoma, such as follicular grade 1 or 2 or monocytoid B cell lymphomas were excluded. Chemoresistant disease was defined as: 1) progressive disease developing during or within 6 weeks of completing initial induction therapy, or 2) failure to achieve at least an overall partial response (> at least a 50% reduction in tumor size assessing the products of the perpendicular diameters of all measurable lesions) to conventional salvage therapy following relapse. Patients required an adequate yield from mobilized peripheral blood harvest. To ensure patient safety, patients had to have adequate organ function (renal, cardiac and pulmonary and absence of fever) to participate in the trial post transplant.
Treatment
Peripheral blood stem cells were collected after mobilization with cyclophosphamide 2.5 g/m² and granulocyte-colony stimulating factor (G-CSF) (10µg/kg/day). Target yield of apheresis was >5×106 CD34 cells/kg with a minimum of 2×106 CD34 cells/kg. After collection, patients were randomized to the treatment arm or to observation. All patients received busulfan and cyclophosphamide or cyclophosphamide and total body irradiation preparative regimen (if there had been prior radiotherapy total body irradiation was not used). Patients assigned to the GVHD induction arm started cyclosporine (Neoral) at 2 mg/kg twice a day (IV formulation was given to those unable to take pills) starting on the day of the BMT and continued until γ-IFN and IL-2 were completed. γ-IFN started when the total white count was >200 cells/ml for 2 consecutive days post-transplant and it was given at a dose of 0.025 mg/m² subcutaneous every other day for 10 doses. The dose of IL-2 started two days later and the dose was 1×106 units/m² subcutaneous for 18 days. G-CSF was given after the transplant until WBC was 1,000 for 3 days, 10,000/mm3 on one occasion, or 5,000/mm3 on two occasions. Should the WBC drop to <1,000 on γ-IFN, G-CSF was restarted until the white count was consistently over 1,000.
If clinical Stage I GVHD(19) was diagnosed, cyclosporine, γ-IFN and IL-2 were discontinued. Clinical GVHD was confirmed by biopsy. GVHD was treated according to the standard practice at the Johns Hopkins Hospital at that time. Cyclosporine levels were not followed since previous studies revealed that a fixed dose of the drug was capable to induce autologous GvHD(8–12). Cyclosporine was adjusted only for renal failure according to the following schedule: creatinine >2.2 mg/dL, decrease dose by 25%; creatinine >3.0, decrease dose by 75%, and creatinine > 4.0; hold drug. The only dose modification of γ-IFN or IL-2 anticipated in this study was discontinuation of therapy. This was done if cyclosporine was discontinued because of GVHD or unexpected toxicity of γ-IFN and/or IL-2. IL-2 and γ-IFN could be held for 48 hours beyond their anticipated administration time point to assess whether a particular toxicity was related to these drugs.
Evaluation
Prior to returning home after the transplant, patients were assessed for response with computed tomography scans of chest, abdomen and pelvis, plus other sites of disease. Bone marrow biopsy in patients with previous bone marrow involvement and bone marrow aspirate for tumor marker studies in patients who had a known tumor marker pre-BMT were also preformed.
Statistical Design
Based on preliminary data, the study was designed to detect a 40% improvement in one-year disease free survival from 10% to 50% in patients randomized to receive autologous GVHD induction. Twenty-five patients per arm were required to detect this difference with 80% power using a two-sided 0.05 alpha level test. Even if the improvement were modestly less (i.e. 35%), this sample size would detect the difference with relatively high power. A 20% improvement in disease free survival was considered to be clinically important. However to detect a 20% improvement with the same power would require 75 patients in each arm. Such a study would require a cooperative group study rather than a single-institution trial.
Two analyses of the randomized trial were performed. The first was based on the intention to treat principle. It compared the outcome in all patients randomized to the intervention arm versus all patients randomized to the other arm. A second analysis of results was also performed. The “per protocol” analysis compared patients randomized to the treatment arm who actually received the treatment. They were compared to those in the control group who met eligibility criteria to receive IL-2 and γ-IFN. These consisted of screening for renal function, liver function, pulmonary function, and absence of fever.
The design of the study allowed for early termination (based on one interim analysis halfway through enrollment) due to evidence of engraftment failure, unexpectedly high transplant related mortality, or poor relapse-free survival. To terminate early for efficacy (as measured by disease-free survival), the interim p-value would have had to be less than or equal to 0.005.
Event-free survival (EFS) was compared between the two arms of the study using a competing risks analysis, where treatment-related mortality was considered a competing risk. Cumulative incidence of relapse at 12 months and their 95% confidence intervals, were calculated in addition to a p-value testing the difference in relapse between the two groups (20). The “per protocol” analysis was performed in the same manner. Kaplan-Meier methods were used for evaluating time to event (defined as relapse or death) and overall survival (OS). Incidence of early mortality (<60 days from randomization) was compared in the two arms using a Fisher’s exact test to compare proportions, and 95% confidence intervals were calculated using an exact binomial procedure. OS was defined as the time from study entry until death by any cause. EFS was defined as the time from study entry until relapse or death. OS was defined as the time from study entry until death by any cause.
Ethical Principles
The study was approved by the Johns Hopkins Institutional Review Board and all patients signed informed consent. A data safety monitoring committee supervised the study.
Results
Patients
Between 10/14/1997 and 4/16/2002, fifty-four patients were screened and fifty-one patients were enrolled. Twenty-four patients were randomized to the treatment arm and 27 to the control arm. Table 1 shows the characteristics of the groups.
Table 1.
Demographics.
| No GVHD arm (27) | GVHD arm (24) | |
|---|---|---|
| Median age | 41 | 42 |
| Females/Males | 11F/16M | 8F/16M |
| Ethnic | 26W/1Asian | 21W/3AA |
| Diagnosis: | ||
| DLBC NHL | 15 | 12 |
| HL | 10 | 6 |
| Anaplastic NHL | 0 | 2 |
| Angioimmunoblastic NHL | 0 | 2 |
| NK NHL | 1 | 0 |
| Burkitt's NHL | 1 | 0 |
| Hepatosplenic NHL | 0 | 1 |
| Follicular Grade 3 NHL | 0 | 1 |
| Conditioning regimen | 17 Bu-Cy/10 Cy-TBI | 15 Bu-Cy/9 Cy-TBI |
| Bone marrow involvement | 3 | 4 (1 unknown) |
| Failed to achieve a PR to salvage therapy following relapse | 14 | 12 |
| Progressive disease less than 6 weeks after completing induction | 13 | 12 |
Characteristics of patients enrolled in study. W: white, AA: African-American, DLBC: diffuse large cell, NHL: non-Hodgkin lymphoma, HL: Hodgkin lymphoma, XRT: radiation therapy, PR: partial response, Bu-Cy: busulfan and cyclophosphamide, Cy-TBI: cyclophosphamide and total body irradiation.
Autologous GVHD
Four patients (16%) in the treatment arm developed a clinically significant rash with biopsy proven GVHD. The first patient developed skin GVHD (stage 2, grade I) while on cyclosporine so did not receive γ-IFN and IL-2. The patient is in a complete remission 6 years after transplant. The second patient received 5 doses of γ-IFN and 6 doses of IL-2 and then developed autologous skin GVHD (stage 2, grade I), but succumbed to VOD. The third patient developed flu-like symptoms and skin GVHD (stage 2, grade I) while on γ-IFN and IL-2 so the drugs were discontinued. This patient died of relapsed lymphoma 6 years after transplant. The fourth patient developed skin GVHD (stage 2, grade I) while on cyclosporine so did not receive γ-IFN and IL-2. The patient died of acute respiratory distress syndrome. No GVHD was observed in the control arm.
Survival and Relapse Analysis
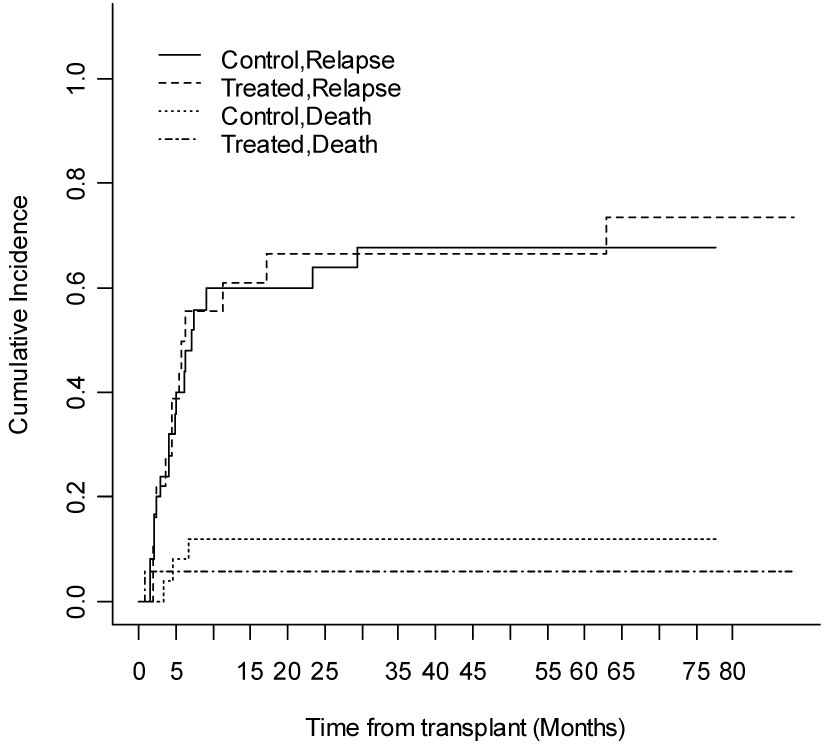
As of April 11, 2006 Thirty-four patients have died, 18/27 (66%) in the control arm and 16/24 (66%) in the GVHD induction arm. Based on a competing risks intent-to-treat analysis, the 12 month incidence of relapse in the control and treated groups are 63% and 50%, respectively (p=0.35) (Table 2). The cumulative incidence of relapse is shown in Figure 1. Treatment related mortality was 21% in the treatment group and 7% in the control group (p=0.23).
Table 2.
12 month cumulative incidence of relapse
| Estimate | 95% Confidence Interval | p-value* | ||
|---|---|---|---|---|
| Intention to treat | Control Group | 0.63 | (0.44, 0.82) | 0.35 |
| Treated Group | 0.50 | (0.29, 0.71) | ||
| “Per protocol” analysis | Control Group | 0.60 | (0.40, 0.80) | 0.84 |
| Treated Group | 0.61 | (0.37, 0.85) |
p-value testing the difference in survival curves
Figure 1.

Competing risks analysis of relapse and death, based on intention to treat analysis. (n=24 patients randomized to the treatment group, n=27 patients randomized to control group).
In the “per protocol” analysis, there are 18 patients in the treated group who completed treatment and they were compared to 25 patients in the control group who would have been eligible to begin the experimental treatment. The per protocol analysis showed no difference in the incidence of relapse as shown in Figure 2, with GVHD induction and control groups having 12 month incidence of relapse of 61% and 60% respectively (p=0.84) as described in Table 2. There were no differences in EFS (death or relapse) or OS between both groups by either analysis (“per protocol” or intent to treat).
Figure 2.
Competing risks analysis of relapse and death, based on “per protocol” analysis (n=18 patients in the treatment group, n=25 patients in the control group).
Adverse Events
Of the 24 patients who started cyclosporine, 6 did not begin IL-2 or γ-IFN: 1 because of renal failure, 1 due to renal failure and skin GVHD, 1 due to capillary leak syndrome, 1 due to skin GVHD, 1 due to veno-occlusive disease of the liver (VOD), and 1 because of VOD-renal failure. Eight patients stopped IL-2 and/or γ-IFN early: due to eosinophilia (15,390/mm³, n=1), flu-like symptoms (n=2), fever (n=1), capillary leak syndrome and renal failure (n=1), VOD and skin GVHD (n=1), mental status changes (n=1), and flu-like symptoms and skin GVHD (n=1). Ten patients tolerated the entire induction therapy (i.e. cyclosporine, γ-IFN and IL-2). Seven deaths occurred prior to day 60: 5 among patients randomized to autologous GVHD and 2 in the control group (p=0.23), however, only one of these patients received γ-IFN and IL-2. Four of the seven deaths were related to known preparative-regimen related toxicities (adult respiratory distress syndrome, interstitial pneumonitis, VOD/liver failure) while three patients died of progressive lymphoma.
Discussion
The group of patients with refractory disease included in the present trial historically has a very poor outcome. Philip et al. studied 100 such patients with intermediate-grade or high-grade NHL(21). Thirty-four percent had disease that had been refractory to primary chemotherapy, and 66 percent had had a complete remission with primary chemotherapy but later relapsed. After high-dose therapy and bone marrow transplantation, the actuarial three-year disease-free survival was zero in the refractory group, 14 percent in the resistant-relapse group, and 36 percent in the sensitive-relapse group. Even when post-transplant therapy may improve the outcome of these patients(4), the results are far from satisfactory. Our experience is similar with poor results in this group of patients. Aksentijevich et al. reported that patients with resistant diffuse large cell NHL at the time of BMT, only 12.5% and 19.1% of patients survived 3-years following allo-or auto-SCT, respectively (p=0.08)(3). Akpek et al. analyzed the outcome of 157 consecutive patients with relapsed or refractory HL, who underwent SCT between March 1985 and April 1998. Disease status before SCT (sensitive relapse if responding to conventional-dose therapy or resistant disease if not) was an independent predictor of EFS and relapse (p < 0.0001)(1).
GVHD is associated with a GVT as evidenced by a decreased relapse rate after allogeneic SCT (22). In animal models and exploratory clinical trials, autologous GVHD also appears to induce a GVT(10;11). Autologous GVHD has been observed using many “induction” regimens. Ratanatharathorn et al. reported on the use of cyclosporine and α-IFN in a small clinical trial(23). The study showed that this approach was feasible, with a majority of patients developing autologous GVHD after bone marrow transplantation BMT. Cyclosporine-induced autologous GVHD has been studied intensively by our group(8;10–12;14;24–27). From the early clinical studies, it was clear that the administration of cyclosporine could predictably induce skin GVHD after BMT(8;10;11). Jones et al. reported that 5/5 patients with lymphoma developed autologous GVHD after exposure to cyclosporine. Later on, patients with leukemia that received a nonpurged(11) or purged(10) SCT exposed to cyclosporine also developed autologous GVHD in high proportions (close to 80%). Vogelsang et al. reported a clinical trial on patients with hematologic malignancies receiving cyclosporine and IFN to induce autologous GVHD in patients receiving 4HC-purged marrow grafts(18). Treatment with cyclosporine and γ-IFN after BMT was well tolerated and did not impair engraftment. EFS with a median of 964 days of follow-up was 44%. Clinically significant GVHD was seen in 20% of cases.
In the current study, only 4 patients developed clinically apparent GVHD. This is in marked contrast to our previous autologous GVHD trials where the majority of patients developed clinical evidence of GVHD(5;8;18). In animal studies of autologous GVHD, immunologic effector cells and antitumor activity was seen only in those animals actually developing the syndrome(12;14). Thus, the lack of clinically significant antitumor effect in the current trial may have resulted from the inability to induce the syndrome in most patients. The relatively low rate of observed autologous GVHD in the present study may result from the use of mobilized peripheral blood instead of BM. Previous studies have also found a much lower incidence of autologous GVHD utilizing mobilized peripheral blood grafts rather than BM(17;26); this primarily appears to be the result of the infusion of a large number of T cells and monocytes that may downregulate the development of autologous GVHD(17;26;28;29). Mobilized peripheral blood contains approximately 10 times more T cells than do bone marrow grafts(30). Interestingly, animal studies suggest that the transfer of mature T cells along with the graft can modify the ability to induce autologous GVHD(31–33). Indeed, it has been reported that autologous GVHD will not occur unless T cells are removed from the peripheral blood graft (17;34). The use of oral cyclosporine (instead of parenteral) may also play an important role, as changes in bioavailability of the drug in the thymus may prevent the induction of auto-reactive T cells(35). Other factors such as the criteria for diagnosis of GVHD and the use of biopsies in patients without symptoms can also affect the expected frequency of GVHD (11;18;36;36–39). In the current study, patients had biopsies only when they developed clinical evidence of GVHD and strict criteria (standard at our institution) for the diagnosis of skin GVHD were followed (lymphocytic infiltrate with dyskeratosis). Certainly, it is possible that a GVT was present but not detected due to the sample size (see the Statistical Design section) or due to a “better than expected” outcome in the control arm(21). This study was designed to detect a 40% improvement in one-year disease free survival from 10% to 50% in patients randomized to receive autologous GVHD induction. The idea behind this ambitious goal was that if a large difference was obtained, we would be confident that the effect found was substantial, which would justify expanding rapidly into other patient groups. Conversely, if the effect was less clear (as it happened), a much larger patient population would be needed to detect any benefit. Further studies would require co-operative group trials as the one currently conducted by the Children’s Oncology Group that hopefully will help to clarify this issue
Figure 3.
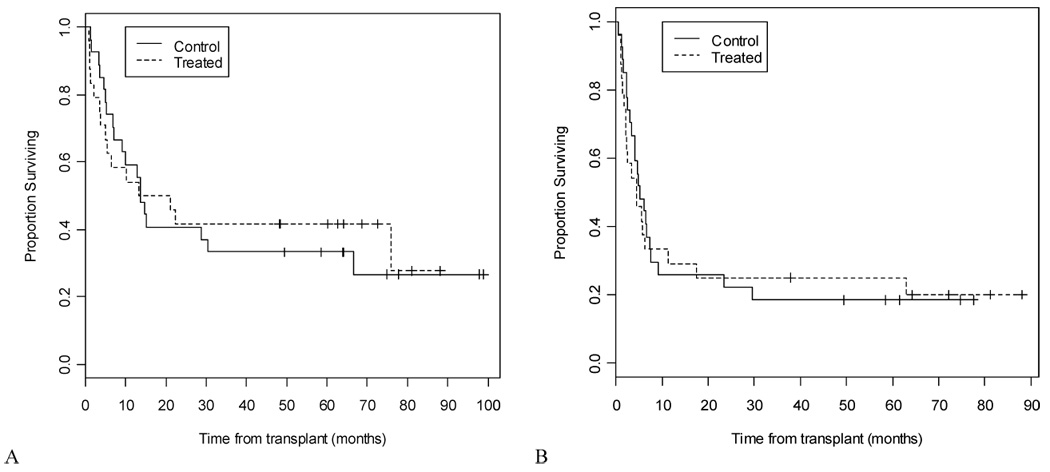
A) Overall survival (intention to treat analysis) and B) event-free (relapse or death) survival (intention to treat analysis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Akpek G, Ambinder RF, Piantadosi S, et al. Long-term results of blood and marrow transplantation for Hodgkin's lymphoma. J Clin Oncol. 2001;19(23):4314–4321. doi: 10.1200/JCO.2001.19.23.4314. [DOI] [PubMed] [Google Scholar]
- 2.Kasamon YL, Jones RJ, Diehl LF, et al. Outcomes of autologous and allogeneic blood or marrow transplantation for mantle cell lymphoma. Biol Blood Marrow Transplant. 2005;11(1):39–46. doi: 10.1016/j.bbmt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Aksentijevich I, Jones RJ, Ambinder RF, Garrett-Mayer E, Flinn IW. Autologous versus Allogeneic Blood and Marrow Transplantation for Relapsed Diffuse Large Cell non-Hodgkin's Lymphoma. Biol Blood Marrow Transplant. 2006;12(9):965–972. doi: 10.1016/j.bbmt.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Rapoport AP, Guo C, Badros A, et al. Autologous stem cell transplantation followed by consolidation chemotherapy for relapsed or refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2004;34(10):883–890. doi: 10.1038/sj.bmt.1704661. [DOI] [PubMed] [Google Scholar]
- 5.Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991;77(3):649–653. [PubMed] [Google Scholar]
- 6.van Besien K, Sobocinski KA, Rowlings PA, et al. Allogeneic bone marrow transplantation for low-grade lymphoma. Blood. 1998;92(5):1832–1836. [PubMed] [Google Scholar]
- 7.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31(8):667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 8.Jones RJ, Vogelsang GB, Hess AD, et al. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet. 1989;333(8641):754–757. doi: 10.1016/s0140-6736(89)92575-0. [DOI] [PubMed] [Google Scholar]
- 9.Vogelsang GB, Jones RJ, Hess AD, Geller R, Schucter L, Santos GW. Induction of autologous graft-versus-host disease. Transplant Proc. 1989;21(1 Pt 3):2997–2998. [PubMed] [Google Scholar]
- 10.Yeager AM, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Santos GW. Cyclosporine-induced graft-versus-host disease after autologous bone marrow transplantation for acute myeloid leukemia. Leuk Lymphoma. 1993;11(3–4):215–220. doi: 10.3109/10428199309086998. [DOI] [PubMed] [Google Scholar]
- 11.Yeager AM, Vogelsang GB, Jones RJ, et al. Induction of cutaneous graft-versus-host disease by administration of cyclosporine to patients undergoing autologous bone marrow transplantation for acute myeloid leukemia. Blood. 1992;79(11):3031–3035. [PubMed] [Google Scholar]
- 12.Hess AD, Horwitz L, Beschorner WE, Santos GW. Development of graft-vs.-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-Ia specificity, including autoreactivity. J Exp Med. 1985;161(4):718–730. doi: 10.1084/jem.161.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noga SJ, Horwitz L, Kim H, Laulis MK, Hess AD. Interferon-gamma potentiates the antitumor effect of cyclosporine-induced autoimmunity. J Hematother. 1992;1(1):75–84. doi: 10.1089/scd.1.1992.1.75. [DOI] [PubMed] [Google Scholar]
- 14.Geller RB, Esa AH, Beschorner WE, Frondoza CG, Santos GW, Hess AD. Successful in vitro graft-versus-tumor effect against an Ia-bearing tumor using cyclosporine-induced syngeneic graft-versus-host disease in the rat. Blood. 1989;74(3):1165–1171. [PubMed] [Google Scholar]
- 15.Soiffer RJ, Murray C, Cochran K, et al. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992;79(2):517–526. [PubMed] [Google Scholar]
- 16.Soiffer RJ, Murray C, Gonin R, Ritz J. Effect of low-dose interleukin-2 on disease relapse after T-cell-depleted allogeneic bone marrow transplantation. Blood. 1994;84(3):964–971. [PubMed] [Google Scholar]
- 17.Miura Y, Ueda M, Zeng W, et al. Induction of autologous graft-versus-host disease with cyclosporin A after peripheral blood stem cell transplantation: analysis of factors affecting induction. J Allergy Clin Immunol. 2000;106(1 Pt 2):S51–S57. doi: 10.1067/mai.2000.106832. [DOI] [PubMed] [Google Scholar]
- 18.Vogelsang G, Bitton R, Piantadosi S, et al. Immune modulation in autologous bone marrow transplantation: cyclosporine and gamma-interferon trial. Bone Marrow Transplant. 1999;24(6):637–640. doi: 10.1038/sj.bmt.1701942. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 20.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of A Competing Risk. Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 21.Philip T, Armitage JO, Spitzer G, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin's lymphoma. N Engl J Med. 1987;316(24):1493–1498. doi: 10.1056/NEJM198706113162401. [DOI] [PubMed] [Google Scholar]
- 22.Bolaños-Meade J, Vogelsang GB. Acute graft-versus-host disease. Clinical Advances in Hematology & Oncology. 2004;2(10):672–682. [PubMed] [Google Scholar]
- 23.Ratanatharathorn V, Uberti J, Karanes C, et al. Phase I study of alpha-interferon augmentation of cyclosporine-induced graft versus host disease in recipients of autologous bone marrow transplantation. Bone Marrow Transplant. 1994;13(5):625–630. [PubMed] [Google Scholar]
- 24.Wu JM, Bensen-Kennedy D, Miura Y, et al. The effects of interleukin 10 and interferon gamma cytokine gene polymorphisms on survival after autologous bone marrow transplantation for patients with breast cancer. Biol Blood Marrow Transplant. 2005;11(6):455–464. doi: 10.1016/j.bbmt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Miura Y, Thoburn CJ, Bright EC, et al. Characterization of the T-cell repertoire in autologous graft-versus-host disease (GVHD): evidence for the involvement of antigen-driven T-cell response in the development of autologous GVHD. Blood. 2001;98(3):868–876. doi: 10.1182/blood.v98.3.868. [DOI] [PubMed] [Google Scholar]
- 26.Miura Y, Ueda M, Takami A, Shiobara S, Nakao S, Hess AD. Enhancement of cyclosporine A-induced autologous graft-versus-host disease after peripheral blood stem cell transplantation by utilizing selected CD34(+) cells. Bone Marrow Transplant. 2003;32(8):785–790. doi: 10.1038/sj.bmt.1704208. [DOI] [PubMed] [Google Scholar]
- 27.Miura Y, Thoburn CJ, Bright EC, Chen W, Nakao S, Hess AD. Cytokine and chemokine profiles in autologous graft-versus-host disease (GVHD): interleukin 10 and interferon gamma may be critical mediators for the development of autologous GVHD. Blood. 2002;100(7):2650–2658. doi: 10.1182/blood-2002-01-0176. [DOI] [PubMed] [Google Scholar]
- 28.Vela-Ojeda J, García-Ruiz Esparza MA, Reyes-Maldonado E, et al. Peripheral blood mobilization of different lymphocyte and dendritic cell subsets with the use of intermediate doses of G-CSF in patients with non-Hodgkin's lymphoma and multiple myeloma. Ann Hematol. 2006;85(5):308–314. doi: 10.1007/s00277-006-0090-8. [DOI] [PubMed] [Google Scholar]
- 29.Condomines M, Quittet P, Lu ZY, et al. Functional Regulatory T Cells Are Collected in Stem Cell Autografts by Mobilization with High-Dose Cyclophosphamide and Granulocyte Colony-Stimulating Factor. J Immunol. 2006;176(11):6631–6639. doi: 10.4049/jimmunol.176.11.6631. [DOI] [PubMed] [Google Scholar]
- 30.Kusnierz-Glaz CR, Still BJ, Amano M, et al. Granulocyte colony-stimulating factor-induced comobilization of CD4- CD8- T cells and hematopoietic progenitor cells (CD34+) in the blood of normal donors. Blood. 1997;89(7):2586–2595. [PubMed] [Google Scholar]
- 31.Fischer AC, Hess AD. Age-related factors in cyclosporine-induced syngeneic graft-versus-host disease: regulatory role of marrow-derived T lymphocytes. J Exp Med. 1990;172(1):85–94. doi: 10.1084/jem.172.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer AC, Laulis MK, Horwitz L, Beschorner WE, Hess A. Host resistance to cyclosporine induced syngeneic graft-versus-host disease Requirement for two distinct lymphocyte subsets. J Immunol. 1989;143(3):827–832. [PubMed] [Google Scholar]
- 33.Wu DY, Goldschneider I. Cyclosporin A-induced autologous graft-versus-host disease: a prototypical model of autoimmunity and active (dominant) tolerance coordinately induced by recent thymic emigrants. J Immunol. 1999;162(11):6926–6933. [PubMed] [Google Scholar]
- 34.Sica S, Chiusolo P, Salutari P, et al. Autologous graft-versus-host disease after CD34+- purified autologous peripheral blood progenitor cell transplantation. J Hematother Stem Cell Res. 2000;9(3):375–379. doi: 10.1089/15258160050079489. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins MK, Schwartz RH, Pardoll DM. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- 36.van der Wall E, Horn T, Bright E, et al. Autologous graft-versus-host disease induction in advanced breast cancer: role of peripheral blood progenitor cells. Br J Cancer. 2000;83(11):1405–1411. doi: 10.1054/bjoc.2000.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giralt S, Weber D, Colome M, et al. Phase I trial of cyclosporine-induced autologous graft-versus-host disease in patients with multiple myeloma undergoing high-dose chemotherapy with autologous stem-cell rescue. J Clin Oncol. 1997;15(2):667–673. doi: 10.1200/JCO.1997.15.2.667. [DOI] [PubMed] [Google Scholar]
- 38.Streetly M, Kazmi M, Radia D, Hoyle C, Schey SA. Second autologous transplant with cyclosporin/interferon alpha-induced graft versus host disease for patients who have failed first-line consolidation. Bone Marrow Transplant. 2004;33(11):1131–1135. doi: 10.1038/sj.bmt.1704484. [DOI] [PubMed] [Google Scholar]
- 39.Marín GH, Menna ME, Bergna MI, et al. Induction of anti-tumor activity following autologous stem cell transplantation: immunotherapeutic implications. Transplant Proc. 2001;33(1–2):2004–2007. doi: 10.1016/s0041-1345(00)02769-x. [DOI] [PubMed] [Google Scholar]


