Abstract
The reproducibility of macular pigment optical density (MPOD) estimates in the elderly was assessed in 40 subjects (age: 79.1 ± 3.5). Test–retest variability was good (Pearson’s r coefficient: 0.734), with an average coefficient of variation (CV) of 18.4% and an intraclass correlation coefficient (ICC) of 0.96. The effect of optical blur on MPOD estimates was investigated in 22 elderly pseudophakic subjects (age: 79.9 ± 3.6) by comparing the baseline MPOD, obtained with an optimal correction, with MPODs obtained with a ±1.00-diopter optical blur. This optical blur did not cause differences in the MPOD estimates, its accuracy, or test duration.
Keywords: Macular pigment optical density, Heterochromatic flicker photometry, Reliability, Optical blur, Aging
1. Introduction
The determination of macular pigment optical density (MPOD) has been the object of increasing interest in the last decade and several methods to estimate it have been developed (Berendschot & van Norren, 2004; Bernstein, Zhao, Sharifzadeh, Ermakov, & Gellermann, 2004; Bone & Landrum, 2004; Davies & Morland, 2004; Delori, 2004; Moreland, 2004). The method that relies on the principle of heterochromatic flicker photometry (HFP) provides a psychophysical estimate of MPOD (Bone & Landrum, 2004; Snodderly & Hammond, 1999), and has emerged as the most widely utilized to date. HFP-based estimates of MPOD have been used as a method to understand the biological determinants of the retinal contents in the macular pigments, lutein and zeaxanthin (Beatty et al., 2001; Bone & Landrum, 2004; Hammond, Wooten, & Snodderly, 1998; Snodderly et al., 2004), and have the potential to develop into an outcome measure in longitudinal observational and interventional studies of macular aging and degeneration.
Validity and reliability are two fundamental properties of any measurement method. In the absence of an alternative gold-standard method for MPOD measurements, it remains a matter of debate which of the various MPOD measurement methods that have been thus far developed may be the best or the most accurate (Bernstein & Gellermann, 2003a, 2003b; Wooten & Hammond, 2003a, 2003b; Berendschot & van Norren, 2004; Berendschot & van Norren, 2005; Bernstein et al., 2004; Bone & Landrum, 2004; Davies & Morland, 2004; Delori, 2004; Moreland, 2004). The spectral properties of the macular pigments, though, are well known and various studies on the validity of the HFP-based determinations based on responses at different wavelengths have been published (Bone, Landrum, & Cains, 1992; Bone & Landrum, 2004; Snodderly, Brown, Delori, & Auran, 1984; Snodderly & Hammond, 1999; Snodderly et al., 2004; Wooten, Hammond, Land, & Snodderly, 1999). Likewise, studies of dietary lutein supplementation have documented its effect on MPOD in both normal (Berendschot et al., 2000; Hammond et al., 1997; Johnson et al., 2000; Koh et al., 2004; Landrum et al., 1997) and diseased eyes (Aleman et al., 2001; Duncan et al., 2002; Koh et al., 2004), further attesting to its validity. Therefore, there is presently little doubt that this method provides a genuine estimate of MPOD.
On the other hand, the test–retest reliability of MPOD psychophysical determinations, i.e., the ascertainment of the reproducibility of a given measurement on the same subject at two distinct points in time, has not been equally investigated. Studies that have formally characterized test–retest reliability in the elderly are particularly limited in number. To the best of our knowledge, the only study that addressed this issue specifically is that of Snodderly et al. (2004), which showed high reproducibility in women between the age of 50 and 79. We recently completed a cross-sectional study on a large biracial sample of elderly subjects from the Memphis metropolitan area participating in the Age-Related Maculopathy Ancillary (ARMA) Study, most of whom were also participants in the prospective Health, Aging, and Body Composition (Health ABC) Study. The strategies that have been utilized to develop a simplified testing protocol more suitable for utilization of the HFP-based method in the elderly and information on the MPOD in this population sample have been recently published (Iannaccone et al., 2007). Here, we present our test–retest reliability data on a sample of the ARMA Study participants over a decade older than in any previous assessment. This is particularly relevant because of the very high occurrence of ARM in this age range (Friedman et al., 2004).
In addition to ARM, also cataract and pseudophakia are highly prevalent among the elderly. By age 75, it has been estimated that over 50% of all Americans will develop cataracts (Congdon et al., 2004). Likewise, presbyopia is another phenomenon that is well known to occur with aging (Croft, Glasser, & Kaufman, 2001; Glasser, Croft, & Kaufman, 2001; Koretz, Kaufman, Neider, & Goeckner, 1989; Krag & Andreassen, 2003; Strenk, Strenk, & Koretz, 2005). While the use of multifocal and pseudo-accommodating intraocular lenses (IOLs) is on the rise, traditional monofocal IOLs are still widely used and highly prevalent in the population, invariably requiring post-operative spectacle correction to focus sharply at near. It has already been shown that MPOD can be reliably estimated in elderly subjects despite dense cataracts, and that subsequent IOL implantation does not lead to MPOD estimates different than baseline ones (Ciulla, Hammond, Yung, & Pratt, 2001). However, to the best of our knowledge, no formal studies of the effects of optical blur on MPOD determinations have been conducted, and specifically not in subjects post-IOL implantation, to understand if pseudophakic subjects may require particular precision in spectacle correction at near for more reliable and/or accurate MPOD testing. Preliminary results of this work have been reported in poster format (Gallaher et al., 2005; Iannaccone et al., 2005).
2. Methods
2.1. Study population
The ARMA Study focused primarily on a sample of participants in the Health ABC study, which consists of a biracial cohort of over 3000 highly functional elderly individuals 70 years old or older at study inception. Health ABC is being conducted at two US sites, Memphis, TN, and Pittsburgh, PA. Our study included a large biracial sample of men and women from the Memphis cohort (n = 340), on average 79 years old. A sample of the ARMA study participants took part in this sub-study. Other details about the ARMA Study population have been provided elsewhere (Iannaccone et al., 2007). All procedures conformed to the Declaration of Helsinki and the study was approved by the Institutional Review Board of the University of Tennessee Health Science Center.
2.2. General MPOD measurement methodology
A commercially available HFP instrument (Macular Metrics Corp., Rehoboth, MA) based on the one developed by, and reported in Wooten et al. (1999), was used to measure the MPOD in our study. Only the 0.5-deg eccentricity target was used in this study for foveal determinations. Further details about our simplified testing protocol and its specific features have been recently published (Iannaccone et al., 2007). During the test, all subjects wore their best correction. The instrument has a default +1.50 D lens, mounted in front of the chin- and headrest, through which subjects observe the test targets. Typically, subjects with intact accommodation at near will not require any additional correction to see the test targets sharply, and we verified this to be the case in a small pre-study assessment sample of younger subjects (unpublished data). However, several of these younger participants reported a subjective impression of increased uncertainty in the identification of the limits of the no-flicker zone, especially with the +1.00 D blurring lens. Therefore, we reasoned that in elderly individuals, and especially pseudophakic ones, the default correction may have not always allowed the sharpest possible perception of the test targets. This could potentially result in suboptimal sharpness of the test targets, increased difficulties in some subjects with the test task, and possibly increased test duration and/or variability. Hence, to make sure that blurry perception of the targets did not jeopardize the outcome of our estimates, we determined systematically the correction in addition to the default +1.50 D one, if any, that allowed participants to detect the edges of a ring test target (1.0-deg eccentricity) the sharpest, and performed MPOD estimations throughout the study with any such supplemental correction in place. These corrections were used as the baseline optimal correction towards which the optical blur study was conducted (see below).
With our simplified testing protocol (Iannaccone et al., 2007), we asked participants to identify the lower and the upper limits of the no-flicker (null) zone, which we termed the minimum and the maximum intensity values for the test target in question. The examiner then calculated the exact average of these two values as the middle of the no-flicker zone, and entered it on the subjects’ behalf. By using this protocol, we have shown that we could limit test repetitions to only three per target. Additional details have been reported elsewhere (Iannaccone et al., 2007).
2.3. Test–retest variability assessment
MPOD test–retest variability was assessed in a sample of 40 healthy subjects (age: 79.1 ± 3.5 years old; range: 69–84). Of these, 25 (63%) were females, 36 (90%) Caucasians, and 18 (45%) were on lutein-containing supplements. In order to gain insight into both short- to mid-term variability as well as long-term variability of the MPOD estimates, retests were performed over a wide range of inter-test time intervals, ranging from as little as one week to as much as approximately 20 months (<6 months, n = 22). Successful completion of a baseline MPOD testing session in at least one eye and self-reported absence of changes in vision, eye health or status (e.g., cataract surgery between sessions) in response to a custom-designed questionnaire administered by study staff were required to be eligible for this test–retest variability study. Prospective retest subjects were approached for retest subsequent to the baseline session (same day or later by phone). In addition to these criteria, which were met by all retest participants, subjects approached to return after ⩾8 weeks from baseline testing (n = 26) were eligible only if they had maintained the same dietary and drinking patterns, had not in the meantime started the use of lutein-containing supplements or changed the dosage thereof, and had not experienced significant general health-related events that could have otherwise modified their nutritional status and/or their ability to absorb carotenoids (e.g., cancer, or surgery of the gastrointestinal or biliary tract). This information was obtained from all subjects by study staff.
2.4. Optical blur assessment
The effect of optical blur was investigated in 22 healthy pseudophakic subjects (age: 79.9 ± 3.6 years old; range: 69–84), 15 of whom were females (68%), 20 Caucasians (91%), and seven (32%) lutein-containing supplement users since baseline. For this purpose, we compared the baseline MPOD, obtained with the optimal correction determined as described above, with MPODs obtained in the presence of a ±1.00 D optical blur. All participants were tested first with their optimal correction, and then adding a +1.00 D and a −1.00 D defocusing lens. All but four of these participants (82%) identified the default +1.50 D lens as the optimal one. The four participants who preferred supplemental correction required the addition of +0.50 D (n = 1) or +1.00 D (n = 3) spherical correction to achieve optimal perception of the ring test target. This is representative of both the proportion of subjects asking for supplemental correction and the amount of correction needed (never more than +1.25 D) throughout the main study (unpublished observation).
All subjects participating in this sub-study were retested on the same day. Therefore, to minimize confounding from a systematic learning effect on the estimate of the impact of the two types of the blurring experimental conditions, repeat testing with the defocusing lenses was performed with an alternate plus/minus and minus/plus lens order on every other participant, so that approximately half of the sample had the +1.00 D (n = 11) and the other half the −1.00 D lens (n = 10) used first, respectively. In order to determine if optical blur increased the variance around the mean of the MPOD estimates and therefore diminished its precision, the SD of each estimate (provided automatically by the instrument) was also used to perform comparisons across the three testing conditions. Lastly, to understand if optical blur had an effect on test duration, test duration was monitored and recorded at the end of each testing session and compared across conditions.
2.5. Statistical methods
To determine if there was a significant difference in baseline and retest MPOD measurements, a two-tailed paired Student’s t-test was used. To determine the level of correlation between the first and the second measure, the Pearson’s r coefficient of correlation was calculated. Lastly, to obtain an estimate of the within-subject variability between measurements, two measures were obtained (Armstrong, White, & Saracci, 1992): the coefficient of variation (CV), and the intraclass correlation coefficient (ICC), estimated via a one-way analysis of variance (ANOVA) for random effects. A low CV and a high ICC are indicative of high reproducibility—i.e., reliability—of the measurement in question (Armstrong et al., 1992). Independent of the CV, a high ICC is also expression of the effectiveness of a measure in discriminating between subjects (Armstrong et al., 1992). Lastly, to ensure that the repeatability of our test results fell within 2 SDs of the average difference in MPOD readings between sessions and to determine if any systematic trend could be detected across the range of measured MPODs, a Bland–Altman plot of the test–retest data was also generated (Bland & Altman, 1986). The question whether the length of the test–retest time interval or the baseline MPOD values correlated with increased variability in the test estimates was approached with a general linear model. Since neither the test–retest interval time nor the CV were normally distributed, they were transformed in their natural log values for these analyses.
To determine if there was an effect of optical blur on the MPOD estimates, on the variance of each estimate, or on test duration, results for the optical blur subgroup were compared across the three test conditions also with a one-way ANOVA. Lastly, to understand if repeated testing on a given day would lead to a systematic and appreciable learning effect that would result in a change in any of the three aforementioned variables despite the optical blur, we re-ranked the sessions by chronological order (baseline being always session no. 1) and re-analyzed the resulting values accordingly.
3. Results
3.1. Test–retest variability
In the 40 healthy elderly subjects in whom MPOD estimates were obtained on two distinct sessions, there was good test–retest correlation (Pearson’s r coefficient: 0.734). The average CV in this sample was 18.4%. The ICC was estimated to be 0.96. The baseline MPOD of the subjects who participated in this sub-study was representative of a wide range (0.06–0.87; mean ± SD: 0.41 ± 0.23). On average, MPOD at retest was virtually identical (0.42 ± 0.23) and the mean change compared to baseline was −0.01 ± 0.16 (p = 0.775 for paired Student’s t-test). Visual inspection of the data plotted in Fig. 1 shows that, in the majority of the subjects, MPOD values between sessions were within ±0.1 units of each other (dashed lines around the diagonal midline) and virtually all of them well within ±0.2 units, whereas two subjects were obvious outliers. Nine of the 40 participants had a CV >20%, whereas in all other cases the CV ranged from as little as 1.3 to as much as 18.1 (in 19 of the 40 subjects, CV <10%).
Fig. 1.
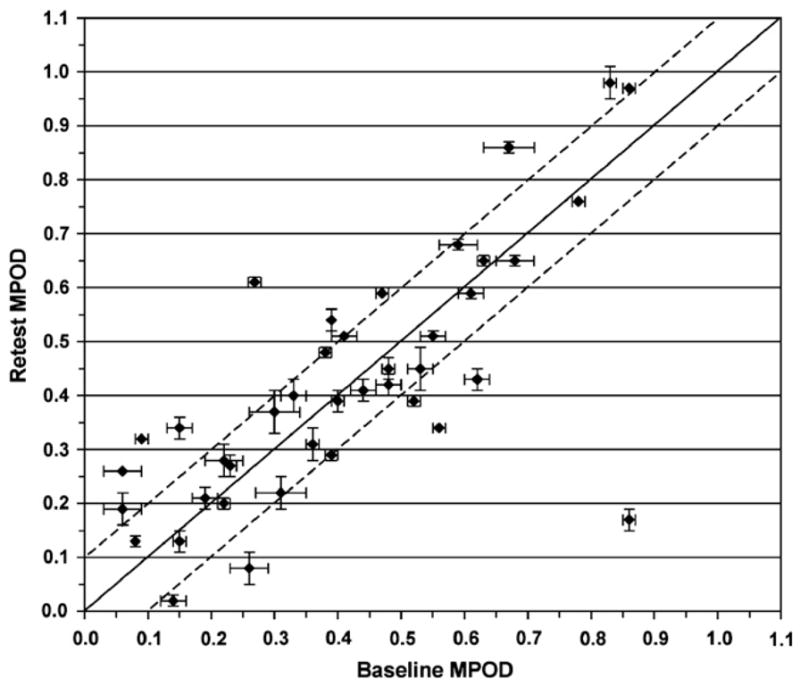
Scatter-plot of MPOD at baseline vs. MPOD at the retest session. Mean ± SD bars are shown for both sessions. The line of equality (solid line) is shown for reference. The two dashed lines identify the ±0.1 MPOD unit limit. Except for two subjects (the data point in the middle of the upper left-hand portion of the panel, and even more clearly, the one at the bottom right-hand corner of the plot), the vast majority of the subjects were with ±0.1 units from baseline and all others were within ±0.2 units.
The agreement between the baseline and the retest MPODs is illustrated in Fig. 2. Thirty-seven of 39 participants were well within the 2 SD limit. One subject was a clear outlier (same as in Fig. 1, bottom right-hand corner), while the other was right at the edge of the −2 SD limit. There was also no evidence of any systematic relationship between test–retest differences and the average of the measured MPOD values, or of any consistent bias.
Fig. 2.
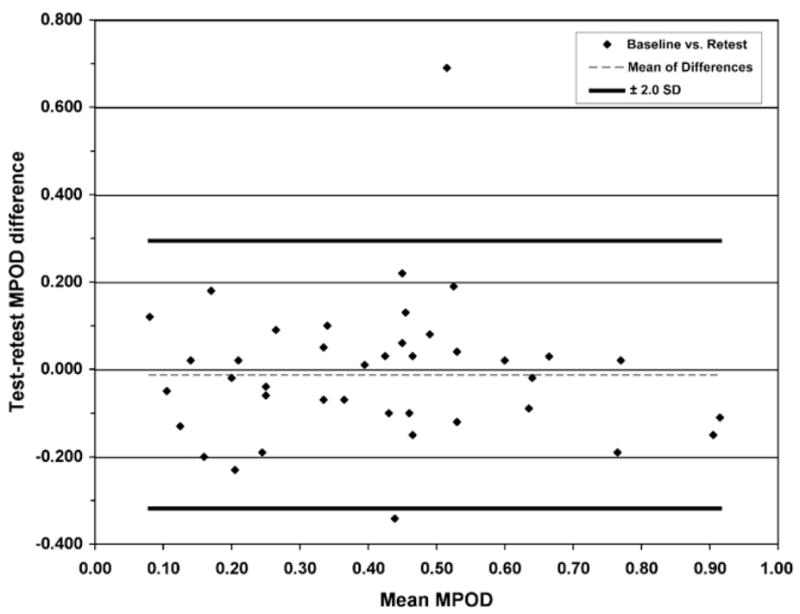
Bland–Altman plot of the difference between baseline and retest MPOD and the average of these measurements. The thick dark lines identify the ±2 SD limit around the mean difference between sessions (dashed thin line). Except for two subjects (top and bottom of the figure, same subjects as in Fig. 1), all subjects were within the ±2 SD limits, and no trends in the data suggestive of systematic relationships or biases across the measured MPOD range were apparent.
We performed supplemental post hoc analyses to understand the reasons for such marked variability in the nine subjects with a CV >20%. Older age was not an explanatory variable, since the mean age of the highly variable subgroup (79 years old) was virtually the same as the highly reproducible one (78.6 years old). Likewise, there were also no systematic differences in gender, race, or use of lutein-containing supplements among these nine subjects (data not shown). In some cases, individual plausible explanatory factors could be identified. For example, the one subject who is an obvious outlier in both Figs. 1 and 2 reported on the retest session marked difficulties in maintaining fixation away from the parafoveal reference target, resulting in an MPOD estimate far lower than baseline. The other outlying subject complained of back pain and had to re-adjust her sitting position multiple times during the retest session, while one additional subject with high CV was extremely talkative during both the baseline and the retest sessions. Both factors may have interfered with the accuracy of the estimates. Lastly, two subjects had clinically detectable macular RPE changes in the tested eye at baseline that did not qualify for any grade of clear-cut ARM (AREDS category 2) (Age-Related Eye Disease Study Research Group, 2001) and that were not associated with visual acuity changes. It is possible that the high variability observed in these two subjects may be expression of impending subclinical macular disease. With these exceptions, we could not document any other potential overt subject-dependent explanatory factor for the higher than average variability observed in those nine participants.
Test–retest time interval was not clearly related to increased variability. Although the mean time interval was somewhat higher in the highly variable subgroup, this difference was not significant (p = 0.816). Accordingly, test–retest time interval was not correlated to the CV in the group as a whole (Pearson’s r for the natural log of both variables = 0.054). When examining the behavior of MPOD estimates for subjects retested within 6 months from baseline (n = 23), the two sessions were more closely correlated (Pearson’s r: 0.829) than for subjects tested more than 6 months apart (Pearson’s r: 0.692; n = 17) and there was slightly more variability in the longer-term test–retest stratum (CV = 21% vs. 16%), although this difference was again not significant (p > 0.25 for both raw and ln-transformed data). The CV remained unrelated to test–retest time interval retested within 6 months from baseline (Pearson’s r = 0.030). Although not statistically significant (p = 0.09), the relationship between test–retest time interval and CV in the subjects retested more than 6 months apart was not in the expected direction. In this group, the former tended to be inversely related to the CV on a log–log scale (Pearson’s r: −0.438), i.e., the longer the test–retest time interval, the lower the variability. Therefore, this observation does not help explain in any plausible fashion the increased variability observed in those nine subjects. Lastly, there was also no systematic direction in MPOD change in the subjects with high CV that could suggest a specific trend in these subjects either, since MPOD at the retest session diminished in four of them and increased in five.
3.2. Effect of optical blur
The results of this sub-study are illustrated in Fig. 3. As for the test–retest sample, also the MPOD of the subjects who participated in this sub-study was representative of a wide range (0.02–0.98). The ±1 D optical blur did not cause differences in the MPOD estimates (0.46 ± 0.27 at baseline, 0.48 ± 0.27 after positive blur, and 0.46 ± 0.24 after negative blur, p > 0.5; Pearson’s r coefficients of 0.890 and 0.945 for positive and negative blur, respectively), nor did it affect test accuracy (MPOD variance: 5.6 × 10−4 at baseline, 4.9 × 10−4 with positive lens blur, and 6.8 × 10−4 with negative blur; p > 0.5 in all cases) or duration (about 15–17 min per eye under either condition; p > 0.05).
Fig. 3.
Optimal correction MPOD vs. optical blur. Panel (a) shows the scatter-plot of MPOD estimates obtained with an optimal correction vs. MPOD estimates obtained at the retest session in the presence of a +1.00 D optical blur. Panel (b) shows the same baseline data vs. MPOD estimates obtained at the retest session in the presence of a −1.00 D optical blur.
When we re-plotted the MPOD estimates in chronological order (i.e., from first test to last regardless of the type of blur, plots not shown), the between-session correlation remained very high (Pearson’s r coefficients of 0.930 and 0.901 for second and third session, respectively), but a tendency towards progressively shorter test durations despite the blur was seen (17.8 ± 5.9 min at baseline, 15.6 ± 5.5 for the second test, and 14.5 ± 4.1 for the third), suggestive of a possible learning effect. The difference in test duration between the first and last test sessions was of borderline significance (p = 0.042). There was also no between-session difference in the MPOD estimates (0.45 ± 0.26 at baseline, 0.46 ± 0.26 on the second test, and 0.47 ± 0.24 on the third) or on in their variance (5.9 × 10−4 at baseline, 6.4 × 10−4 with positive lens blur, and 5.3 × 10−4 with negative blur; p > 0.6 in all cases).
4. Discussion
To the best of our knowledge, to date the only study that has formally addressed the issue of MPOD short-term test–retest reliability in the elderly is that of Snodderly et al. (2004), who studied a group of 54 elderly, mainly Caucasian, highly educated women, on average 66 years old (range: 50–79) participating in CAREDS, an ancillary study to Women’s Health Initiative at the University of Wisconsin, Madison site. In this study, the between-session repeatability (CV) was, on average, between 17% and 22%. For the entire sample retested in our study, the mean CV was 18.4%, the ICC was very high, and very few subjects exhibited variability within ±2 SDs around the mean of the test–retest differences (Fig. 2). Therefore, our findings are nicely in agreement with those of Snodderly et al. (2004) and indicate that reproducible foveal MPOD measurements can be obtained in elderly subjects within a reasonably short testing time not only between 50 and 79 years of age (Snodderly et al., 2004), but also well into the 8th and 9th decade of life, when the risk of ARM is highest (Friedman et al., 2004) and MPOD estimates for the study of macular aging and degeneration are more likely to be used.
Possible reasons for high test–retest variability have been identified in our participants. For example, the subject whose second MPOD was most different from baseline (outlier in Figs. 1 and 2) experienced difficulties during the retest session with maintaining fixation away from the parafoveal target. Indeed, this aspect of the test was often reported (unpublished observation) as the most challenging of the tasks by several of the participants also in the main study (Iannaccone et al., 2007). This finding suggests that future studies should pay special attention to this procedural aspect of the test, and that further improvements in the testing methodology may help make MPOD testing an even more patient-friendly task for all ages.
Subclinical retinal changes of possible functional significance towards the MPOD estimates were also seen in two subjects, but these did not correlate with the direction of the change between sessions, nor would they correlate well with the physical location of the MPs in the retina (Snodderly et al., 1984). To our knowledge, there are also no published prospective studies of MPOD changes over time in elderly subjects with minimal retinal changes or with early ARM. Therefore, the significance of this observation, if any, is presently unknown.
The direction of change in the MPOD values was not correlated to increased test–retest variability. We cannot entirely exclude that the self-reported information provided by participants with high test–retest variability was incorrect and that, e.g., changes in ocular status, dietary patterns, general health, or use of lutein-containing supplements had in fact occurred between sessions in these subjects. Lastly, although all examiners were extensively trained and supervised in the course of the study, it cannot be entirely excluded that measurement error was introduced at either session by the examiners themselves. However, we have no evidence to believe that data inaccuracy or technical errors contributed to the observed variability, which is more likely to be, in this particular age range, intrinsic to the challenges posed to some participants by the test itself.
The results of our optical blur study also show that MPOD testing was insensitive to a ±1.00 D optical blur in pseudophakic subjects with respect to MPOD estimates, their variance, or the duration of the testing session. None of the subjects who participated in this sub-study required more than a +1.00 D correction in addition to the default +1.50 D lens of the instrument, and the vast majority did not require any supplemental correction. Therefore, provided that subjects are tested with their best correction at distance in place, there appears to be no compelling requirement for time-consuming supplemental correction at near beyond the default one. These results further extend the findings of Ciulla et al. that MPOD can be reliably measured in elderly subjects despite the presence of dense cataracts and that subsequent IOL implantation does not produce significant and/or systematic differences compared to baseline MPOD values (Ciulla et al., 2001).
In summary, our study provided evidence that reproducible MPOD estimates can be obtained not only in elderly women between the age of 50 and 79 years old (Snodderly et al., 2004), but also in elderly subjects of both genders ranging between 69 and 84 years in age. We also showed that MPOD remains fairly stable in elderly subjects not only in the immediate short-term but also over a relatively extended period of time, provided that no conditions developed or dietary/supplementation pattern were changed in the interim period. Lastly, we verified that MPOD estimates by HFP are a robust measure that is insensitive not only to the presence of lens opacities (Ciulla et al., 2001) but also to a ±1.00 D optical blur. On the aggregate, these findings position well HFP-based techniques for the estimation of MPOD for large-scale utilization also in the epidemiologic geriatric setting.
Acknowledgments
The authors gratefully acknowledge helpful comments and suggestions in the early stages of this study from Drs. Billy R. Wooten (Brown University, Providence, RI), B. Randy Hammond Jr. (Medical College of Georgia, Augusta, GA) and Julie A. Mares (University of Wisconsin, Madison, WI) and the helpful comments to this manuscript made by Dr. Ronald Shorr (UTHSC Dept. Preventive Medicine) and the Health ABC Publications Committee members; the assistance of the entire Health ABC staff at the Memphis study site; the technical assistance of Salwa M. Ahmed, Jeremy Racey, Deon Cistrunck, and Mary Lane; the assistance of Songmei Meng, Hongtao Zhai and Wentao Du with database creation and management; and the enthusiasm of all ARMA Study participants. Sources of Support: NEI grant K23 EY000409; NIA contracts N01 AG62101, N01 AG62103, N01 AG62106 and the Intramural NIA Research Program; International Retinal Research Foundation, Birmingham, AL and RPB, New York, NY (CDA to AI and unrestricted grant to the UTHSC Department of Ophthalmology). KG was the recipient of an intramural Medical Student Summer Research Fellowship, supported by training grant T35 DK07405 (UTHSC), and of an ARVO Foundation/Retina Research Foundation/Joseph M. and Eula C. Lawrence Travel Grant Award (ARVO 2005 meeting). TLH was the recipient of a Memphis McNair Program Summer Research Fellowship, supported by a grant from the US Department of Education P217A030229 to UTHSC. The MacularMetrics instrument utilized to measure macular pigment density in this study was purchased with the generous joint funding of Bausch & Lomb and Kemin Foods Corp.
References
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. American Journal of Ophthalmology. 2001;132(5):668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, Gardner LM, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Investigative Ophthalmology & Visual Science. 2001;42(8):1873–1881. [PubMed] [Google Scholar]
- Armstrong BK, White E, Saracci R. Monographs in epidemiology and biostatistics. Vol. 21. Oxford: Oxford University Press; 1992. Principles of exposure measurement in epidemiology; pp. 49–114. [Google Scholar]
- Beatty S, Murray IJ, Henson DB, Carden D, Koh HH, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a northern European population. Investigative Ophthalmology & Visual Science. 2001;42:439–446. [PubMed] [Google Scholar]
- Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Investigative Ophthalmology & Visual Science. 2000;41(11):3322–3326. [PubMed] [Google Scholar]
- Berendschot TT, van Norren D. Objective determination of the macular pigment optical density using fundus reflectance spectroscopy. Archives of Biochemistry and Biophysics. 2004;430(2):149–155. doi: 10.1016/j.abb.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Berendschot TT, van Norren D. On the age dependency of the macular pigment optical density. Experimental Eye Research. 2005;81(5):602–609. doi: 10.1016/j.exer.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Gellermann W. Assessment of the Raman method of measuring human macular pigment (e-letter) Investigative Ophthalmology & Visual Science. 2003a #x0005B;serial online]. Published: August 13, 2003. Available from: http://www.iovs.org/cgi/eletters/39/11/2003#74.
- Bernstein PS, Gellermann W. Assessment of the Raman method of measuring human macular pigment. II (e-letter) Investigative Ophthalmology & Visual Science. 2003b [serial online]. Published: November 17, 2003. Available from: http://www.iovs.org/cgi/eletters/39/11/2003#94.
- Bernstein PS, Zhao DY, Sharifzadeh M, Ermakov IV, Gellermann W. Resonance Raman measurement of macular carotenoids in the living human eye. Archives of Biochemistry and Biophysics. 2004;430(2):163–169. doi: 10.1016/j.abb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- Bone R, Landrum J, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Research. 1992;32(1):105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT. Heterochromatic flicker photometry. Archives of Biochemistry and Biophysics. 2004;430(2):137–142. doi: 10.1016/j.abb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Hammond BR, Jr, Yung CW, Pratt LM. Macular pigment optical density before and after cataract extraction. Investigative Ophthalmology & Visual Science. 2001;42(6):1338–1341. [PubMed] [Google Scholar]
- Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Archives of Ophthalmology. 2004;122(4):487–494. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- Croft MA, Glasser A, Kaufman PL. Accommodation and presbyopia. International Ophthalmology Clinics. 2001;41(2):33–46. doi: 10.1097/00004397-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Davies NP, Morland AB. Macular pigments: their characteristics and putative role. Progress in Retinal and Eye Research. 2004;23(5):533–559. doi: 10.1016/j.preteyeres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Delori FC. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Archives of Biochemistry and Biophysics. 2004;430(2):156–162. doi: 10.1016/j.abb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Duncan JL, Aleman TS, Gardner LM, De Castro E, Marks DA, Emmons JM, et al. Macular pigment and lutein supplementation in choroideremia. Experimental Eye Research. 2002;74(3):371–381. doi: 10.1006/exer.2001.1126. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Archives of Ophthalmology. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Gallaher K, Mura M, Todd WA, Kenyon E, Harris TL, Satterfield S, et al. Test–retest variability of macular pigment optical densitometry in the elderly: findings from the health ABC ARMA Study [ARVO Abstract] Investigative Ophthalmology & Visual Science. 2005;46(5):1570. E-Abstract. [Google Scholar]
- Glasser A, Croft MA, Kaufman PL. Aging of the human crystalline lens and presbyopia. International Ophthalmology Clinics. 2001;41(2):1–15. doi: 10.1097/00004397-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum K, Edwards RB, et al. Dietary modification of human macular pigment density. Investigative Ophthalmology & Visual Science. 1997;38(9):1795–1801. [PubMed] [Google Scholar]
- Hammond BR, Jr, Wooten BR, Snodderly DM. Preservation of visual sensitivity of older subjects: association with macular pigment density. Investigative Ophthalmology & Visual Science. 1998;39:397–406. [PubMed] [Google Scholar]
- Iannaccone A, Gallaher K, Mura M, Kenyon E, Harris TL, Todd WA, et al. Macular pigment optical densitometry in the elderly: findings in a large biracial mid-south sample and effect of optical blur in pseudophakic subjects [ARVO Abstract] Investigative Ophthalmology & Visual Science. 2005;46(5):1567. E-Abstract. [Google Scholar]
- Iannaccone A, Mura M, Gallaher K, Todd WA, Kenyon E, Harris TL, et al. Macular pigment optical density in the elderly. Findings in a large biracial mid-south sample. Investigative Ophthalmology & Visual Science. 2007;48(4):1458–1465. doi: 10.1167/iovs.06-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EJ, Hammond BR, Jr, Yeum KJ, Qin J, Wang XD, Casteneda C, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. American Journal of Clinical Nutrition. 2000;71(6):1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- Koh HH, Murray IJ, Nolan D, Carden D, Feather J, Beatty S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Experimental Eye Research. 2004;79(1):21–27. doi: 10.1016/j.exer.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye–aging of the anterior segment. Vision Research. 1989;29(12):1685–1692. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- Krag S, Andreassen TT. Mechanical properties of the human lens capsule. Progress in Retinal and Eye Research. 2003;22(6):749–767. doi: 10.1016/s1350-9462(03)00063-6. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Experimental Eye Research. 1997;65(1):57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- Moreland JD. Macular pigment assessment by motion photometry. Archives of Biochemistry and Biophysics. 2004;430(2):143–148. doi: 10.1016/j.abb.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investigative Ophthalmology & Visual Science. 1984;25(6):660–673. [PubMed] [Google Scholar]
- Snodderly DM, Hammond BR., Jr . In vivo psychophysical assessment of nutritional and environmental influences on human ocular tissues: lens and macular pigment. In: Taylor AJ, editor. Nutritional and environmental influences on the eye. Boca Raton, FL: CRC Press; 1999. pp. 251–273. [Google Scholar]
- Snodderly DM, Mares JA, Wooten BR, Oxton L, Gruber M, Ficek T. Macular pigment measurement by heterochromatic flicker photometry in older subjects: the Carotenoids and Age-Related Eye Disease Study. Investigative Ophthalmology & Visual Science. 2004;45(2):531–538. doi: 10.1167/iovs.03-0762. [DOI] [PubMed] [Google Scholar]
- Strenk SA, Strenk LM, Koretz JF. The mechanism of presbyopia. Progress in Retinal and Eye Research. 2005;24(3):379–393. doi: 10.1016/j.preteyeres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wooten BR, Hammond BR. Assessment of the Raman method of measuring human macular pigment (e-letter) Investigative Ophthalmology & Visual Science. 2003a [serial online]. Published: August 7, 2003. Available from: http://www.iovs.org/cgi/eletters/39/11/2003#73. [PubMed]
- Wooten BR, Hammond BR. Assessment of the Raman method of measuring human macular pigment. II (e-letter) Investigative Ophthalmology & Visual Science. 2003b [serial online]. Published: November 17, 2003. Available from: http://www.iovs.org/cgi/eletters/39/11/2003#92. [PubMed]
- Wooten BR, Hammond BR, Jr, Land RI, Snodderly MD. A practical method for measuring macular pigment optical density. Investigative Ophthalmology & Visual Science. 1999;40:2481–2489. [PubMed] [Google Scholar]



