Abstract
Objective
To study the effects of a short-term very-low calorie diet (VLCD) on intramyocellular lipid (IMCL), total body fat, and insulin sensitivity in a group of obese non-diabetic and Type 2 Diabetic (T2DM) patients.
Research Methods and Procedures
Seven untreated T2DM and 5 obese non-diabetic individuals were studied before and after a 6-day VLCD using proton-magnetic resonance spectroscopy to quantify IMCL, DXA to assess body fat, and hyperinsulinemic-euglycemic clamps to measure peripheral insulin sensitivity.
Results
In both groups, decrements in total body fat mass and BMI were small but statistically significant. In contrast, the diet resulted in a pronounced reduction in IMCL compared to baseline values in non-diabetics (56% decrease) and T2DM (40% decrease), P<0.05, and this was accompanied by an overall 9.3% increase in maximally-stimulated glucose disposal rate (P<0.01). IMCL was significantly correlated with insulin sensitivity, (r=−0.69; P<0.01) and waist circumference (r = 0.72 and 0.83, baseline and post-diet respectively, both P < 0.01), but neither IMCL nor insulin sensitivity was related to measures of general adiposity such as BMI, % body fat, or total body fat (P=NS).
Conclusions
Short-term VLCD is accompanied by small decrements in general adiposity, marked decrease in IMCL, and an increase in insulin sensitivity in non-diabetic and T2DM subjects. Therefore, rapid amelioration of insulin resistance by VLCD can be partially explained by loss of IMCL in both non-diabetics and in T2DM in the absence of substantial changes in total body fat. These observations are consistent with the idea that insulin resistance is more directly related to IMCL rather than body fat per se.
Keywords: body fat distribution, caloric restriction, metabolic syndrome
Introduction
Skeletal muscle insulin resistance is a key metabolic abnormality that characterizes Type 2 Diabetes (T2DM) and the Metabolic Syndrome, and elucidation of causal mechanisms is critical to development of effective therapy and prevention. Obesity is commonly evoked as a cause or contributor to insulin resistance despite the fact that generalized obesity can only explain a relatively small portion of individual variability in insulin sensitivity in cross sectional studies (1). Nevertheless, there is a strong epidemiological link between obesity and the development of T2DM, and moderate weight reduction has consistently been reported to improve insulin sensitivity. This paradox points to the possibility that other factors, perhaps associated with obesity, may play a more direct role in the pathogenesis of insulin resistance. For example, relative upper body fat distribution, alterations in adipokine secretion, or increased intramyocellular lipid (IMCL) may directly influence insulin sensitivity in a manner that is only partially or indirectly related to overall adiposity. In support of this hypothesis, obese insulin-resistant adolescents were found to have greater quantities of IMCL and visceral fat and lower adiponectin values compared with matched obese insulin sensitive counterparts, despite similar degrees of adiposity (2).
In recent years, new evidence has shed insights on the interrelationship involving IMCL and the development of insulin resistance associated with abnormalities in fatty acid metabolism. For example, healthy subjects showed a decrease in insulin sensitivity and an increase in IMCL following acute intralipid infusion (3, 4), and, conversely, long-term hypocaloric diets or malabsorption induced by biliopancreatic diversion were found to lead to enhanced insulin sensitivity and decreased IMCL (5–7). These observations have suggested a causal effect of IMCL on the development of insulin resistance. However, the link between IMCL and insulin resistance in T2DM is not so clear. Petersen et al (8) found that obese and insulin resistant T2DM patients had improved insulin sensitivity despite no significant decrease in IMCL after a moderate hypocaloric diet (1,200 kcal/day) over an average of 7 weeks. Tamura et al. (9) found that weight loss produced by a moderate hypocaloric diet alone (25 to 30 kcal/kg/day) over 2 weeks reversed hepatic steatosis but did not improve insulin sensitivity or affect IMCL. Thus, in these two studies involving T2DM patients, moderate hypocaloric diets per se did not significantly decrease IMCL over 2–7 weeks. However, there have been no studies examining whether short-term substantial caloric restriction would affect IMCL accumulation and insulin sensitivity in T2DM patients or in non-diabetics. The aim of the current study was to test whether a short-term very-low calorie diet (700 kcal/day for 6 days) would significantly decrease IMCL in both T2DM and non-diabetic subjects, and use this perturbation to study the interrelationships between IMCL, insulin sensitivity, and generalized adiposity.
Research Design and methods
Subject characteristics
We studied 12 (10 females, 2 males) overweight and obese subjects with and without T2DM (10), and the clinical characteristics of the study group are listed in Table 1. Mean HbA1c in T2DM was 8.6 ± 1.3. Prior to study, all patients with T2DM (n = 7) were being treated with diet or sulfonylurea and/or metformin, but were withdrawn from therapy for at least 3 weeks and followed on an outpatient basis. Mean baseline weight had to be stable (±3%) for at least 3 months before study, and none of the study subjects engaged in regular exercise. None of the volunteers had cardiovascular, renal, or hepatic disease, and all were chemically euthyroid. No subjects were ingesting any pharmacological agents known to affect carbohydrate homeostasis, lipids, or lipoprotein metabolism. Protocols were approved by the Institutional Review Board, and written informed consent was obtained from every subject.
TABLE 1.
Changes in body composition and metabolic parameters at baseline and after 6-day of very low calorie diet in the study groups
| Non-diabetics
(n = 5§, F) |
Type 2 diabetics
(n = 7§, 5F/2M) |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Age | 38 ± 12 | 43 ± 6 | ||
| BMI (kg/m2) | 36 ± 5 | 35 ± 5* | 37 ± 7 | 35 ± 7† |
| Fat mass (kg) | 44 ± 10 | 43 ± 10 | 45 ± 19 | 44 ± 18 |
| Lean body mass (kg) | 43 ± 4 | 42 ± 4 | 58 ± 10 | 56 ± 9 |
| % fat | 49 ± 5 | 49 ± 5 | 41 ± 11 | 41 ± 11 |
| Waist (cm) | 99 ± 10 | 96 ± 9* | 117 ± 14 | 113 ± 15* |
| IMCL (a.u.) | 7.9 ± 3.0 | 3.6 ± 2.0† | 18.6 ± 13.7 | 11.2 ± 10.9* |
| Fasting plasma glucose(mmol/l) | 5.05 ± 0.55 | 4.77 ± 0.22 | 11.93 ± 4.44‡ | 9.82 ± 3.71 |
| Fasting plasma insulin(pmol/l) | 144 ± 72 | 84 ± 18 | 90 ± 72 | 78 ± 72 |
| GDR (mg · kg−1· min) | 13.3 ± 2.2 | 14.7 ± 1.8 | 6.4 ± 1.5‡ | 7.5 ± 1.1 |
| HOMA-IR | 5.5 ± 2.6 | 3.8 ± 1.4* | 6.9 ± 4.0 | 5.4 ± 5.1* |
| Adiponectin (μg/ml) | 7.4 ± 1.8 | 7.8 ± 0.8 | 7.8 ± 2.1 | 6.2 ± 2.1 |
| RQ | 0.85 ± 0.02 | 0.79 ± 0.06 | 0.78 ± 0.05‡ | 0.80 ± 0.02 |
| Fat oxidation(g/kg lbm/day | 1.3 ± 0.2 | 1.9 ± 0.6 | 2.0 ± 0.7‡ | 1.8 ± 0.1 |
| Glucose oxidation(g/kglbm/day) | 3.7 ± 1.2 | 2.5 ± 1.4 | 1.5 ± 1.2‡ | 1.9 ± 0.7 |
| FFA (mmol/l) | 400 ± 120 | 680 ± 90† | 570 ± 100 | 710 ± 180* |
Significantly different from baseline within the group:
P < 0.05.
P < 0.01.
Significantly different from non-diabetics P < 0.05.
Except for GDR, where n=5 (before) and n=4 (after), for both non-diabetics and Type 2 diabetics. F = females, M = males. RQ = respiratory quotient.
Hypocaloric Feeding Protocol
After medical screening, volunteers were admitted as inpatients to the GCRC in the afternoon and, over the next 3 days, equilibrated on an isocaloric diet containing 50% carbohydrate, 30% fat, and 20% protein with the help of a registered dietician who worked with the patients during the length of the protocol. Baseline studies were performed including anthropometrics, oral glucose tolerance test (OGTT), dual energy X-ray absorptiometry (DEXA) for body composition, proton magnetic resonance spectroscopy (1H MRS) assessment of IMCL, indirect calorimetry, and a hyperinsulinemic clamp study to assess insulin sensitivity. On day 4 of admission the patients started the very low calorie-diet (VLCD) containing 700 kcal/day, with the same macronutrient distribution as in the initial diet. Patients remained on this diet for 6 days. At the end of this period, the metabolic studies performed at baseline were repeated to assess effects of the dietary intervention. During these repeat measurements, the VLCD was continued to assure that the subjects were maintained in a negative energy balance state. Throughout the study, participants remained as inpatients and all meals were prepared and provided by the metabolic kitchen of the GCRC.
OGTT
Standard 75-g oral glucose tolerance tests were performed after a 12-h overnight fast (10). Among the 5 non-diabetic subjects, 4 subjects were classified as having a normal OGTT and 1 was found to have impaired fasting glucose. Seven patients had T2DM.
Insulin sensitivity
In vivo insulin sensitivity was assessed using the euglycemic-hyperinsulinemic glucose clamp technique at a maximally effective steady-state serum insulin concentration as previously described (11). Briefly, after a 12-h fast, a catheter was inserted into the brachial vein to administer insulin, glucose, and KPO4. A dorsal hand vein was cannulated in a retrograde manner and kept in a warming device (65°C) to provide arterialized venous blood for sampling. To maximally stimulate glucose uptake and suppress hepatic glucose production, regular insulin (Humulin; Eli Lilly, Indianapolis, IN) was administered at a rate of 200 mU · m-2 · min- 1, producing a mean steady-state insulin concentration of 3,480 ± 138 pmol/l, which is maximally effective for stimulating glucose uptake into skeletal muscle (11). Serum glucose was clamped at 5.0 mmol/l for at least 3 h, and maximal glucose uptake for each individual was calculated from the mean glucose infusion rate over the final three 20-min intervals. Whole-body glucose uptake was calculated based on the glucose infusion rate corrected for changes in the glucose pool size, assuming a distribution volume of 19% body weight and a pool fraction of 0.65. Glucose uptake was normalized per kilogram lean body mass (excluding bone mass) determined by DEXA to yield the GDR per kilogram of lean body mass. Lower GDR values indicate greater insulin resistance. During initial baseline studies, only 10 out of 12 subjects were able to complete the hyperinsulinemic euglycemic clamp and only 8 out of those 10 subjects were able to complete this test at the end of the dietary period due to problems with venous access.
HOMA insulin sensitivity index
The homeostatic model assessment index was used to determine levels of insulin resistance using fasting insulin and glucose levels and the formula = [glucose (in millimoles per liter) x insulin (in microunits per milliliter)]/22.5. HOMA index was calculated for all subjects before and after intervention, and are presented to complement data on hyperinsulinemic clamps that were not available for all patients as explained above.
Intramyocellular lipid
IMCL was quantified using 1H MRS. Due to logistical circumstances, different MR scanners were used to study the non-diabetic and diabetic subjects. For any given subject, all measurements were performed on the same scanner and with the same acquisition parameters, which permitted baseline and post-diet comparisons in these individuals. For instance, all T2DM patients (n = 7) were run on a 1.5T clinical MR scanner with a commercially provided 1H transmit/receive knee coil (Philips Gyroscan 1.5T ACS-NT PT6000 with actively shielded compact gradients and the 1H spectroscopy software package f/ACS-NT; Philips Medical Systems). All studies on this 1.5T system utilized a Point Resolved Spectroscopy (PRESS) single voxel acquisition sequence in which three water-suppressed PRESS voxels (TE = 33 msec, TR = 5000 msec, 2 × 2 × 1 cm3 voxel size) were acquired from three different locations in the soleus. These locations were chosen to give an overall characterization of soleus muscle lipid content. Separate non-water suppressed spectra were also collected under fully relaxed conditions so that the water signal could be used as an amplitude reference (TE = 33 msec, TR = 5000 msec, 2 × 2 × 1 cm3 voxel size). On all follow-up studies in this group of patients the location of the PRESS voxels were kept constant.
In a similar manner, all studies in obese non-diabetic subjects (n = 5) were run on a 4.1 T whole body imaging and spectroscopy system interfaced to a Bruker console (Bruker Instruments, Billerica, MA). All subject’s legs were positioned inside a laboratory built 1H birdcage coil with the knee in extension and the ankle in a neutral position. IMCL was measured on this system using a commercially provided slice selective 2D magnetic resonance spectroscopic imaging (MRSI) sequence. Details concerning this technique and the reproducibility of these methods have been previously published (12). In brief, following the acquisition a series of 36 spectra from a 6×6 voxel region of interest (ROI) was summed to provide a single spectrum that represented a 2.25 cc area of soleus muscle. This single summed spectrum is what was analyzed for each subject. On all follow-up studies in this group of patients the MRSI slice location and the location of the summed ROI were kept constant.
All spectra were analyzed by fitting the peak positions and areas through time domain fitting using jMRUI (Java-Based Magnetic Resonance User Interface) (13). The same fitting procedures were used regardless of the system used for acquiring the spectra and all fitting models and sets of prior knowledge information have been previously published (14–17). To account for day-to-day variation in system performance, our protocol normalized the IMCL peak amplitudes to the corresponding internal water peak amplitude in that same muscle location. This is similar to the methodology described by Krssak et al. (18). All peak areas in this study are expressed in arbitrary units per pixel area relative to internal water and have been corrected for relaxation effects by using published T1’s and T2’s for water and IMCL at 1.5T (19) and 4T (20). Using the combination of the internal water as an intensity reference with correcting for T1 and T2 relaxation effects allows us to compare the 1.5T and 4.1T measurements of IMCL in our subject groups.
Indirect calorimetry
After an overnight fast, resting energy expenditure, fat oxidation, and carbohydrate oxidation were measured by indirect calorimetry using a Deltratrac metabolic monitor (Deltratrac II, SensorMedics, Yorba Linda, CA) as previously described (21). Measurements began after 30 minutes of rest, while supine in bed. Expired air was collected using the adult-size ventilated canopy system for 20 minutes after a 10-minute equilibration. Whole body oxygen consumption (VO2) and CO2 production (VCO2) were calculated by measuring gradients across the face and the flow rates of air using Haldane transformation. Rates of lipid and carbohydrate oxidation were determined from the respiratory quotient, and normalized per kg metabolically active body mass, as previously described (21).
Anthropometric and body composition
Body mass index (BMI) was measured as weight in kilograms divided by the height in meters square (kg/m2). Fat distribution was assessed by waist and hip circumferences (cm) using a tension-controlled tape measure by Novel Products. DEXA scanning was performed using DPX-L (Lunar Radiation Corp., Madison, WI) with the use of software version 1.33 (Lunar Corp.), and provided body composition measures including total body fat, % body fat, and lean body mass independent of bone mass. Lean body mass was used to normalize rates of glucose disposal and fuel oxidation.
Other assays
Plasma glucose was measured by the glucose oxidase method using a glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH). Serum insulin levels were measured using an electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). In our laboratory, this assay has a mean intra-assay coefficient of variation of 5%, and a mean interassay coefficient of variation of 6%. Plasma adiponectin levels were measured using an ELISA kit (Linco Research Co®). Plasma free fatty acids were measured using a NEFA C kit (Wako Chemicals, Richmond, VA) as described by the manufacturer, the intra-assay coefficient of variation is 0.8%.
Statistical analyses
All data are given as means ± SD unless otherwise indicated. Logarithmic transformation was used for normalization of the variables of interest when appropriate. The correlations between baseline insulin sensitivity and different metabolic and anthropometric variables were examined using Spearman correlation coefficients. Changes in outcomes of interest before and after diet were compared using paired t-test. These analyses were performed separately for the entire group, and in subgroups consisting of non-diabetic and T2DM subjects. The SAS program version 8.0 (SAS Institute, Cary, NC) was used for analyses. Differences were accepted as significant at P < 0.05.
Results
Baseline Characteristics
A total of 12 subjects were studied, 5 non-diabetics and 7 with T2DM. Baseline and post-diet characteristics of the study subjects are shown in Table 1.
Effects of a 6-day very low calorie diet in non-diabetics and T2DM
Over the course of the 6-day diet, obese non-diabetic lost an average of 2.3 kg (P < 0.05) and T2DM subjects lost an average of 3.7 kg of body weight (P < 0.01). Table 1 shows mean baseline and post-diet levels of anthropometric and metabolic variables for non-diabetics and T2DM subjects. BMI and waist circumference decreased modestly after the diet; BMI decreased by 2.5% (P < 0.05) and 5% (P < 0.01) in non-diabetics and T2DM respectively, and was accompanied by decreases in waist circumference of around 3% in both groups (both, P < 0.05). Importantly, the diet produced more profound decrements in IMCL in all patients ranging from 16% to 74% compared to baseline values. These decrements averaged 56% (P=0.006) and 40% (P = 0.04) in non-diabetics and T2DM patients respectively, as shown in Figure 1. Changes in IMCL in these two groups are also outlined in Table 1. In the entire group, these changes in body composition and IMCL were accompanied by a significant increase in insulin sensitivity, characterized by a 14% increase in maximally stimulated glucose disposal rate (GDR) from 9.8 ± 4.0 to 11.1 ± 4.1 mg/kg lbm/min at the end of the dietary period (P < 0.01) (Figure 1). Significant increments in insulin sensitivity were also documented in both non-diabetic and T2DM by HOMA-IR as shown in Table 1. Overall, these metabolic and anthropometric changes occurred for all subjects regardless of diabetic status, and were not proportionally different between these two groups. Levels of plasma free fatty acids increased significantly in non-diabetics (from 400 ± 120 to 680 ± 90 μmol/L, P < 0.01) and T2DM (from 570 ± 100 to 710 ± 180 μmol/L, P < 0.05), reflecting increased lipolysis secondary to caloric restriction. Whole body rates of fat or carbohydrate oxidation did not change significantly after the intervention.
Figure 1.
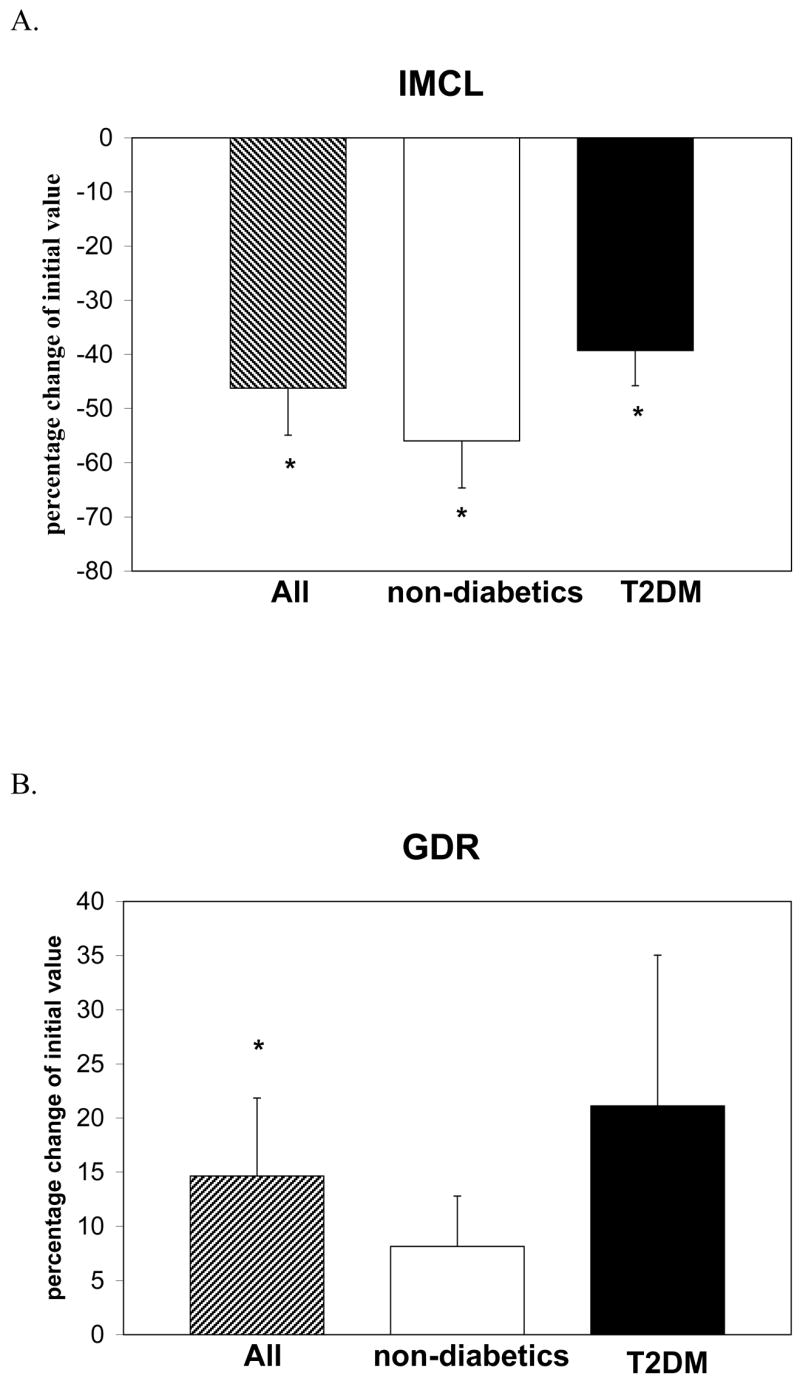
Percentage change in intramyocellular lipid (IMCL) and GDR (glucose disposal rate) in all subjects (n = 12), non-diabetic subjects (n = 5), and Type 2 Diabetics (n = 7) after short-term very low calorie diet. IMCL was assessed in the soleus muscle of each individual using proton-nuclear magnetic resonance spectroscopy and GDR was assessed using the euglycemic-hyperinsulinemic glucose clamp technique. *Significantly different from baseline values P < 0.05.
Relationships between insulin Sensitivity and measures of regional and total adiposity before and after 6-day very low calorie diet
Insulin sensitivity measured by GDR was found to be negatively correlated with IMCL (r = −0.69; P < 0.01) as shown in Fig 2A, and waist circumference (r = −0.59; P < 0.01), Fig 2B. In contrast, insulin sensitivity in these subjects was not correlated with BMI (r = −0.12, P = ns), Fig. 2C. When analyzed before and after VLCD, the correlations between IMCL and insulin sensitivity showed a trend to significance (r = −0.58 and r = −0.67, before and after respectively; both P = 0.06). Similarly, waist circumference showed borderline correlations with insulin sensitivity (r = −0.55 and −0.63, before and after respectively, both P = 0.09). IMCL was highly correlated with waist circumference both at baseline and post-diet (r = 0.72 and 0.83, respectively, both P < 0.01). However, IMCL was not related to measures of total adiposity including total fat mass (r = 0.32, and 0.42 baseline and post-diet, respectively, P = ns) or BMI (r = 0.45, and 0.53 baseline and post-diet respectively, P = ns). Interestingly, the changes in IMCL were inversely correlated with the changes in plasma free fatty acids (r = −0.71, P < 0.05), since IMCL levels decreased while free fatty acid levels increased as a result of the hypocaloric dietary intervention. Thus, greater decrements in IMCL were progressively associated with higher levels of circulating free fatty acids. Baseline plasma adiponectin levels did not differ from post-diet values 7.6 ± 1.8 vs. 6.9 ± 1.8μg/ml, respectively.
Figure 2.
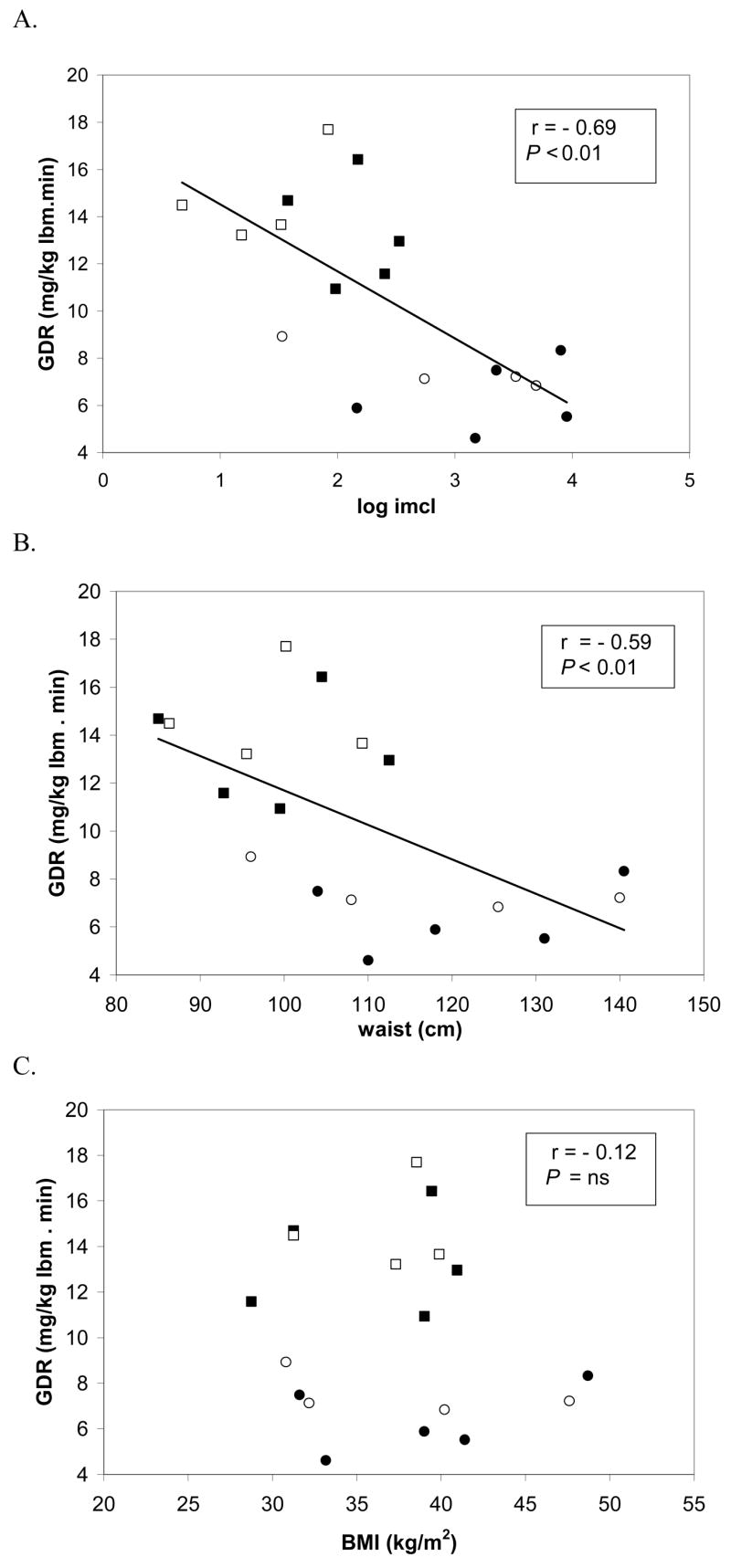
Relationships between insulin sensitivity and intramyocellular lipid (IMCL), waist circumference, and body mass index before and after very low calorie diet (VLCD) in the group of non-diabetic and diabetic subjects. The figures show correlations between glucose disposal rate (GDR), as measured by hyperinsulinemic euglycemic clamp, and IMCL measured by proton nuclear magnetic resonance spectroscopy (A), waist circumference (B), and body mass index (C). Circles represent diabetics, before (●) and after (○) VLCD; and squares represent non-diabetics before (■) and after (□) VLCD.
Discussion
To the best of our knowledge, this is the first study investigating effects of short-term caloric restriction on IMCL and the relationships between changes in IMCL and whole body metabolism in this context. In addition, our data address the effects of a very-low calorie diet on IMCL in both T2DM and non-diabetic subjects. Results from this study indicate that IMCL was decreased concomitant with an increase in insulin sensitivity after a short-term diet providing 700 kcal/day, and that these changes occurred in both diabetics and non-diabetics. Furthermore, insulin sensitivity was significantly correlated with IMCL and waist circumference in the entire group. In contrast, diet-induced changes in IMCL and insulin sensitivity were accompanied by only small effects on total body fat and BMI, and these measures of general adiposity were not correlated with IMCL and insulin sensitivity. Therefore, increments in insulin sensitivity observed with short-term VLCD are associated with loss of IMCL in the absence of marked changes in total body fat.
Several intervention studies, varying in length from weeks to months have explored the effects of mild to moderate caloric restriction on IMCL (3–9, 22). Most of these studies have focused on obese non-diabetic subjects who achieved weight loss through surgically-induced malabsorptive procedures, or through moderate dietary caloric restriction. Most of these studies established that IMCL was reduced in response to these interventions in conjunction with a parallel increase in insulin sensitivity. A cause-effect relationship between intramyocellular lipid accumulation and insulin signaling has been previously hypothesized (6), and a feed-back mechanism involving effects of intracellular triglycerides and long-chain fatty acyl-CoA on insulin action and glucose metabolism has been posited (23). Fewer studies have addressed effects of weight loss on IMCL and insulin sensitivity in T2DM, and have only then employed mild to moderate caloric restriction (5, 8, 9). Petersen et al (8) found that T2DM undergoing moderate weight reduction on a low-fat diet providing ~ 1200 kcal/day experienced hepatic but not peripheral changes in insulin sensitivity. These experimental conditions led to a marked reduction in hepatic triglyceride and an increase in hepatic insulin sensitivity with no changes in IMCL or peripheral glucose metabolism. In this study, the length of the dietary intervention was variable, between 3 to 12 weeks including a period of weight stabilization, and was targeted towards achievement of normoglycemia rather than a pre-defined amount of weight loss. Tamura et al (9) randomized subjects with T2DM to either diet (1700kcal/day for 2 weeks) or diet plus exercise for two weeks. Again, in contrast to the current findings, the diet-only arm resulted in a decrease in intrahepatic lipid but did not affect IMCL or peripheral insulin sensitivity. These previous studies suggest that IMCL may not be responsive to hypocaloric diets in T2DM. Differences between previous intervention studies and the one reported here include degree of caloric restriction and length of the intervention. For instance, our 6-day inpatient protocol provided fewer calories (700/day) and was aimed to determine the short-term effect of a severe caloric restriction on levels of IMCL and its relation to insulin sensitivity. Overall, our findings suggest that a more robust caloric restriction may be necessary to initiate mobilization of intramyocellular lipid stores and further activate signaling pathways aimed to improve muscle glucose uptake. Another pertinent factor in study design is whether the dietary intervention was followed by a period of weight stabilization prior to post-diet assessment. The study by Petersen et al did feature isocaloric equilibration while the current study and the study by Tamura et al did not. In the current study, the observed decrements in IMCL on a VLCD reflect active mobilization of IMCL stores under predominantly negative energy balance conditions, and illustrate the dynamic nature of this highly active lipid pool (3, 4). An important finding was that even though obese and T2DM subjects are characterized by defects in substrate oxidation (7, 24) including impaired lipid oxidation (25, 26), we observed that both non-diabetics and T2DM responded to a significant caloric restriction by decreasing IMCL stores. Under conditions of reduced energy intake resulting in lipolysis and elevated circulating FFA, there is increased reliance on fat oxidation by muscle as the predominant source of fuel (27). Our results indicate that IMCL responds acutely to caloric manipulation (28, 29), and that IMCL represents a dynamic fuel compartment that provides readily available energy for muscle function in both T2DM and non-diabetics.
While IMCL was severely depleted after the short-term dietary intervention in both T2DM and non-diabetic subjects, these changes were accompanied by only small changes in general adiposity, which was assessed by BMI and measures of total fat mass and % body fat. Moreover, before and after the intervention, measures of generalized adiposity were not correlated with either IMCL or insulin sensitivity, probably as a result of the small sample size of the study. On the other hand, upper body fat distribution as reflected by waist circumference was borderline correlated with insulin sensitivity, and did vary as a function of IMCL. These observations suggest that measures of regional adiposity, IMCL and abdominal fat, rather than measures of generalized adiposity might be more pathophysiologically relevant to insulin resistance. However, two functional aspects of adipose tissue do not appear to be involved in the loss of IMCL and increase in muscle glucose disposal in response to the diet. First, IMCL was reduced despite an increase in circulating free fatty acids and free fatty acid availability, with no measurable change in basal rates of lipid and carbohydrate oxidation. Infusion of lipid emulsions have been previously shown to acutely induce insulin resistance together with augmentation in IMCL (3, 4); however, in the current setting on the hypocaloric diet, increments in free fatty acids brought about by lipolysis during hypocaloric feeding were associated with a decrease rather than an increase in IMCL. The absence of change in whole body basal lipid oxidation indicates that IMCL was reduced by preferential oxidation of IMCL over circulating free fatty acids in skeletal muscle, and supports previous observations indicating that free fatty acids taken up by resting muscle are not oxidized directly, but probably enter the intramuscular pool which is the immediate source of oxidized lipid substrate (30). Secondly, circulating levels of the adipokine, adiponectin, were unchanged after the VLCD diet. Other studies have demonstrated that adiponectin levels are increased following substantial weight loss; however, in the current study, short-term caloric restriction itself, without major loss of body fat, was not sufficient to increase adiponectin concentrations. Our results are in agreement with a few other studies showing that small to moderate weight loss after conventional caloric restriction was not correlated with changes in adiponectin levels (31, 32). In contrast, studies reporting major weight loss after bariatric surgery consistently report increases in adiponectin levels (33, 34) supportive of the idea that more severe or chronic weight loss may be necessary to alter plasma adiponectin. In our study, the absence of correlations between adiponectin and insulin sensitivity or IMCL suggests that under short-term caloric restriction adiponectin does not play a direct role in altering peripheral insulin sensitivity or IMCL levels.
The current results underscore the relevance of IMCL as a target for treatment and prevention of T2DM and the Metabolic Syndrome. Limitations of the study include the small sample size when non-diabetics and T2DM subjects are considered as separate subgroups. Also, studies at the end of the dietary period were performed while subjects were continued on the hypocaloric diet without a period of isocaloric equilibration. It will be important to observe whether the decrease in IMCL due to short-term VLCD is retained during isocaloric stabilization and to examine the relationship between IMCL and insulin sensitivity under these conditions. In addition, we did not assess hepatic fat content changes and their contribution to the improved metabolic condition, as well as the role of other markers with the potential to affect insulin sensitivity and cellular energy balance (i.e., TNF-α, AMP-kinase).
In conclusion, short-term VLCD resulted in marked decrements in IMCL together with increases in insulin sensitivity, in both non-diabetic and T2DM subjects. At the same time, the dietary intervention produced small to minimal changes in measures of general adiposity, such as BMI, % body fat, or total fat mass, which were not related to effects on IMCL or insulin sensitivity. Adiponectin levels did not change in response to the diet, and circulating fasting free fatty acid levels were elevated indicative of increased delivery to skeletal muscle. Therefore, the increase in insulin sensitivity and reduction in IMCL reflected responses that were primarily to skeletal muscle, and could not be explained by effects originating in adipose tissue as reflected by adiponectin secretion or free fatty acid availability. By inference, a partial component of the increase in insulin sensitivity observed following longer term diets may be related to changes in IMCL and other fat depots than to reductions in total body fat. Thus, following short-term VLCD, IMCL appears to be an important correlate of insulin sensitivity in both non-diabetics and T2DM.
Acknowledgments
This work was supported from grants from the National Institutes of Health (DK-38765, PO1 HL-55782) and by the Merit Review program of the Department of Veterans Affairs. We gratefully acknowledge the support of the UAB General Clinical Research Center (MO1 RR-00032), research core facilities in the UAB Clinical Nutrition Research Unit (P30-DK56336), and the participation of the research volunteers.
Funding support: National Institutes of Health (DK-38765, PO1 HL-55782), Merit Review program of the Department of Veterans Affairs. UAB General Clinical Research Center (MO1 RR-00032), research core facilities in the UAB Clinical Nutrition Research Unit (P30-DK56336).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lara-Castro C, Garvey WT. Diet, insulin resistance, and obesity: zoning in on data for Atkins dieters living in South Beach. J Clin Endocrinol Metab. 2004;89:4197–205. doi: 10.1210/jc.2004-0683. [DOI] [PubMed] [Google Scholar]
- 2.Weiss R, Taksali SE, Dufour S, et al. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab. 2005;90:3731–7. doi: 10.1210/jc.2004-2305. [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–7. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann OP, Dahl DB, Brechtel K, et al. Effects of Intravenous and Dietary Lipid Challenge on Intramyocellular Lipid Content and the Relation With Insulin Sensitivity in Humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–72. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 6.Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–51. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 7.Fabris R, Mingrone G, Milan G, et al. Further lowering of muscle lipid oxidative capacity in obese subjects after biliopancreatic diversion. J Clin Endocrinol Metab. 2004;89:1753–9. doi: 10.1210/jc.2003-031343. [DOI] [PubMed] [Google Scholar]
- 8.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of Nonalcoholic Hepatic Steatosis, Hepatic Insulin Resistance, and Hyperglycemia by Moderate Weight Reduction in Patients With Type 2 Diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura Y, Tanaka Y, Sato F, et al. Effects of Diet and Exercise on Muscle and Liver Intracellular Lipid Contents and Insulin Sensitivity in Type 2 Diabetic Patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- 10.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Diabetes Care. 2002;25:5S–20. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 11.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222–34. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 12.Newcomer BR, Lawrence JC, Buchthal S, Den Hollander J. High-resolution chemical shift imaging for the assessment of intramyocellular lipids (In Press) Magn Reson Med. 2007 doi: 10.1002/mrm.21209. [DOI] [PubMed] [Google Scholar]
- 13.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–86. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 14.Larson-Meyer DE, Newcomer BR, Hunter GR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. Am J Physiol Endocrinol Metab. 2002;282:E95–106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- 15.Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1H-MRS. J Appl Physiol. 1999;87:2068–2072. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- 16.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 17.Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14:73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–6. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 19.Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn Reson Med. 1993;29:158–67. doi: 10.1002/mrm.1910290203. [DOI] [PubMed] [Google Scholar]
- 20.Hwang JH, Pan JW, Heydari S, Hetherington HP, Stein DT. Regional differences in intramyocellular lipids in humans observed by in vivo 1H-MR spectroscopic imaging. J Appl Physiol. 2001;90:1267–74. doi: 10.1152/jappl.2001.90.4.1267. [DOI] [PubMed] [Google Scholar]
- 21.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–59. [PubMed] [Google Scholar]
- 22.Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA. Effect of weight loss on muscle lipid content in morbidly obese subjects. Am J Physiol Endocrinol Metab. 2003;284:E726–732. doi: 10.1152/ajpendo.00371.2002. [DOI] [PubMed] [Google Scholar]
- 23.Oakes N, Bell K, Furler S, et al. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes. 1997;46:2022–2028. doi: 10.2337/diab.46.12.2022. [DOI] [PubMed] [Google Scholar]
- 24.Kim J-Y, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 25.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–41. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005;115:1699–702. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decombaz J, Schmitt B, Ith M, et al. Postexercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. Am J Physiol Regul Integr Comp Physiol. 2001;281:R760–9. doi: 10.1152/ajpregu.2001.281.3.R760. [DOI] [PubMed] [Google Scholar]
- 29.Schrauwen-Hinderling VB, Kooi ME, Hesselink MKC, et al. Intramyocellular Lipid Content and Molecular Adaptations in Response to a 1-Week High-Fat Diet. Obes Res. 2005;13:2088–2094. doi: 10.1038/oby.2005.259. [DOI] [PubMed] [Google Scholar]
- 30.Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976;58:421–31. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannopoulou I, Fernhall B, Carhart R, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–75. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 32.Mousavinasab F, Tahtinen T, Jokelainen J, et al. Lack of increase of serum adiponectin concentrations with a moderate weight loss during six months on a high-caloric diet in military service among a young male Finnish population. Endocrine. 2005;26:65–9. doi: 10.1385/ENDO:26:1:065. [DOI] [PubMed] [Google Scholar]
- 33.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–83. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 34.Vendrell J, Broch M, Vilarrasa N, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–71. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]


