Abstract
Intravenous drug use is a major vector of HIV transmission. We assessed whether contingency management (CM), in which participants earn reinforcers for drug abstinence, reduces such risk behaviors in methadone-maintained opiate- and cocaine-using outpatients. Participants (n = 116) were randomly assigned to prize-based CM or to receipt of prize draws noncontingently on a schedule yoked to the CM group. Both groups received methadone and individual counseling throughout treatment. The HIV-Risk Taking Behaviour Scale (HRBS; Darke et al., 1991) was administered in written questionnaire form at 2-week intervals. A mediation analysis was conducted to determine whether abstinence from opiates and cocaine mediated the effect of CM on HRBS scores. Changes in HRBS scores over time differed significantly by treatment (F(9,334)=2.4, p<0.05), with HRBS scores decreasing over time in the CM group to a greater extent than in the noncontingent control group. Participants in the CM group had significantly lower rates of simultaneous cocaine/opiate-positive urine specimens than those in the noncontingent control group during CM treatment (F(1,111)=6.8, p=0.01). The relationship between treatment condition and HRBS scores was mediated by abstinence. CM targeted toward cocaine and heroin use produces significant reductions in injection-related drug-taking behaviors associated with heightened risk for getting or transmitting HIV.
Keywords: contingency management, substance abuse treatment, HIV, opiate, cocaine, methadone maintenance
1. Introduction
Injection drug use is a major risk factor in the transmission of HIV. In a survey conducted from 2001 to 2004 in 33 states of the United States, 21% of females and 21% of males among adults and adolescents newly diagnosed with HIV/AIDS were injection drug users (IDUs) (CDC, 2005). There is an urgent public health need to reduce high risk behaviors associated with HIV transmission. The World Health Organization estimates 39.5 million people are living with HIV. There were 4.3 million new infections in 2006, and 2.9 million people died of AIDS-related illnesses (World Health Organization, 2006).
Methadone maintenance is an effective treatment for opioid dependence (Ball and Ross, 1991). Among IDUs, methadone maintenance has been shown to lead to reductions in HIV risk behaviors (Gibson et al., 1999; Metzger et al., 1998) by reducing drug-related risk behaviors and sexual risk-taking behaviors (Kwiatkowski and Booth, 2001; Nadeau et al., 2000; Schroeder et al., 2006; Sorensen and Copeland, 2000; Thiede et al., 2000). Because the pharmacological effects of methadone are specific to opioids, behavioral therapies are key components of successful treatment, especially for reducing use of non-opioid drugs such as cocaine. Cocaine use is a common problem among patients in methadone maintenance.
Contingency management (CM) is a behavioral treatment in which participants earn reinforcers in exchange for objective evidence of drug abstinence (Higgins et al., 1993; Higgins et al., 1991; Stitzer et al., 1992). CM has been shown to decrease use of cocaine, opioids, and other drugs in patients in methadone maintenance (see Griffith et al., 2000; Lussier et al., 2006; Prendergast et al., 2006 for review). Our laboratory recently completed an evaluation of two reinforcement schedules in the prized-based CM procedure developed by Petry and colleagues (Petry et al., 2005a; Petry and Martin, 2002; Petry et al., 2004). In prize-based CM, drug abstinence is reinforced with opportunities to draw for prizes; in the most studied application of prize-based CM, the prize/draw ratio is about 50%. Our study compared the 50% (standard) prize/draw schedule to a schedule in which the probability of winning a prize was increased, approximately 78% (Ghitza et al., 2007). Participants randomized to the higher-prize-probability group had significantly greater abstinence compared to participants in the standard-prize-probability groups and to participants who received prize draws independent of their drug use (noncontingent control group).
A clinically important question is whether the efficacy of CM in enhancing abstinence from illicit drug use translates into a reduction in the frequency of HIV-risk behaviors. Such a reduction has not been demonstrated in dual cocaine and opiate users. If found, it would have clinically significant public health implications and could further support the addition of CM into standard practice in community programs.
Surprisingly little work has been published on CM interventions and HIV-related behaviors (Haug and Sorensen, 2006). Several studies have shown that contingency management techniques can improve adherence to HIV medications (Sorensen et al., 2007; Rosen et al., 2007) and compliance with HIV-related prevention and counseling sessions (Petry et al., 2001; Deren et al., 1994; Kamb et al., 1998). Two studies have shown decreases in HIV risk behaviors in clinical trials comparing CM and cognitive behavioral therapy (CBT), though in neither study was the decrease specific to either treatment (Schroeder et al., 2006; Shoptaw et al., 2005).
The objective of the present study was to evaluate the efficacy of prize-based CM in reducing HIV-risk behaviors in methadone-maintained cocaine and opiate users and to assess whether such a reduction is mediated by enhanced abstinence from both opiate and cocaine use, as assessed by urinalysis, or mediated by behavioral changes independent of drug abstinence. We hypothesized that prize-based CM would reduce injection-related HIV risk-taking behaviors and that this reduction would be mediated by enhanced abstinence from illicit drug use.
2. Methods
2.1 Study participants
Participants were 201 patients consecutively admitted to a clinical trial evaluating the efficacy of prize-based CM and methadone maintenance for dual cocaine and heroin use. The trial was conducted at an outpatient inner-city treatment research clinic in Baltimore, MD and was approved by the local Institutional Review Board for human research. Participants were recruited through advertisements in a variety of local newspapers and television stations selected to ensure exposure to both sexes and all ethnicities. Eligibility criteria for enrollment were: age 18-65, cocaine and opiate use (by self-report and urine screen), and physical dependence on opiates. Participants gave informed consent prior to their participation. Participants with alcohol dependence or major psychiatric disorders were excluded.
Screening conducted before admission to the trial included medical, psychiatric, and drug-use histories, a physical examination, urine and blood screens, and a battery of assessment instruments, including the Addiction Severity Index (ASI; (McLellan et al., 1985)) and the Diagnostic Interview Schedule (DIS-IV; (Robins, 1995)). Laboratory testing included screening for HIV, hepatitis C and hepatitis B. All participants were told the results of hepatitis and HIV testing; those who tested positive were also given advice by a physician's assistant or nurse practitioner on how to reduce the medical consequences of their infection. Participants were excluded from the study and given treatment referrals if their CD4 counts were below 200.
2.2 Standard Treatment
All participants began methadone maintenance upon enrollment in the study and received, without charge, daily methadone and weekly individual counseling (standard treatment) throughout the study. Methadone HCl (Mallinckrodt, Inc., St. Louis, MO) was administered orally in 35 ml of a cherry-flavored solution. The daily maintenance dose ranged from 70 to 100 mg, adjusted based on feedback from the participant and on the clinical judgment of the staff. During counseling, counselors completed a semistructured psychosocial assessment and formulated a treatment plan for each participant. Reduction of substance use was the primary goal. Individual-counseling sessions were devoted to discussion of cessation of all illicit drug use, reducing HIV risk behaviors, and addressing psychosocial problem areas (e.g., vocational, medical, educational, and emotional).
2.3 Data Collection
Mondays, Wednesdays, and Fridays, urine specimens were collected under the observation of laboratory technicians and tested for cocaine and opiates (Enzyme Multiplied Immunoassay Technique); cutoff concentrations for positive specimens were 300 ng/ml for cocaine, opiates (morphine), benzodiazepines (oxazepam), phencyclidine, barbituates, and 50 ng/ml for marijuana. Breath alcohol levels were determined with an Alco-Sensor III (Intoximeters, Inc., St. Louis, MO). Participants completed the HIV-Risk Taking Behaviour Scale (HRBS; (Darke et al., 1991)) in written questionnaire form at 2-week intervals, from intake up until week 30. This instrument has been shown to have satisfactory psychometric properties for measuring HIV-risk behaviors in substance abusers (Petry, 2001).
The HRBS is an 11-item scale comprising two subscales: drug use and sexual behavior. We added a 12th item to assess how often condoms were used during anal sex. The first question on each subscale is a screening question to determine whether the remaining items in that subscale need to be answered. The 6 items from the drug-use scale were: (1) How many times have you injected any drugs in the last 2 weeks? (2) How many times in the last 2 weeks have you used a needle after someone else had already used it? (3) How many different people have used a needle before you in the last 2 weeks? (4) How many times in the last 2 weeks has someone used a needle after you used it? (5) How often, in the last 2 weeks, have you cleaned needles before re-using them? (6) Before using needles again, how often in the last 2 weeks did you use bleach to clean them? The 6 items from the sexual-behavior scale were: (1) How many people, including clients, have you had sex with in the last 2 weeks? (2) How often have you used condoms when having sex with your regular partner(s) in the last 2 weeks? (3) How often have you used condoms when you had sex with casual partners in the last 2 weeks? (4) How often have you used condoms when you have been paid for sex in the last 2 weeks? (5) In the last 2 weeks, how many times did you have anal sex? (6) In the last 2 weeks, how often have you used condoms when having anal sex? Scores on these individual items (with responses anchored 1-6) are summed to produce subscale scores, which can thus range from 1 to 36, with a higher score indicating a greater frequency of high-risk behaviors.
2.4 Study timeline, groups, and contingency management procedures
The study was conduced in three phases: a 5-week baseline of standard treatment during which eligibility for randomization was determined, a 12-week phase of experimental intervention (plus standard treatment), and an 8-week maintenance/post-intervention phase in which baseline conditions resumed (prize-based CM ceased but standard treatment continued).
Each subject was randomly assigned to one of four groups: three contingent conditions (lower reinforcement probability with manual draws, n=20; lower reinforcement probability with computerized draws, n=36; or higher reinforcement probability with computerized draws, n=20) or a noncontingent control group, n=40. Because the three contingent conditions (lower probability with manual draws, lower probability with computerized draws, higher probability with computerized draws) did not differ with respect to HRBS scores, data from subjects in these groups (n=76) were combined for the analyses reported here. Subjects were eligible for randomization if at least four of 15 urine specimens tested positive for opiates and cocaine (not necessarily on the same days) during the first 5 weeks of treatment (baseline). Randomization was stratified by race, sex, employment status, probation status, and frequency of opiate- and cocaine-positive urine specimens during baseline.
Rules for earning draws were modeled after those used by Petry and Martin (2002) and were the same for all contingent-reinforcement groups. Each urine specimen negative for either opiates or cocaine earned one draw; each specimen negative for both drugs earned four draws. Missed specimens counted as positive. Weekly bonus draws were earned if all specimens that week tested negative for both drugs. The number of bonus draws increased with each consecutive week of abstinence: five the first week, six the second week, up to 16 for the twelfth week. Positive or missed urine specimens reset the bonus draws to five. The maximum possible number of draws was 270. Prize draws for participants in the noncontingent group were determined by a yoking procedure, such that the number of draws, drawing method (manual or computerized), and probability of winning was matched to a participant in a contingent group.
In the manual drawing procedure, subjects drew from a rotating drum that contained 250 wooden balls marked with a symbol that indicated the prize type. In the computerized drawing procedure (Automated Contingency Management; ACM; Vahabzadeh et al., 2007), subjects clicked on an icon on the computer to draw and received a prompt indicating the outcome of the draw. Probabilities of winning a prize were 50% no prize ($0), 43.6% small prize ($1-5), 6% large prize ($20), and 0.4% jumbo prize ($100) in the lower probability reinforcement conditions and 22% no prize, 65.2% small , 12% large , and 0.8% jumbo prize in the higher probability conditions. Examples of small prizes were restaurant gift certificates, household items such as dish detergent, grocery-store gift certificates, and phone cards. Examples of large prizes were mini food choppers, gift certificates to home-improvement stores, makeup kits, blenders, and large denim purses. Examples of jumbo prizes were television sets, portable phones, grills, large hair dryers, and CD players. Prize-reinforcement probability was blind to subjects (and staff). Prizes were selected and received immediately following the draws.
2.5 Data Analysis
For all analyses, the alpha level was p≤0.05 (two-tailed). Analyses were performed using SAS version 9.1 (Cary, NC, USA). Intake measures were analyzed by ANOVA or t-test (for continuous variables) or by Pearson χ2 (for categorical variables). Study retention was analyzed with a log-rank test (SAS Proc Lifetest) of time until provision of the final urine sample. Scores from the HRBS drug and sex subscales over time were analyzed by repeated-measures mixed-regression models (SAS Proc Mixed), which produce an output like that of a repeated-measures ANOVA but do not require imputation of missing data points. Mixed-regression models have been widely accepted in the CM literature as appropriate analytical tools for longitudinal data since they were introduced in the late 1980s. They have been shown to compare favorably with traditional repeated-measures approaches (Nich & Carroll, 1997). These likelihood-based models use iterative methods that utilize all of the existing data, both on an individual and on a group level, to estimate treatment outcomes over time. They facilitate intent-to-treat analyses by interpolating missing values (with appropriate penalties reflected in larger standard errors) rather than deleting participants with missing values or coding all missing values identically. They also allow correlations between repeated measurements to be specified; in our case an unstructured covariance structure was used. This covariance structure allows random intercepts and slopes to covary and estimates the covariance between them.
In two separate repeated-measures mixed-regressions of the HRBS scores, the dependent variable was: (1) each participant's total drug-subscale score or (2) each participant's total sexsubscale score, collected at 2-week increments over the 12-week intervention phase and the 8-week maintenance/post-intervention phase. The independent variables were contingency group (CM or noncontingent control), week of treatment (2 through 24), a covariate for each participant's drug-subscale or sex-subscale score during the first 2 weeks of the study before the CM intervention commenced, and study dropout (coded as the last urine specimen collected for each participant). The term for dropout was included based on the pattern-mixture approach to controlling for the nonrandom nature of missing data—i.e. for the possibility that dropouts differed in some systematic way from study completers (Hedeker and Gibbons, 1997). In order to rule out the possibility that changes in drug-related risk behaviors among groups are merely due to differences in injection drug use (i.e., whether participants were injection drug users), analyses included only occasions when participants reported having injected in the last 2 weeks (item 1). Therefore, the question addressed in these analyses was the degree of risk associated with each potentially risky behavior when it occurred at all. The same type of analysis was done with the sex subscale: the analysis included only occasions when participants reported having had sex in the last 2 weeks.
Urine-screen results were analyzed by repeated-measures logistic regression (SAS GLIMMIX macro); the repeated outcome measures were urines simultaneously negative for opiates and cocaine at each of up to 36 time points during the 12 weeks of CM treatment. The analysis of urines simultaneously negative for opiates and cocaine, and not of each separately, was based on a previous analysis showing CM effects on combined abstinence from cocaine and opiates, but not on abstinence from opiates alone. Methadone and counseling decreased opiate use comparably in both noncontingent control and CM groups. The independent variables in the present urine-screen analyses were group (CM or noncontingent control), a covariate for dropout (continuous variable: the number of the last urine specimen collected during the study), and covariates for baseline drug use, expressed two ways: (1) percentage of urine specimens negative for cocaine, and (2) percentage negative for opiates. Baseline drug use was included as a covariate because, although baseline drug use was not significantly different across groups, we have found that baseline drug use is a major predictor of treatment response (Preston et al., 1998).
To determine the mediators of observed reductions in risk behaviors, we conducted a four-step mediation analysis (Baron and Kenny, 1986; Morgenstern and Longabaugh, 2000) (Figure 1). In step 1 (described above), we assessed whether risk behaviors were reduced more in the CM group than in the control group. In step 2 (described above), we assessed whether simultaneous abstinence from cocaine and opiate was enhanced more in the CM group than in the control group. In step 3, we assessed whether simultaneous abstinence from cocaine and opiate use predicted reductions in risk behaviors, using repeated-measures mixed-regression models similar to the ones described above. The repeated outcome variables were HRBS drug-subscale scores collected at 2-week intervals and the explanatory variables were urines simultaneously negative for opiates and cocaine at each of up to 36 time points during the 12 weeks of CM. In step 4, we assessed whether the relationship seen in Step 1 (between CM group and HRBS scores) remained significant after inclusion of simultaneous abstinence from cocaine and opiates as a between-participants predictor. If Steps 1 through 3 yield significant results yet step 4 yields nonsignificant results, changes in HRBS scores over time can be attributed to enhanced abstinence during CM.
Figure 1.
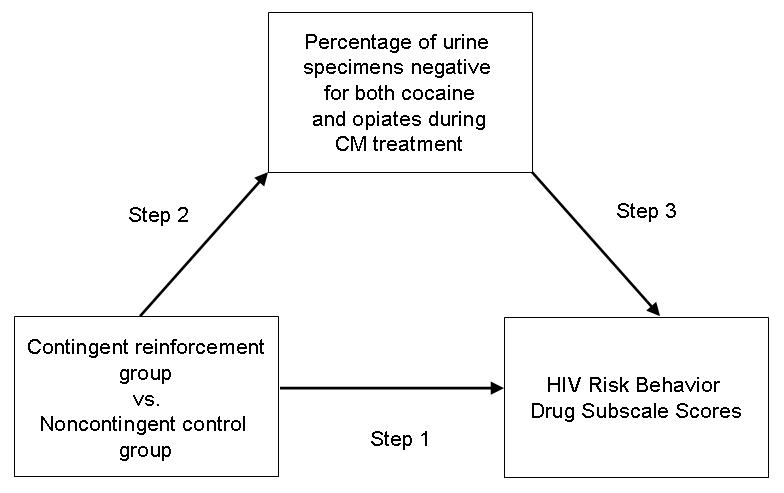
Schematic diagram of mediation analysis assessing whether abstinence from opiate and cocaine use mediated the relationship between CM intervention and HIV risk behavior scores.
3. Results
3.1 Participant characteristics
Among the 201 individuals who qualified for the study and were enrolled, 48 dropped out or were discontinued from the study before being randomized, 33 completed baseline but failed to meet drug-use criteria for randomization, and 4 completed baseline but, due to a technician error, were counted as having failed to meet drug-use criteria for randomization. The remaining 116 were randomized and were included in the analyses. Table 1 lists the demographic characteristics for participants in the CM (n=76) and noncontingent control (n=40) groups. Pearson χ2 and ANOVA analyses showed that demographic, ASI, and DIS-IV characteristics at intake did not differ significantly between groups. Length of time in treatment did not differ between groups (log-rank χ2=0.1, df=1, p=0.75). The proportion of participants completing at least 25 weeks of the study and the mean weeks of retention in the study were not significantly different between the noncontingent control and CM groups (Table 1). Nonetheless, our mixed-regression analyses controlled for length of time in treatment by including a covariate term for study dropout. The mean total prize amounts received per group over the 12-week intervention period were $195 for the CM group and $171 for the noncontingent control group; these amounts were not significantly different from each other (F(1,114)=0.3, p=0.62).
Table 1.
Mean (SD) intake demographic characteristics and study retention
| All | Experimental Groups | ||
|---|---|---|---|
| Participants | Noncontingent Controls | Contingent | |
| N | 116 | 40 | 76 |
| Age (Years) | 37.0 (8.4) | 36.5 (8.2) | 37.9 (8.2) |
| Male (%) | 56 | 55 | 57 |
| African-American (%) | 47 | 40 | 50 |
| Years of Education | 11.4 (1.3) | 11.5 (1.4) | 11.3 (1.3) |
| Heroin Use (Years) | 10.3 (7.5) | 9.0 (5.8) | 10.6 (8.2) |
| Cocaine Use (Years) | 9.4 (7.4) | 9.1 (7.2) | 8.0 (6.5) |
| Days of Use in last 30 | |||
| Heroin | 29.0 (4.3) | 29.7 (0.9) | 29.2 (3.3) |
| Cocaine | 17.4 (10.2) | 17.1 (10.6) | 17.5 (10.0) |
| Retention (weeks) | 21.3 (6.4) | 22.0 (5.7) | 21 (6.8) |
| Completed 25 weeks (%) | 69 | 73 | 68 |
3.2 Association between HIV risk-taking behavior drug subscale scores and treatment group
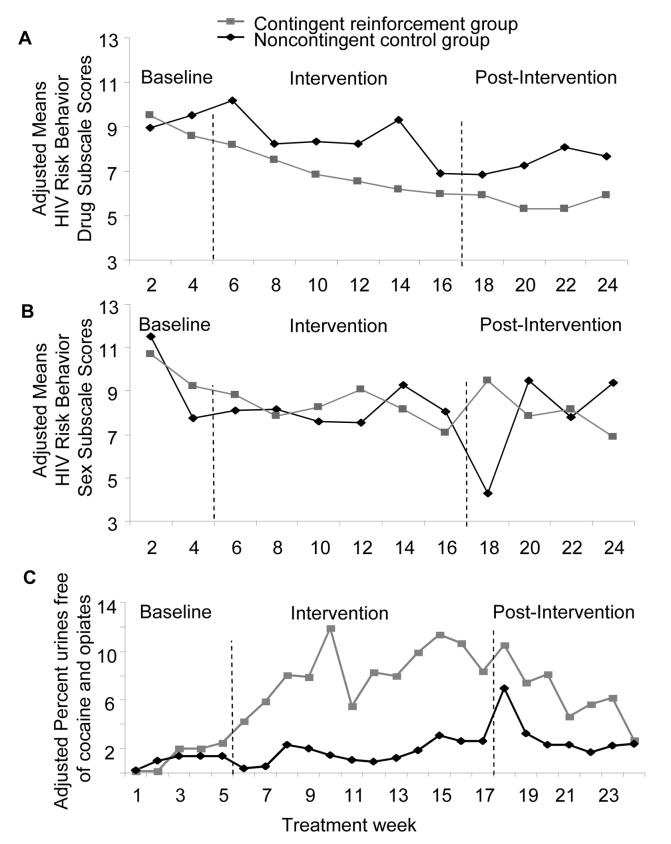
Figure 2 panel A shows the adjusted HIV risk-taking behavior scores by treatment week and group throughout the study. During baseline, before the CM intervention commenced, there was no difference in drug-related risk behaviors between subjects who would eventually be randomized into the CM group or the noncontingent control group (F(1,92)=0.1, p=0.79). Mean (SD) unadjusted scores were 8.9 (3.66) in the control group and 9.45 (3.49) in the CM group. Participants in the CM group reported a greater reduction in drug-related risk behaviors than participants in the noncontingent control group during the treatment weeks encompassing the intervention and post-intervention phases (F(1,29)=5.8, p<0.05). In addition, there was a significant Group × Treatment Week interaction (F(9,334)=2.4, p<0.05), reflecting a greater decrease in HRBS scores over time in the CM group.
Figure 2.
A) Adjusted means of HIV risk behavior drug subscale scores by group and treatment week. Each adjusted mean is from a mixed-regression model (SAS Proc Mixed procedure), controlling for baseline HIV risk behavior drug subscale scores and for study dropout (expressed as the last urine specimen collected during the study). B) Adjusted means of HIV risk behavior sex subscale scores by group and treatment week. Each adjusted mean is from a mixed-regression model (SAS Proc Mixed procedure), controlling for baseline HIV risk behavior sex subscale scores and for study dropout (expressed as the last urine specimen collected during the study). C) Adjusted percentages of urine specimens by treatment group that were negative from both cocaine and opiate use. Each adjusted percentage is a mu value from a repeated-measures logistic regression model (SAS GLIMMIX macro), controlling for baseline drug use (expressed as: 1) the percentage of urine specimens negative for cocaine, 2) negative for opiates) and for study dropout (coded as the last urine specimen collected for each participant).
In each panel, the baseline values are from an analysis that includes only baseline, whereas the intervention and maintenance values are from an analysis that controls for baseline values. In panel C, the adjusted percentages should not be taken as reflections of actual frequency of drug use, but are useful for between-group comparisons.
3.3 Association between HIV risk-taking behavior sex subscale scores and treatment group
Figure 2 panel B shows the adjusted scores for sex-related risk behaviors by treatment week and group throughout the study. There was also no preexisting difference during baseline (F(1,86)=0.1, p=0.80). Mean (SD) unadjusted scores were 11.9 (7.48) in the control group and 10.9 (5.68) in the CM group. There was no group difference in sex-related risk behaviors during the intervention and post-intervention phases (F(1,55)=2.1, p=0.15) and there was no Group × Treatment Week interaction (F(8,208)=1.6, p=0.12).
3.4 Proportion of urine specimens negative for both opiates and cocaine mediates the effects of CM on HIV drug-related risk-taking behaviors
To evaluate whether the relationship between CM and risk reduction was mediated by abstinence from opiates and cocaine, we first assessed the relationship between CM and the proportion of urine specimens negative for both opiates and cocaine. There were no preexisting group differences during baseline; the raw percentages of urine specimens negative for both cocaine and opiates were 6.0% for the CM group and 6.8% for the noncontingent control group (F(1,111)=2.4, p=0.13). During the CM intervention period, the raw percentages of urine specimens negative for both cocaine and opiates were 23% for the CM group and 17.5% for the noncontingent control group. Using a repeated-measures logistic regression (SAS GLIMMIX macro) that controlled for baseline cocaine use, baseline opiate use, and length of time in treatment, we found that CM had a significant impact on simultaneous abstinence from opiates and cocaine use (F(1,111)=6.8, p=0.01). Adjusted percentages of urine specimens negative for both cocaine and opiates from the repeated-measures logistic regression model were 7.2% (95% confidence intervals: 3.9-13.1%) in the CM group and 1.6% (95% confidence intervals: 0.6-4.2%) in the noncontingent control group (Figure 2 panel C). (Adjusted percentages from GLIMMIX analyses are not readily interpretable in absolute terms, but are useful for comparing groups while controlling for all covariates in the model.)
The percentage of urine specimens negative for both cocaine and opiates during the 12-week intervention was also significantly related to the frequency of drug-related risk behaviors (F(1,57)=46.7, p<0.001). When we controlled for the urine data, CM group was no longer related to the frequency of drug-related risk behaviors (F(1,44)=2.1, p=0.16), and there was no longer a significant Group × Treatment Week interaction (F(18,1046)=1.0, p=0.51). From this mediation analysis, we can infer that opiate and cocaine abstinence mediated the effect of CM on drug-related risk reduction.
4. Discussion
The present study investigated whether prize-based CM for opiate and cocaine dependence is effective in reducing HIV-risk behaviors in methadone-maintained outpatients. Our new analysis of data from our randomized, controlled trial showed that compared to a noncontingent control condition, prize-based CM produces significant reductions in injection-related risk behaviors that are associated with heightened risk for HIV transmission. We wish to emphasize that the decrease in injection-related risk behaviors was not merely secondary to a decrease in overall use by injection (though such a decrease did occur); rather, when participants used by injection, they did so in ways less likely to transmit disease. Thus, these data suggest an important benefit of CM not previously shown.
An earlier study in our clinic evaluated HIV-risk behaviors in a clinical trial comparing CM alone, CBT alone, CM and CBT combined, and a noncontingent control condition for treatment of cocaine use in methadone-maintained outpatients (Schroeder et al., 2006). That study showed decreases in both injection-related and sexual behaviors in all groups, including the control group, but none of the decreases were specific to the CM alone condition. (Direct comparison between that study and the present one is difficult due to the use of different risk-assessment questionnaires and due to other procedural differences, such as the use of group therapy for all participants in the earlier study.) In a study of CM and CM plus CBT to treat methamphetamine use in urban gay and bisexual men, sexual risk behaviors were decreased in all treatment groups (Shoptaw et al., 2005); these results complement ours in that the type of risk behavior reduced (injection-related or sex-related) corresponded to the behavior that was most salient for each study's population.
Our mediation analysis indicates that the decrease in HIV-risk behaviors can be attributed specifically to abstinence from both opiates and cocaine. The finding complements the results of a recently published mediation analysis showing that abstinence from opiates, cocaine, and alcohol mediated the effects of prize-based CM on quality of life in cocaine abusers (Petry et al., 2007). One practical implication of our findings is that they empirically justify the inclusion of the likelihood of a decrease in bloodborne-disease transmission in cost-benefit analyses of CM.
The present study had several strengths. The data were collected in a controlled, randomized clinical trial. Our use of likelihood-based mixed-regression models enabled us to analyze all of our repeated-measures data, without filling in missing data points, to estimate treatment outcomes over time; the models also enabled us to control for study dropout, baseline risk behaviors, and baseline cocaine and opiate use. The differential effects seen on drug-related versus sex-related risk behaviors support the validity of the finding; that is, differences do not appear to be related to random group differences or to a generalized decrease in reporting of all risky behaviors, and they are consistent with the CM-targeted behavior, injection drug use.
The study had several limitations. Generalizability may be limited by our having excluded participants with alcohol dependence and major psychiatric disorders. These disorders are likely to be prevalent in a non-research, treatment population. There was a relatively high attrition rate before randomization. (However, it should be noted that the attrition rate in the present study is comparable to that of previous studies in methadone-maintained patients receiving CM (Preston et al., 2000; Preston et al., 2001; Silverman et al., 1998).)
Nonetheless, the present study shows that CM tartgeted to enhance abstinence from cocaine and opiates in methadone-maintained patients reduces the frequency of injection-related risk behaviors. This broad therapeutic effect represents a clinically important public-health benefit. The modest cost of prize-based CM ($195 in mean total prizes received over a 12-week period) makes the procedure amenable to implementation in community-based outpatient treatment settings.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NIDA.
Financial Support: The work was supported by the Intramural Research Program of the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amass L, Kamien J. A tale of two cities: financing two voucher programs for substance abusers through community donations. Experimental and Clinical Psychopharmacology. 2004;12:147–155. doi: 10.1037/1064-1297.12.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment: Patients, Programs, Services, and Outcome. Springer-Verlag; New York: 1991. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control Online at: http://www.cdc.gov/hiv/topics/testing/resources/slidesets/pdf/testing_healthcare.pdf. [Accessed July 5, 2007]
- Chutuape MA, Silverman K, Stitzer M. Contingent reinforcement sustains post-detoxification abstinence from multiple drugs: a preliminary study with methadone patients. Drug and Alcohol Dependence. 1999;54:69–81. doi: 10.1016/s0376-8716(98)00144-6. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS. 1991;5:181–185. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Deren S, Stephens R, Davis WR, Feucht TE, Tortu S. The impact of providing incentives for attendance at AIDS prevention sessions. Public Health Reports. 1994;109:548–554. [PMC free article] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Experimental and Clinical Psychopharmacology. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Epstein DH, Schmittner J, Vahabzadeh M, Lin J-L, Preston KL. Randomized trial of prize-based reinforcement density for simultaneous abstinence from cocaine and heroin. Journal of Consulting and Clinical Psychology. 2007;75:765–774. doi: 10.1037/0022-006X.75.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DR, Flynn NM, McCarthy JJ. Effectiveness of methadone treatment in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 1999;13:1807–1818. doi: 10.1097/00002030-199910010-00002. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone maintenance treatment: a meta-analysis. Drug and Alcohol Dependence. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Haug NA, Sorensen JL. Contingency management intervention for HIV-related behaviors. Current HIV/AIDS Reports. 2006;3:154–159. doi: 10.1007/s11904-006-0010-5. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;2:64–78. [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G. Achieving cocaine abstinence with a behavioral approach. American Journal of Psychiatry. 1993;150:763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Kamb ML, Rhodes F, Hoxworth T, Rodgers J, Lentz A, Kent C, MacGowen R, Peterman TA. What about money? Effect of small monetary incentives on enrollment, retention, and motivation to change behaviour in an HIV/STD prevention counselling intervention. The Project RESPECT Study Group. Sexually Transmitted Infections. 1998;74:253–255. doi: 10.1136/sti.74.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KC, Marlowe DB, Festinger DS, Lamb RJ, Platt JJ. Schedule of voucher delivery influences initiation of cocaine abstinence. Journal of Consulting and Clinical Psychology. 1998;66:761–767. doi: 10.1037//0022-006x.66.5.761. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Booth RE. Methadone maintenance as HIV risk reduction with street-recruited injecting drug users. Journal of Acquired Immune Deficiency Syndromes. 2001;26:483–489. doi: 10.1097/00126334-200104150-00014. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Navaline H, Woody GE. Drug abuse treatment as AIDS prevention. Public Health Reports. 1998;113(Suppl 1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Longabaugh R. Cognitive-behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction. 2000;95:1475–1490. doi: 10.1046/j.1360-0443.2000.951014753.x. [DOI] [PubMed] [Google Scholar]
- Nadeau L, Truchon M, Biron C. High-risk sexual behaviors in a context of substance abuse: a focus group approach. Journal of Substance Abuse Treatment. 2000;19:319–328. doi: 10.1016/s0740-5472(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Nich C, Carroll K. Now you see it, now you don't: a comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. Journal of Consulting and Clinical Psychology. 1997;65:252–261. doi: 10.1037//0022-006x.65.2.252. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Schwartz M, Krasnansky J, Pencer E, Silva-Vazquez L, Kirby KC, Royer-Malvestuto C, Roll JM, Cohen A, Copersino ML, Kolodner K, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM. Reliability of drug users' self-reported HIV risk behaviors using a brief, 11-item scale. Substance Use and Misuse. 2001;36:1731–1741. doi: 10.1081/ja-100107576. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Finocche C. Contingency management in group treatment: a demonstration project in an HIV drop-in center. Journal of Substance Abuse Treatment. 2001;21:89–96. doi: 10.1016/s0740-5472(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005a;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Obert J, Killeen T, Saladin ME, Cowell M, Kirby KC, Sterling R, Royer-Malvestuto C, Hamilton J, Booth RE, Macdonald M, Liebert M, Rader L, Burns R, DiMaria J, Copersino M, Stabile PQ, Kolodner K, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Archives of General Psychiatry. 2005b;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T. Contingency management improves abstinence and quality of life in cocaine abusers. Journal of Consulting and Clinical Psychology. 2007;75:307–315. doi: 10.1037/0022-006X.75.2.307. [DOI] [PubMed] [Google Scholar]
- Piotrowski NA, Tusel DJ, Sees KL, Reilly PM, Banys P, Meek P, Hall SM. Contingency contracting with monetary reinforcers for abstinence from multiple drugs in a methadone program. Experimental and Clinical Psychopharmacology. 1999;7:399–411. doi: 10.1037//1064-1297.7.4.399. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. Journal of Consulting and Clinical Psychology. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Abstinence reinforcement maintenance contingency and one-year follow-up. Drug and Alcohol Dependence. 2002;67:125–137. doi: 10.1016/s0376-8716(02)00023-6. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM., III . The Diagnostic Interview Schedule, version IV. Washington University; St. Louis, MO: 1995. [Google Scholar]
- Robles E, Silverman K, Preston KL, Cone EJ, Katz E, Bigelow GE, Stitzer ML. The brief abstinence test: voucher-based reinforcement of cocaine abstinence. Drug and Alcohol Dependence. 2000;58:205–212. doi: 10.1016/s0376-8716(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Rosen MI, Dieckhaus K, McMahon TJ, Valdes B, Petry NM, Cramer J, Rounsaville B. Improved adherence with contingency management. AIDS Patient Care and STDS. 2007;21:30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- Schroeder JR, Epstein DH, Umbricht A, Preston KL. Changes in HIV risk behaviors among patients receiving combined pharmacological and behavioral interventions for heroin and cocaine dependence. Addictive Behaviors. 2006;31:868–879. doi: 10.1016/j.addbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug and Alcohol Dependence. 2005;78:125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996a;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, Umbricht-Schneiter A, Schuster CR, Preston KL. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug and Alcohol Dependence. 1996b;41:157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Copeland AL. Drug abuse treatment as an HIV prevention strategy: a review. Drug and Alcohol Dependence. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, Tulsky JP, Barnett P, Hall S. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug and Alcohol Dependence. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Iguchi MY, Felch LJ. Contingent take-home incentive: effects on drug use of methadone maintenance patients. Journal of Consulting and Clinical Psychology. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- Thiede H, Hagan H, Murrill CS. Methadone treatment and HIV and hepatitis B and C risk reduction among injectors in the Seattle area. Journal of Urban Health. 2000;77:331–345. doi: 10.1007/BF02386744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahabzadeh M, Lin J-L, Epstein DH, Mezghanni M, Schmittner J, Preston KL. Computerized contingency management for motivating behavior change: automated tracking and dynamic reward reinforcement management; Proc. 20th IEEE International Symposium on Computer-Based Medical Systems (CBMS 2007); 2007. pp. 85–90. [Google Scholar]
- Willenbring ML, Hagedorn HJ, Postier AC, Kenny M. Variations in evidence-based clinical practices in nine United States Veteran Administration opioid agonist therapy clinics. Drug and Alcohol Dependence. 2004;75:97–106. doi: 10.1016/j.drugalcdep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- World Health Organization Online at: http://www.who.int/hiv/mediacentre/news62/en/index.html <Accessed July 5, 2007>.