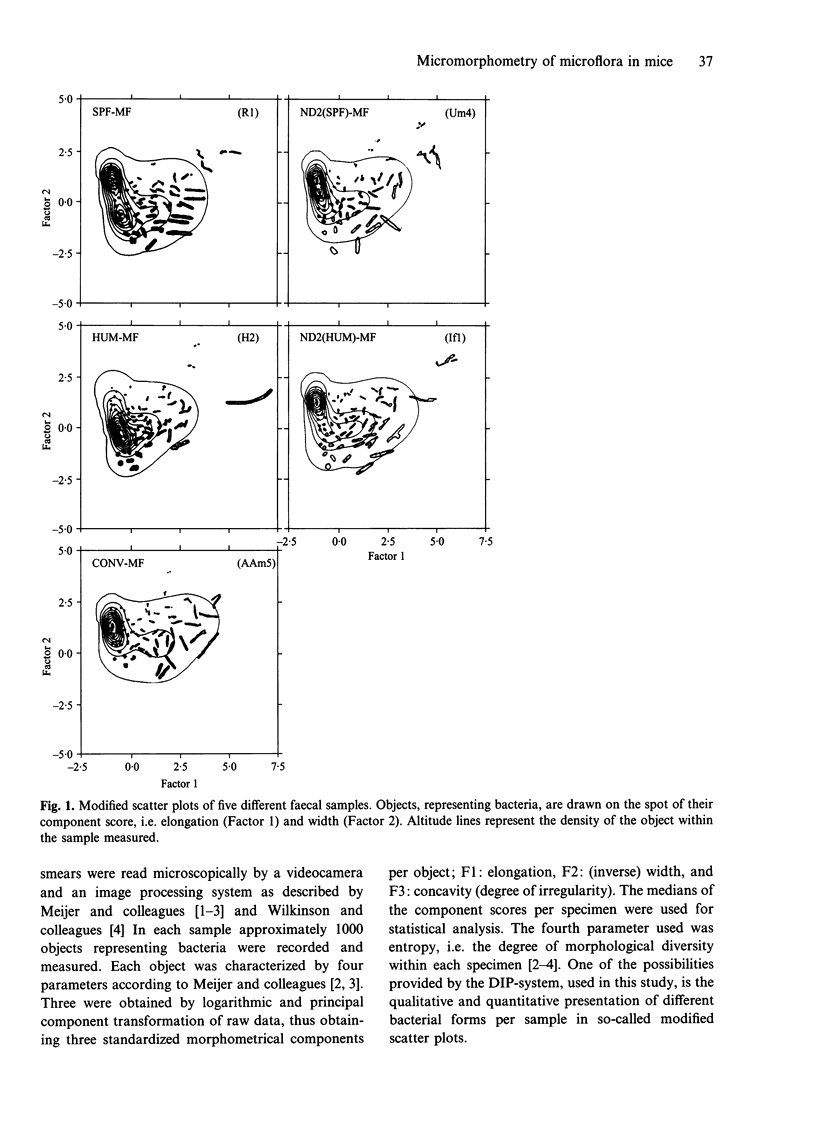
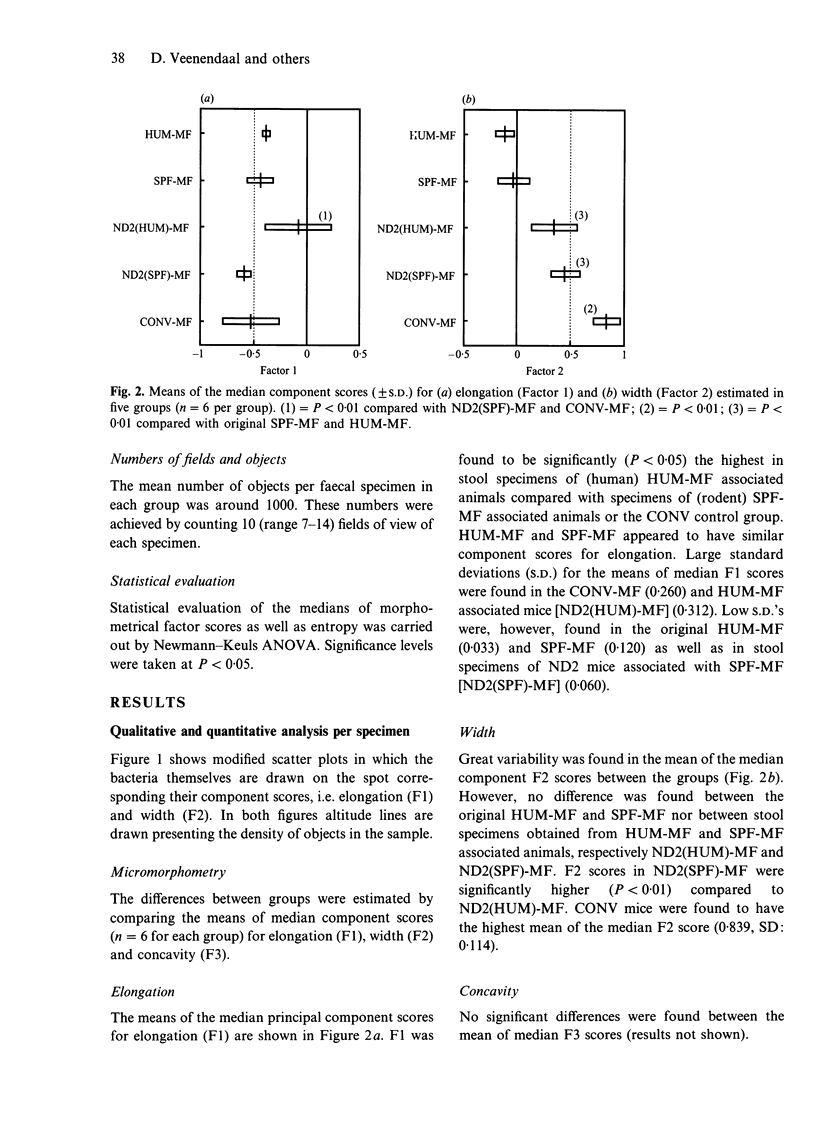
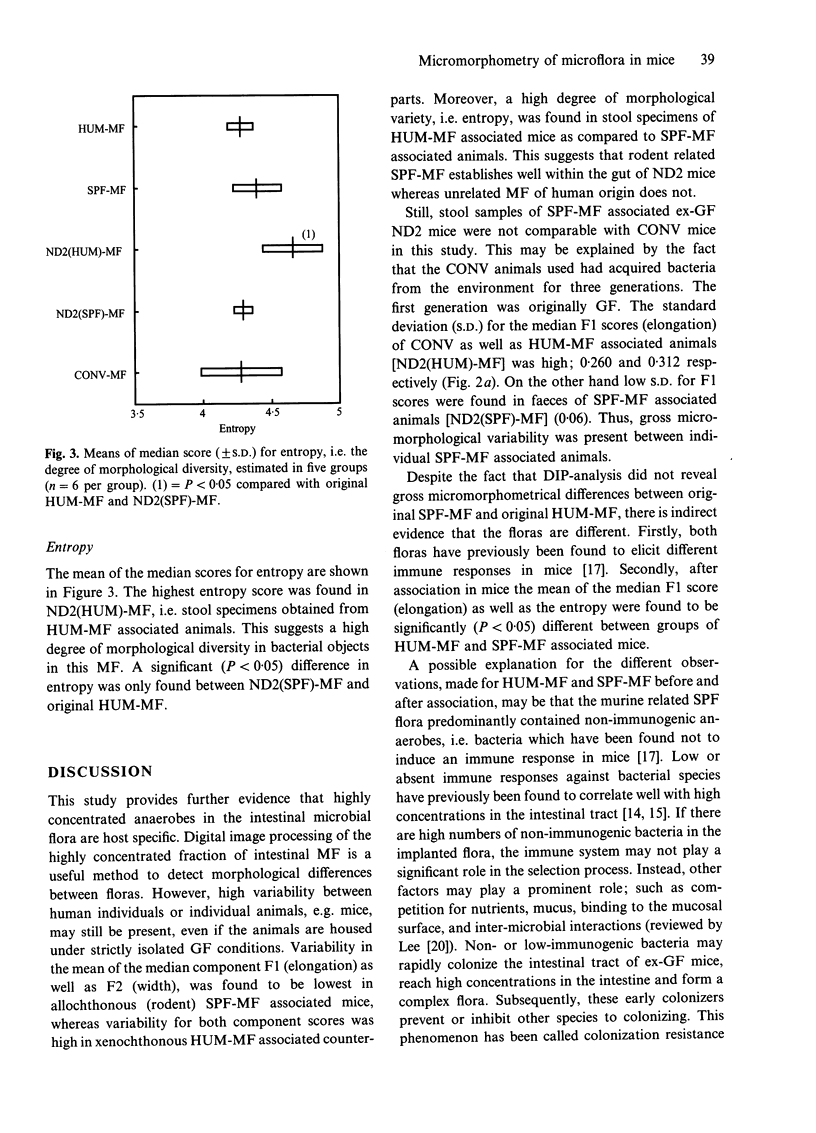
Abstract
Digital image processing (DIP) of bacterial smears is a new method of analysing the composition of the gut microbial flora. This method provides the opportunity to compare and evaluate differences in the complex highly concentrated anaerobic fraction of gut microbial flora, based on micromorphological differences. There is ample evidence that this fraction can be characterized as related or unrelated to the host organism by its immunogenicity. In this study germfree ND2 mice were associated with either related (rodent) SPF microflora (SPF-MF) or unrelated human MF (HUM-MF). DIP analysis was performed on original SPF-MF and HUM-MF and on the faeces of ex-germfree mice 4 weeks after association. The micromorphological pattern of highly concentrated anaerobic bacteria in faeces of HUM-MF associated ex-germfree mice was significantly different from SPF-MF associated counterparts with regard to the scores for elongation (P < 0.01) and morphological variety (P < 0.05). Moreover, gross morphological variability was present between individual HUM-MF associated mice but not between individual SPF-MF associated animals. No differences were found between original SPF and HUM-MF. The data are discussed with regard to differences in the presence of (non-)immunogenic bacteria and the ability for related and unrelated flora to colonize the murine gut. This study provides evidence that murine host specificity of microbial flora may not only be reflected in the number of non-immunogenic bacteria but also in the micromorphological pattern of highly concentrated anaerobic bacteria in faeces measured by DIP analysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. D., Savage D. C. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975 Feb;11(2):320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo M. C., Lee A. Immunological response of mice to members of the autochthonous intestinal microflora. Infect Immun. 1972 Oct;6(4):525–532. doi: 10.1128/iai.6.4.525-532.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer-Severs G. J., van Santen E. Variations in the anaerobic faecal flora of ten healthy human volunteers with special reference to the Bacteroides fragilis-group and Clostridium difficile. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Feb;261(1):43–52. doi: 10.1016/s0176-6724(86)80061-x. [DOI] [PubMed] [Google Scholar]
- Meijer B. C., Kootstra G. J., Geertsma D. G., Wilkinson M. H. Effects of ceftriaxone on faecal flora: analysis by micromorphometry. Epidemiol Infect. 1991 Jun;106(3):513–521. doi: 10.1017/s0950268800067571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer B. C., Kootstra G. J., Wilkinson M. H. Morphometrical parameters of gut microflora in human volunteers. Epidemiol Infect. 1991 Oct;107(2):383–391. doi: 10.1017/s0950268800049025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Kager L. The normal flora of the gastrointestinal tract. Neth J Med. 1984;27(7):249–252. [PubMed] [Google Scholar]
- Van de Merwe J. P., Stegeman J. H., Hazenberg M. P. The resident faecal flora is determined by genetic characteristics of the host. Implications for Crohn's disease? Antonie Van Leeuwenhoek. 1983 Jun;49(2):119–124. doi: 10.1007/BF00393669. [DOI] [PubMed] [Google Scholar]
- Veenendaal D., de Boer F., Van der Waaij D. Effect of selective decontamination of the digestive tract of donor and recipient on the occurrence of murine delayed-type graft-versus-host disease. Med Microbiol Immunol. 1988;177(3):133–144. doi: 10.1007/BF00232893. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. H., Jansen G. J., van der Waaij D. Computer processing of microscopic images of bacteria: morphometry and fluorimetry. Trends Microbiol. 1994 Dec;2(12):485–489. doi: 10.1016/0966-842x(94)90653-x. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971 Sep;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij D. Evidence of immunoregulation of the composition of intestinal microflora and its practical consequences. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):103–106. doi: 10.1007/BF01962193. [DOI] [PubMed] [Google Scholar]
- van der Waaij D. The persistent absence of Enterobacteriaceae from the intestinal flora of mice following antibiotic treatment. J Infect Dis. 1968 Feb;118(1):32–38. doi: 10.1093/infdis/118.1.32. [DOI] [PubMed] [Google Scholar]


