Abstract
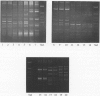
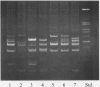
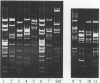
Several typing systems have been described for Campylobacter jejuni and C. coli, to assess the complex epidemiology of these important enteric pathogens. In the present study two typing methods, slide agglutination according to the Lior scheme, and the demonstration of restriction-fragment length polymorphisms (RFLP) of flagellar genes, have been used in parallel on a set of 194 strains. This set comprised 118 sero-reference strains of C. jejuni and C. coli of the Lior scheme, as well as 76 clinical isolates. All isolates were serotyped and subjected to PCR for amplification of flagellar genes, and the PCR product was restricted with Alu I. Flagellar genes could be amplified in 152 strains. Among 85 seroreference strains, 74 different RFLP patterns were observed, and among 67 clinical isolates, there were 36 patterns. There was only limited correlation between flagellar RFLP and the Lior serogroup, and the variability of patterns in serogroups HL2 and HL4 were as marked as the variability between serogroups. Flagellar gene RFLP patterns are shown to be stable, highly discriminatory epidemiologic markers.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm R. A., Guerry P., Power M. E., Lior H., Trust T. J. Analysis of the role of flagella in the heat-labile Lior serotyping scheme of thermophilic Campylobacters by mutant allele exchange. J Clin Microbiol. 1991 Nov;29(11):2438–2445. doi: 10.1128/jcm.29.11.2438-2445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm R. A., Guerry P., Trust T. J. Distribution and polymorphism of the flagellin genes from isolates of Campylobacter coli and Campylobacter jejuni. J Bacteriol. 1993 May;175(10):3051–3057. doi: 10.1128/jb.175.10.3051-3057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- Fischer S. H., Nachamkin I. Common and variable domains of the flagellin gene, flaA, in Campylobacter jejuni. Mol Microbiol. 1991 May;5(5):1151–1158. doi: 10.1111/j.1365-2958.1991.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Guerry P., Logan S. M., Thornton S., Trust T. J. Genomic organization and expression of Campylobacter flagellin genes. J Bacteriol. 1990 Apr;172(4):1853–1860. doi: 10.1128/jb.172.4.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. A., Logan S. M., Guerry P., Trust T. J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987 Nov;169(11):5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Skjerve E., Vik L., Hauge K., Lysaker A., Aalmen I., Ostroff S. M., Potter M. Epidemiological investigation of risk factors for campylobacter colonization in Norwegian broiler flocks. Epidemiol Infect. 1993 Oct;111(2):245–255. doi: 10.1017/s0950268800056958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilger G., Grimont P. A. Differentiation of Salmonella phase 1 flagellar antigen types by restriction of the amplified fliC gene. J Clin Microbiol. 1993 May;31(5):1108–1110. doi: 10.1128/jcm.31.5.1108-1110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lior H., Woodward D. L., Edgar J. A., Laroche L. J., Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 1982 May;15(5):761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I., Bohachick K., Patton C. M. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J Clin Microbiol. 1993 Jun;31(6):1531–1536. doi: 10.1128/jcm.31.6.1531-1536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten P. J., van Asten F. J., Gaastra W., van der Zeijst B. A. Structural and functional analysis of two Campylobacter jejuni flagellin genes. J Biol Chem. 1990 Oct 15;265(29):17798–17804. [PubMed] [Google Scholar]
- Owen R. J., Fayos A., Hernandez J., Lastovica A. PCR-based restriction fragment length polymorphism analysis of DNA sequence diversity of flagellin genes of Campylobacter jejuni and allied species. Mol Cell Probes. 1993 Dec;7(6):471–480. doi: 10.1006/mcpr.1993.1070. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Fitzgerald C., Sutherland K., Borman P. Flagellin gene polymorphism analysis of Campylobacter jejuni infecting man and other hosts and comparison with biotyping and somatic antigen serotyping. Epidemiol Infect. 1994 Oct;113(2):221–234. doi: 10.1017/s0950268800051657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C. M., Nicholson M. A., Ostroff S. M., Ries A. A., Wachsmuth I. K., Tauxe R. V. Common somatic O and heat-labile serotypes among Campylobacter strains from sporadic infections in the United States. J Clin Microbiol. 1993 Jun;31(6):1525–1530. doi: 10.1128/jcm.31.6.1525-1530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C. M., Wachsmuth I. K., Evins G. M., Kiehlbauch J. A., Plikaytis B. D., Troup N., Tompkins L., Lior H. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter strains. J Clin Microbiol. 1991 Apr;29(4):680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Congi R. V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983 Aug;2(4):378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]