Abstract
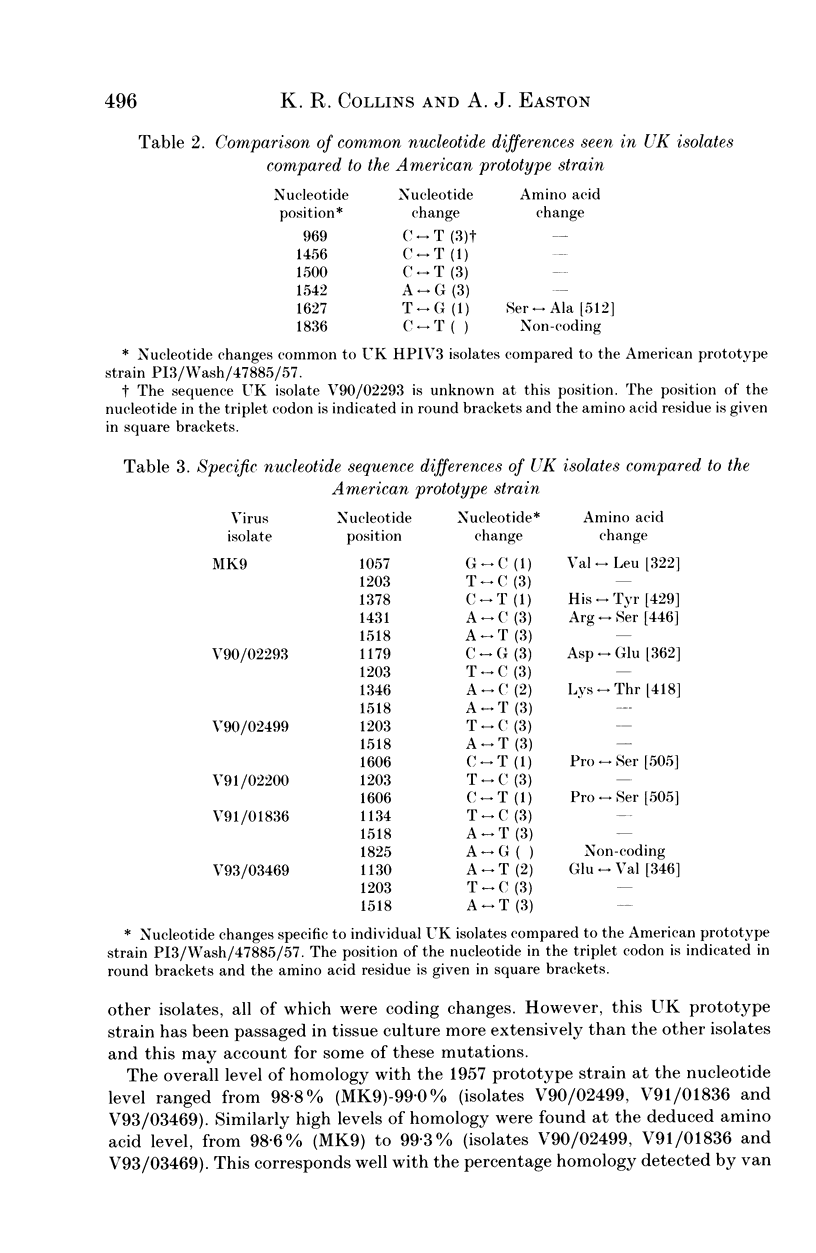
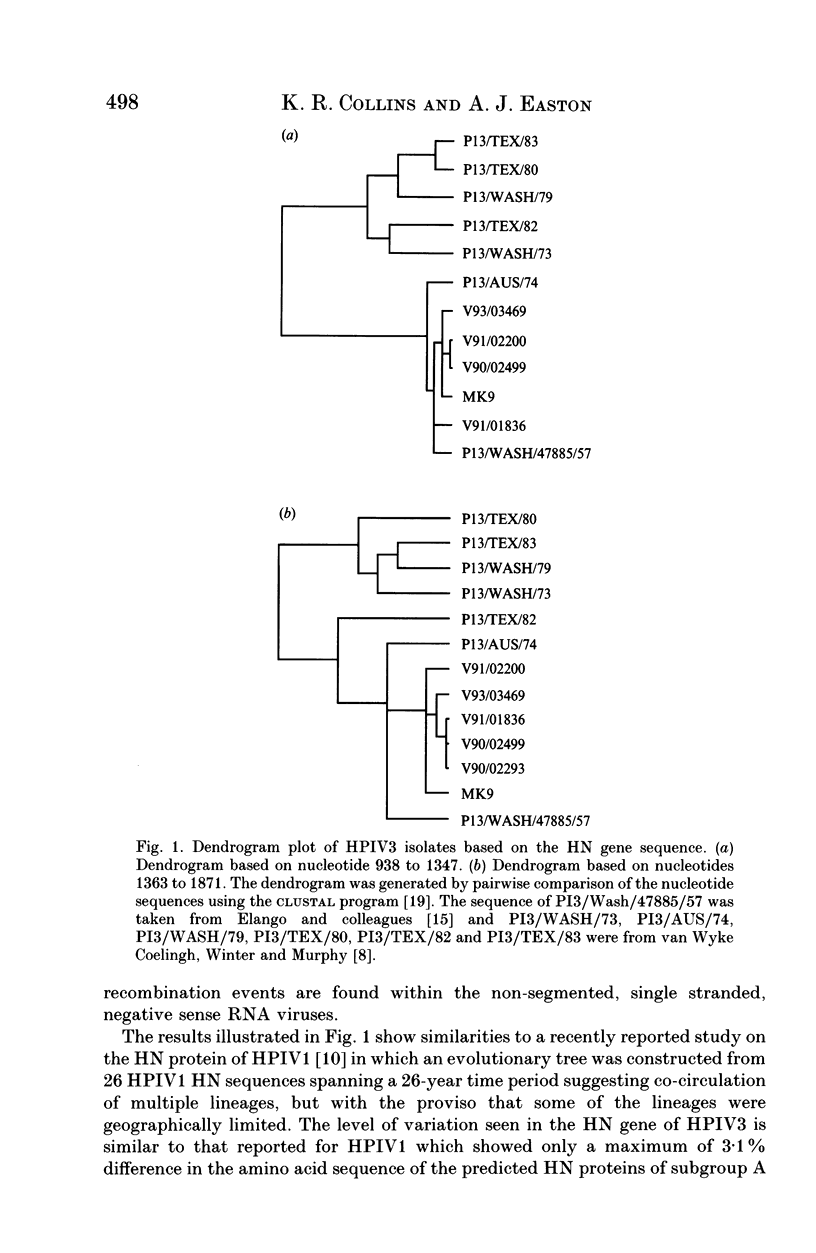
The sequence variation in a 934 base-pair region of the gene encoding the haemagglutinin-neuraminidase of five human parainfluenza virus type 3 (HPIV3) isolates was determined together with that of a prototype UK strain. All of the clinical isolates were from the Manchester area of the UK and were obtained in 1990, 1991 and 1993. The gene segment was amplified by the polymerase chain reaction using HPIV3-specific oligonucleotide primers. The nucleotide homology of the strains was high, around 99% and specific differences in the UK sequences when compared with that of the US prototype strain were identified. In addition, a number of isolate-specific differences were seen. No correlation was detected between the observed nucleotide mutations and the year of isolation, which supports the hypothesis that HPIV3 shows cocirculation of a heterogeneous population of viruses rather than varying with time in a linear fashion. However, the data suggested that geographically-defined genetic lineages of HPIV3 may exist.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buonagurio D. A., Nakada S., Desselberger U., Krystal M., Palese P. Noncumulative sequence changes in the hemagglutinin genes of influenza C virus isolates. Virology. 1985 Oct 30;146(2):221–232. doi: 10.1016/0042-6822(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Cane P. A., Matthews D. A., Pringle C. R. Analysis of respiratory syncytial virus strain variation in successive epidemics in one city. J Clin Microbiol. 1994 Jan;32(1):1–4. doi: 10.1128/jcm.32.1.1-4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Hoyne P. A., Lawrence M. C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993 Jun;67(6):2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. J., Eglin R. P. Epidemiology of parainfluenza virus type 3 in England and Wales over a ten-year period. Epidemiol Infect. 1989 Jun;102(3):531–535. doi: 10.1017/s0950268800030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Coligan J. E., Jambou R. C., Venkatesan S. Human parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein: nucleotide sequence of mRNA and limited amino acid sequence of the purified protein. J Virol. 1986 Feb;57(2):481–489. doi: 10.1128/jvi.57.2.481-489.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Frank A. L., Taber L. H., Kasel J. A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984 Dec;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Henrickson K. J., Savatski L. L. Genetic variation and evolution of human parainfluenza virus type 1 hemagglutinin neuraminidase: analysis of 12 clinical isolates. J Infect Dis. 1992 Nov;166(5):995–1005. doi: 10.1093/infdis/166.5.995. [DOI] [PubMed] [Google Scholar]
- Hetherington S. V., Watson A. S., Scroggs R. A., Portner A. Human parainfluenza virus type 1 evolution combines cocirculation of strains and development of geographically restricted lineages. J Infect Dis. 1994 Feb;169(2):248–252. doi: 10.1093/infdis/169.2.248. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Jorgensen E. D., Collins P. L., Lomedico P. T. Cloning and nucleotide sequence of Newcastle disease virus hemagglutinin-neuraminidase mRNA: identification of a putative sialic acid binding site. Virology. 1987 Jan;156(1):12–24. doi: 10.1016/0042-6822(87)90431-4. [DOI] [PubMed] [Google Scholar]
- Klippmark E., Rydbeck R., Shibuta H., Norrby E. Antigenic variation of human and bovine parainfluenza virus type 3 strains. J Gen Virol. 1990 Jul;71(Pt 7):1577–1580. doi: 10.1099/0022-1317-71-7-1577. [DOI] [PubMed] [Google Scholar]
- Ling R., Easton A. J., Pringle C. R. Sequence analysis of the 22K, SH and G genes of turkey rhinotracheitis virus and their intergenic regions reveals a gene order different from that of other pneumoviruses. J Gen Virol. 1992 Jul;73(Pt 7):1709–1715. doi: 10.1099/0022-1317-73-7-1709. [DOI] [PubMed] [Google Scholar]
- Ray R., Compans R. W. Monoclonal antibodies reveal extensive antigenic differences between the hemagglutinin-neuraminidase glycoproteins of human and bovine parainfluenza 3 viruses. Virology. 1986 Jan 15;148(1):232–236. doi: 10.1016/0042-6822(86)90420-4. [DOI] [PubMed] [Google Scholar]
- Raymond F. L., Caton A. J., Cox N. J., Kendal A. P., Brownlee G. G. The antigenicity and evolution of influenza H1 haemagglutinin, from 1950-1957 and 1977-1983: two pathways from one gene. Virology. 1986 Jan 30;148(2):275–287. doi: 10.1016/0042-6822(86)90325-9. [DOI] [PubMed] [Google Scholar]
- Rydbeck R., Löve A., Orvell C., Norrby E. Antigenic analysis of human and bovine parainfluenza virus type 3 strains with monoclonal antibodies. J Gen Virol. 1987 Aug;68(Pt 8):2153–2160. doi: 10.1099/0022-1317-68-8-2153. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Krystal M., Fitch W. M., Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988 Mar;163(1):112–122. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Murphy B. R. Nucleotide and deduced amino acid sequence of hemagglutinin-neuraminidase genes of human type 3 parainfluenza viruses isolated from 1957 to 1983. Virology. 1988 Jan;162(1):137–143. doi: 10.1016/0042-6822(88)90402-3. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., Hall S. L., London W. T., Kim H. W., Chanock R. M., Murphy B. R. Antibody responses of humans and nonhuman primates to individual antigenic sites of the hemagglutinin-neuraminidase and fusion glycoproteins after primary infection or reinfection with parainfluenza type 3 virus. J Virol. 1990 Aug;64(8):3833–3843. doi: 10.1128/jvi.64.8.3833-3843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C., Murphy B. R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985 Jun;143(2):569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]


