Abstract
The purpose of our study was to examine the neuroanatomical correlates of late-onset minor and major depression and to compare them with similar measures obtained from nondepressed controls. Our study groups were comprised of 18 patients with late-onset minor depression, 35 patients diagnosed with late-onset major depression, and 30 nondepressed controls. All subjects were scanned by using a 1.5-tesla MRI scanner. Absolute whole brain volume and normalized measures of prefrontal and temporal lobe volumes were obtained and used for comparison among groups. Our findings indicate that patients with minor depression present with specific neuroanatomical abnormalities that are comparable with the major depression group but significantly different from the controls. Normalized prefrontal lobe volumes show a significant linear trend with severity of depression, with volumes decreasing with illness severity. Whole brain volumes did not differ significantly among groups. These findings have broad implications for the biology of late-life depression and suggest that there may be common neurobiological substrates that underlie all clinically significant forms of late-onset mood disturbances.
Minor and other nonmajor forms of clinical depression are more prevalent in adult and elderly populations than the more readily recognized major depressive disorder (MDD) (1–9). The higher prevalence of minor depression has been observed in primary care populations, community samples, and in more traditional psychiatric settings (1, 3, 4, 9). Minor depression consistently results in an increased use of health services and absenteeism together with loss of productivity and a decline in psychosocial well being (10–14). In addition, minor depression is a well recognized risk factor for MDD in adult and geriatric populations (15, 16). Additionally, family studies indicate that there may be a genetic predisposition to both major and minor forms of depression (17–20). Despite these observations, there is little understanding about the neurobiological substrates of minor depression, especially in the elderly (21). Minor depression is often conceptualized as a transient emotional response to life stressors with few biological underpinnings (22–27).
The neuroanatomical and physiological correlates of major depression in late-life have been relatively well characterized (28–41). Widespread reductions in glucose use and cerebral blood flow have been demonstrated in late-life MDD by using positron emission tomography (PET), Xenon 133 inhalation, and single photon emission-computed tomography (SPECT) (29, 30, 39–41). Glucose hypometabolism in MDD occurs in neocortical and subcortical regions (29). Both computerized tomography (CT) and MRI studies have shown that late-life MDD also is associated with widespread neuroanatomical changes in neocortical areas and subcortical nuclei (28, 31–38). However, the neuroanatomic and physiologic correlates of minor depression in late-life remain largely unknown.
In an earlier report (21), we demonstrated that patients with late-life minor depression presented with smaller prefrontal lobe volumes when compared with age-matched nondepressed controls. That was the first report to establish neurobiologic correlates to late-onset mood disorders hitherto categorized as “minor” by using standard psychiatric nosology. However, there are no studies that have specifically examined the neuroanatomical correlates of minor depression in relation to those with patients diagnosed with MDD. Therefore, the magnitude and extent of neuroanatomic changes in minor depression relative to the more widely studied MDD remain unclarified. In addition, the role of common neuroanatomical substrates to late-onset mood disorders in general remains unknown. Published reports on the genetic, pharmacologic, and other clinical aspects of minor depression focus largely on younger, adult populations (14, 16–19). We are not aware of other published data on the neurobiological correlates of minor depression occurring for the first time in late-life—late-onset minor depression (21).
The purpose of our current study was to examine estimates of global and focal brain volumes by using quantitative MRI in subjects belonging to three distinct categories: late-onset minor depression, late-onset MDD, and nondepressed older controls. Based on our earlier findings, we hypothesized that patients with late-onset minor depression would present with focal brain volume abnormalities that were comparable with, although of lesser magnitude than, similar measures in patients with late-onset MDD. More specifically, our a priori hypothesis was that patients with minor depression would present with prefrontal lobe volumes midway between the MDD and control groups.
METHODS
Subjects.
Our minor depression sample included 18 patients who met modified Diagnostic and Statistical Manual for Mental Disorders DSM IV research criteria for minor depression (42). All patients received a structured interview (SCID) based on the DSM-IV. Minor depression was defined operationally as the presence of low mood and/or loss of interest in activities and at least one additional depressive symptom from the DSM-IV checklist. A duration of illness of 1 mo or more also was required. The purpose of this criterion was to focus on patients in whom the minor depression was sustained for a period of time and did not merely represent transient dysphoria. In addition, a Hamilton Rating Scale for Depression score (HRSD) in the 8–16 range inclusive, by using the 17-item Hamilton scale, also was required (43). Six patients concurrently met DSM criteria for dysthymic disorder (minor depression of at least 2 years duration). All of the patients included in this minor depression group reported the index episode as their first episode of an affective disturbance. Information on prior episodes and the age of onset of current episode was obtained from patients and caregivers. Patients with minor depression and the controls were recruited from the community in response to advertisements in local newspapers and were part of a larger study designed to examine the neuroanatomic and neuropsychological profiles of elderly patients with minor depression. Patients with MDD were recruited from the ambulatory and inpatient geropsychiatry programs of the University of Pennsylvania. To meet our inclusion criteria, all MDD patients had to meet DSM IV criteria for the disorder and have a 17-item Hamilton Rating Scale for Depression score of 15 or greater. Quantitative MRI measures reported here are only from patients with late-onset minor and major depression—operationally defined as onset of the first episode occurring after age 60. This classification permits comparison of neuroanatomic measures between two groups of patients with mood disorders of different severity but with certain comparable phenomenologic characteristics. All subjects received comprehensive medical/neurologic exams and a battery of laboratory tests. None of the subjects had clinical evidence of dementia or any other brain disorder based on history and mental status examinations, and their mini-mental state exam scores (44) were in the normal range (Table 1). The Cumulative Illness Rating Scale (CIRS) was used to quantify comorbid medical disorders in both groups (45). The Cumulative Illness Rating Scale is a validated instrument that rates the dysfunction of six primary organ systems (cardiorespiratory, gastrointestinal, genitourinary, musculoskeletal, neurologic, and general systems) on a 0–4 severity scale and is commonly used in studies involving elderly subjects. The minor depression and control groups had a few stable comorbid medical disorders of comparable severity such as hypertension, diabetes, and arthritis, whereas patients with MDD had greater medical comorbidity (Table 1). A psychiatric exam and a structured interview (SCID) for normals were administered to all control subjects to rule out current or past psychopathology. Controls and patients were comparable in socioeconomic characteristics.
Table 1.
Principal demographic and clinical characteristics of study groups
| Index | Controls, n = 30 | Minors, n = 18 | Majors, n = 35 |
|---|---|---|---|
| Age | 69.43 (6.09) | 70.94 (8.69) | 74.57 (6.91) |
| Age onset | N/A | 69.44 (8.93) | 71.26 (6.58) |
| Gender | 23 F, 7 M | 9 F, 9 M | 25 F, 10 M |
| MMSE | 29.54 (0.69) | 28.53 (2.03) | 27.29 (2.67) |
| CIRS | 2.29 (1.44) | 2.75 (2.11) | 4.42 (3.65) |
| HAMD | N/A | 12.33 (2.03) | 19.74 (3.78) |
MMSE, Mini Mental State Exam; CIR, Cumulative Illness Rating Scale; HAMD, Hamilton Depression Scale Score; N/A, not applicable.
MRI Methods.
MRI scans were obtained on a 1.5-tesla General Electric scanner with head coil in planes parallel to the canthomeatal line. Both T2 and proton density weighted images were obtained in all subjects [reception time (TR) = 3,000, echo time (TE) = 30 and 80 msec], and the images were displayed on a 256×256 matrix, with pixel size of 0.86 mm, and field of view of 22 cm (46, 47). Axial slices were 5-mm thick and contiguous. A segmentation program developed within the Department of Radiology at the University of Pennsylvania and previously used to examine neuroanatomical changes in subjects with major depression, schizophrenia, and dementia of the Alzheimer type (DAT) was used in our image analysis (46, 47). The temporal lobe outlined in our analysis included both lateral and mesial temporal structures, and the prefrontal cortex did not include the sensorimotor region (48). Details of the technique used to segment brain from cerebrospinal fluid and the anatomic boundaries and landmarks used to delineate the prefrontal and temporal lobes have been described (48). Adequate interrater reliability was established between raters for all anatomical analyses (intraclass correlation coefficient of 0.80). Quantitative measures of whole brain volume were obtained together with estimates of prefrontal and temporal lobe volumes. Measures of focal brain volume were additionally normalized to the intracranial volume (brain plus cerebrospinal fluid) to correct for individual differences in head size.
Statistics.
A linear regression was used to compare the absolute whole brain and normalized prefrontal and temporal lobe volumes between the two depressed groups and between each of these groups and the control group while adjusting for current age. To control for potential gender and age influences, we adjusted for gender, age, and possible age by gender interaction in our models. The predictor gender and the age by gender interaction were not significant in any of the regression models. We also used a linear regression to compare normalized focal brain volume (prefrontal and temporal) in all three groups while also adjusting for age, gender, and age by gender interaction. In a secondary analysis, we introduced total Cumulative Illness Rating Scale scores as another covariate in the regression, in addition to existing controls for age and gender, to control for the impact of overall medical burden on atrophy.
RESULTS
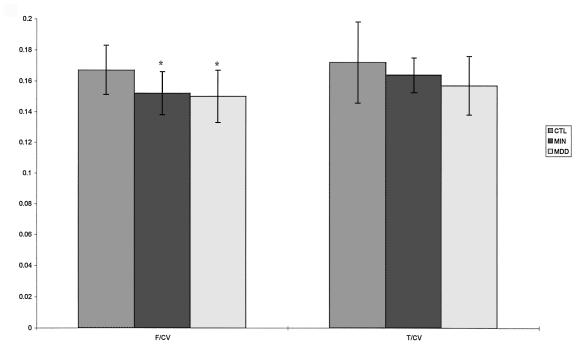
Normalized prefrontal lobe volumes in both the major and minor depression groups were significantly smaller (P < 0.05) when compared with the control group (Table 2). Prefrontal lobe volumes in the minor depression group were in between the means for the control and the MDD groups (Fig. 1). Normalized temporal lobe volumes in the minor depression group were also smaller than the controls and lie between the MDD and control means, although this trend only approached statistical significance (Fig. 1). There were no statistically significant differences between the major and minor depression groups on any measure of brain volume. Although we present absolute prefrontal and temporal lobe volumes in Table 2, statistical analyses of focal volumes were performed only on normalized measures. Whole brain volumes did not differ across groups.
Table 2.
Absolute and normalized brain volumes for the three groups
| Index | Controls, n = 30 | Minors, n = 18 | Majors, n = 35 |
|---|---|---|---|
| Total brain volume, cc | 1008.65 (128.7) | 1031.34 (147.78) | 950.26 (139.54) |
| Intracranial volume | 1182.65 (137.98) | 1206.89 (145.44) | 1138.20 (160.63) |
| Frontal brain, cc | 197.51 (31.09) | 183.62 (33.21) | 171.50 (31.83) |
| Temporal brain, cc | 202.5 (35.1) | 197.82 (31.74) | 178.08 (31.63) |
| Frontal brain/CV | 0.167 (0.016) | 0.152* (0.014) | 0.150* (0.017) |
| Temporal brain/CV | 0.172 (0.026) | 0.164 (0.011) | 0.157 (0.019) |
Significant difference from controls, P < 0.05 after controlling for age, gender, and age × gender interactions. CV, intracranial volume.
Figure 1.
A bar graph depicting normalized prefrontal and temporal lobe volumes in the three groups. CTL, controls; MIN, minor depression; MDD, major depression; F/CV, prefrontal volume/cranial volume; T/CV, temporal lobe volume/cranial volume. ∗, significantly different from controls, P < 0.05. SD bars are depicted in the figure.
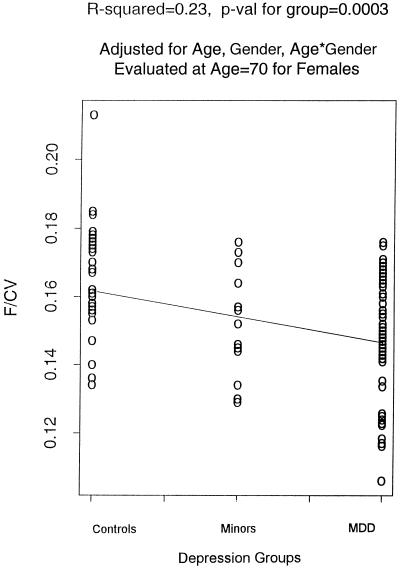
Normalized prefrontal lobe volumes showed a significant linear trend with severity of clinical depression, i.e., volumes of prefrontal lobes decrease with increasing severity of illness from the controls to the minor depression group to the group with MDD, after controlling for age, gender, and age by gender interactions (Fig. 2). When we controlled for differences in overall medical comorbidity using total Cumulative Illness Rating Scale scores, the linear gradation in prefrontal lobe volumes among the three groups remained significant.
Figure 2.
A regression plot for normalized prefrontal volume in all of the three groups after age and gender corrections. F/CV, prefrontal volume/cranial volume
DISCUSSION
This report demonstrates that patients with late-onset minor depression present with neuroanatomical changes that are comparable to, although of smaller magnitude, than those observed in patients diagnosed with MDD. Further, the neuroanatomical abnormalities detected by using quantitative MRI show a linear relationship to severity of clinical depression, with the minor depression group presenting with prefrontal lobe volumes that lie between the late-onset MDD and control groups. The prefrontal volume differences across groups remained statistically significant after controlling for the potential influence of age, gender, and overall medical burden. Normalized temporal lobe volumes also show a decrease with depression severity, although this trend was not statistically significant.
The prefrontal cortex has undergone more phylogenetic evolution than the rest of the cortex, reaching a maximum size and anatomical complexity in humans (49–51). The prefrontal cortex has reciprocal connections with several limbic, diencephalic, and other neocortical structures (49–51). Either directly or through the thalamus, the prefrontal cortex receives afferents from the hypothalamus, subthalamus, mesencephalon, and the limbic system. The prefrontal cortex also sends efferent connections to several structures including the basal ganglia from which it does not receive any input (49–51). Although the precise nature of these inputs is unclear, inputs from the hypothalamus and the amygdala are presumed to be involved in the regulation and modulation of affect and motivation. Evidence from primate and human studies demonstrates an important role for the prefrontal region in behavioral regulation consistent with its extensive interconnections with other brain regions (49–51). Prefrontal atrophy, by disrupting these neural circuits, could result in a broad array of behavioral and cognitive abnormalities. In vivo imaging in humans corroborates these neurobiologic observations and demonstrates prefrontal involvement in diverse psychiatric disorders such as schizophrenia, frontotemporal dementia, attention deficit, and obsessive compulsive disorders (52–56). Although the precise mechanisms by which neuroanatomical compromise leads to mood disturbances remain unknown, it is likely that gray and white matter compromise leads to impairment in several stages of neurotransmitter metabolism and other functional links between critical brain regions. These may lead to a reduced neuronal reserve that increases vulnerability to mood disorders. A combination of mechanisms may therefore be involved, with clinical depression representing the end point of the various neurobiological aberrations.
Smaller brain volumes and larger high intensity lesion volumes have been demonstrated in patients with major depression when compared with controls (28, 31–34). The atrophy demonstrated in patients with MDD by using MRI is more pronounced in, but not restricted to, the prefrontal lobe (28, 33). Earlier findings from our laboratory indicate that an increase in cerebrospinal fluid volume (serving as an indirect measure of atrophy) and overall medical burden, increases the odds ratio for the prevalence of existing MDD (28, 57). The primary finding of our current study, that prefrontal brain volumes decrease with severity of illness in late-life, extends our earlier observations to indicate that atrophy probably has a pathophysiologic role in all clinically significant late-onset mood disorders. Depression in late-life is frequently associated with medical comorbidity (58–60). Other investigators have found that selected samples of MDD patients, free of medical comorbidity, show a focal decrease in hippocampal volume without a concomitant increase in lesion volumes when compared with controls (38). In our study, statistically controlling for overall medical comorbidity does not reduce the significance of brain volume gradation across our study groups. Collectively, these findings suggest that the role of atrophy in the pathophysiology of late-onset mood disorders may be independent of medical comorbidity that is often associated with it clinically.
Several longitudinal studies indicate that the relationship of minor/nonmajor forms of depression to MDD is dynamic and complex (61–78). Patients diagnosed as having either disorder cross-sectionally may at various points during the course of their illness meet criteria for the other disorder (61, 71, 77, 78). Minor depression is a risk factor for MDD in both adult and elderly populations (15, 16, 61). In some study samples, up to 25% of patients diagnosed with minor depression developed major depression within 2 years (9). Other studies show either a persistence or episodic emergence of symptoms, with striking functional and psychological compromise, in patients with an initial presentation consistent with minor depression (70, 75, 77, 79–81). In addition, minor depression also appears as a sequelae of major depression in partial remission (61, 71, 72). In elderly study samples, late-onset major depression is a risk factor for clinical dementia, especially dementia of the Alzheimer type (DAT) (82). The natural history of late-onset minor depression, especially its relationship to dementia, remains unknown. It is tempting to speculate that minor depression patients with preexisting neuronal compromise, such as the subgroup with smaller prefrontal lobe volumes, are more likely to develop dementia on follow up. Longitudinal studies specifically designed to answer these related sets of questions are necessary before a definitive statement can be made.
Genetic studies assessing morbidity risks for mood disorders in first degree relatives of index cases with depression (major and minor), demonstrate comparably elevated risks in relatives of patients with both major and minor depression (17, 18). Other studies indicate that the risk of minor depression is elevated in families of probands with major unipolar depression (20). These reports suggest that at least in certain nonelderly adult populations, major and minor forms of depression may be genetically indistinguishable. Neuropsychological studies demonstrate that patients diagnosed with minor depression, like those with MDD, present with compromise in similar cognitive domains such as executive functions and memory (unpublished observations). Similarities in neurophysiological measures, family histories, and clinical features, collectively indicate that a common set of neurobiological substrates may underlie all degrees of clinically significant mood disorders in adult and more elderly populations (62). Our findings additionally suggest that major, minor, and possibly other commonly encountered clinical variants of depression may be better conceptualized as a continuum of disorders rather than as distinct clinical entities. Nonmajor forms of depression probably represent a heterogenous group of disorders that have one primary feature in common: clinically significant mood disorders that do not meet the currently accepted severity threshold for MDD (21, 83, 84). Under this rubric, there are likely to be several subcategories such as subsyndromal depression and dysthymic disorder with relatively subtle clinical distinctions demarcating the boundaries between them (83, 84). The DSM IV definition of minor depression and our adaptation of these criteria are attempts to provide an operational definition and some clarity to this otherwise nebulous entity.
In summary, our data indicate that patients with late-onset minor depression present with neuroanatomical abnormalities on MRI that are comparable to those observed in patients with late-onset MDD. These data, in addition to providing evidence in support of a neuroanatomic basis to different degrees of late-onset mood disturbance, also corroborate genetic and other epidemiologic reports, in younger adult populations, that establish biological similarities between major and minor forms of depression. Neuroanatomical abnormalities may represent one aspect of a broader neurobiological diathesis to mood disorders in late-life. In patients with such a genetic/biologic predisposition to an affective disorder, the clinical variants of depression, frequently encountered, may represent different phenotypic endpoints of neuronal compromise that emerge in the presence of other central nervous system insults like medical comorbidity and psychosocial stressors. Additional studies combining neuroimaging with focused postmortem and other neurochemical studies are required to further elucidate the biological basis of mood disorders occurring in late-life.
Acknowledgments
We wish to thank Drs. Steven Arnold and Ira Katz and Jeanine Makara for their support and technical assistance. Supported by National Institute of Mental Health Grant MH 55115, a Clinical Research Center in Late-Life Mood Disorders grant (MH 52129), and support from the Charles A. Dana Foundation.
ABBREVIATIONS
- MDD
major depressive disorder
References
- 1.Blazer D G. N Engl J Med. 1989;320:164–166. doi: 10.1056/NEJM198901193200306. [DOI] [PubMed] [Google Scholar]
- 2.Blazer D G. Am J Psychiatry. 1994;151:1567–1569. doi: 10.1176/ajp.151.11.1567. [DOI] [PubMed] [Google Scholar]
- 3.Blacker C V R, Clare A W. Br J Psychiatry. 1987;150:737–751. doi: 10.1192/bjp.150.6.737. [DOI] [PubMed] [Google Scholar]
- 4.Parmalee P A, Katz I R, Lawton M P. J Gerontol. 1989;44:M22–M29. doi: 10.1093/geronj/44.1.m22. [DOI] [PubMed] [Google Scholar]
- 5.Blazer D. Curr Opin Psychiatry. 1991;4:596–599. [Google Scholar]
- 6.Barrett J E, Barrett J A, Oxman T E, Gerber P D. Arch Gen Psychiatry. 1988;45:1100–1106. doi: 10.1001/archpsyc.1988.01800360048007. [DOI] [PubMed] [Google Scholar]
- 7.Tollefson G, Hughes E, Derro R A, Teubner-Rhodes D, Tuason V B. Compr Psychiatry. 1983;24:144–153. doi: 10.1016/0010-440x(83)90102-5. [DOI] [PubMed] [Google Scholar]
- 8.Oxman T E, Barrett J E, Barrett J, Gerber P. Gen Hosp Psychiatry. 1987;9:167–173. doi: 10.1016/0163-8343(87)90002-8. [DOI] [PubMed] [Google Scholar]
- 9.Beck D A, Koenig H G. Int J Psychiatry Med. 1996;26(2):177–209. doi: 10.2190/AC30-P715-Y4TD-J7D2. [DOI] [PubMed] [Google Scholar]
- 10.Broadhead W E, Blazer D G, George L K, Tse C K. J Am Med Assoc. 1990;264:2524–2528. [PubMed] [Google Scholar]
- 11.Johnson J, Weissman M M, Klerman G L. J Am Med Assoc. 1992;267:1478–1483. [PubMed] [Google Scholar]
- 12.Wells K B, Burnam M A, Rogers W, Hays R D, Camp P. Arch Gen Psychiatry. 1992;49:788–794. doi: 10.1001/archpsyc.1992.01820100032007. [DOI] [PubMed] [Google Scholar]
- 13.Judd L L, Paulus M P, Wells K B, Rapaport M H. Am J Psychiatry. 1996;153:1411–1417. doi: 10.1176/ajp.153.11.1411. [DOI] [PubMed] [Google Scholar]
- 14.Skodol A E, Schwartz S, Dohrenwend B P, Levav I, Shrout P E. Arch Gen Psychiatry. 1994;54:542–551. doi: 10.1001/archpsyc.1994.03950070034008. [DOI] [PubMed] [Google Scholar]
- 15.Parmalee P A, Katz I R, Lawton M P. J Gerontol. 1992;47:M189–M196. doi: 10.1093/geronj/47.6.m189. [DOI] [PubMed] [Google Scholar]
- 16.Sherbourne C D, Wells K B, Hays R D, Rogers W, Burnam M A, Judd L L. Am J Psychiatry. 1994;151:1777–1784. doi: 10.1176/ajp.151.12.1777. [DOI] [PubMed] [Google Scholar]
- 17.Horwath E, Johnson J, Klerman G L, Weissman M M. Arch Gen Psychiatry. 1992;49:817–823. doi: 10.1001/archpsyc.1992.01820100061011. [DOI] [PubMed] [Google Scholar]
- 18.Maier W, Lichtermann D, Minges J, Heun R, Hallmayer J. Eur Arch Psychiatry Clin Neurosci. 1992;242:89–92. doi: 10.1007/BF02191553. [DOI] [PubMed] [Google Scholar]
- 19.Akiskal H S, Weise R E. Am J Psychother. 1992;46:9–22. doi: 10.1176/appi.psychotherapy.1992.46.1.9. [DOI] [PubMed] [Google Scholar]
- 20.Remick R A, Sadovnick A D, Lam R W, Zis A P, Yee I M L. Am J Med Genet Suppl (New York) 1996;67:347–353. doi: 10.1002/(SICI)1096-8628(19960726)67:4<347::AID-AJMG6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, Schweizer E, Zhisong J, Miller D, Bilker W, Swan L L, Gottlieb G. Arch Neurol. 1997;54:613–617. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- 22.Brown G W, Craig T K J, Harris T O. Br J Psychiatry. 1985;147:612–622. doi: 10.1192/bjp.147.6.612. [DOI] [PubMed] [Google Scholar]
- 23.Philipp, M. (1993) Int. Clin. Psychopharmacol. 8, Suppl.1, 35–37. [DOI] [PubMed]
- 24.Himmelhoch J M, Auchenbach R, Fuchs C Z. J Clin Psychiatry. 1982;43:26–32. [PubMed] [Google Scholar]
- 25.Gold M S, Pottash A L C, Extein I, Martin D M, Howard E, Mueller E A, Sweeney D R. Psychoneuroendocrinology. 1981;6:159–169. doi: 10.1016/0306-4530(81)90008-1. [DOI] [PubMed] [Google Scholar]
- 26.Beekman A T F, Deeg D H J, van Tilburg T, Smit J H, Hooijer C, van Tilburg W. J Affect Disord. 1995;36:65–75. doi: 10.1016/0165-0327(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 27.Draper B, Anstey K. Aust N Z J Psychiatry. 1996;30:567–572. doi: 10.3109/00048679609062651. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Miller D, Ewbank D. Am J Geriatr Psychiatry. 1997;5:15–25. [PubMed] [Google Scholar]
- 29.Kumar A, Newberg A, Alavi A, Berlin J, Smith R, Reivich M. Proc Natl Acad Sci USA. 1993;90:7019–7023. doi: 10.1073/pnas.90.15.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackheim H A, Prohovnik I, Moeller J R. Arch Gen Psychiatry. 1990;47:60–70. doi: 10.1001/archpsyc.1990.01810130062009. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan K R R, Goli V, Ellinwood E H, France R D, Blazer D G, Nemeroff C B. Biol Psychiatry. 1988;25:519–522. doi: 10.1016/0006-3223(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 32.Coffey C E, Figiel G S, Djang W T, Weiner R D. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- 33.Coffey C E, Wilkinson W E, Weiner R D. Arch Gen Psychiatry. 1993;50:7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- 34.Rabins P V, Pearlson G D, Aylward E, Kumar A J, Dowell K. Am J Psychiatry. 1991;148:617–620. doi: 10.1176/ajp.148.5.617. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan K R R. J Geriatr Psychiatry Neurol. 1993;6:39–58. doi: 10.1177/002383099300600107. [DOI] [PubMed] [Google Scholar]
- 36.Pearlson G E, Rabins P V, Kim W S, Speedie L J, Moberg P J, Burns A, Bascom M J. Psychol Med. 1989;19:573–584. doi: 10.1017/s003329170002417x. [DOI] [PubMed] [Google Scholar]
- 37.Pearlson G E, Rabins P V, Burns A. Psychol Med. 1991;21:321–328. doi: 10.1017/s0033291700020420. [DOI] [PubMed] [Google Scholar]
- 38.Sheline Y I, Wang P W, Gado M H, Csernansky J G, Vannier M W. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesser I M, Mena I, Boone K B, Miller B L, Mehringer C M, Wohl M. Arch Gen Psychiatry. 1994;51:677–686. doi: 10.1001/archpsyc.1994.03950090009002. [DOI] [PubMed] [Google Scholar]
- 40.Upadhyaya, A. K., Abou-Saleh, M. T., Wilson, K., Grime, S. J. & Critchley, M. (1990) Br. J. Psychiatry 157, Suppl. 9, 76–81. [PubMed]
- 41.Kumar A, Mozley P D, Dunham C, Velchik M, Reilly J, Gottlieb G, Alavi A. Int J Geriatr Psychiatry. 1991;6:775–777. [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. 4th Ed. Washington, DC: Am. Psychiatric Assoc.; 1994. [Google Scholar]
- 43.Hamilton M. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 44.Folstein M F, Folstein S E, McHugh P R. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Linn B S, Linn M W, Gurel L. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 46.Kohn M, Tanna N, Herman G. Radiology. 1991;178:115–122. doi: 10.1148/radiology.178.1.1984289. [DOI] [PubMed] [Google Scholar]
- 47.Kumar A, Newberg A, Alavi A. Am J Geriatr Psychiatry. 1994;2:21–31. doi: 10.1097/00019442-199400210-00005. [DOI] [PubMed] [Google Scholar]
- 48.Cowell P C, Turetsky B I, Gur R C, Grossman R I, Shtasel D L, Gur R E. J Neurosci. 1994;14:4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuster J M. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Philadelphia: Lippincott; 1997. [Google Scholar]
- 50.Stuss D T, Benson D F. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- 51.Goldman-Rakic P. In: Handbook of Physiology: The Nervous System. Plum F, Mountcastle V, editors. Bethesda, MD: Am. Phy. Soc.; 1987. pp. 373–413. [Google Scholar]
- 52.Modell J G, Mountz J M, Curtis G C, Greden J F. J Neuropsychiatry. 1989;1:27–36. doi: 10.1176/jnp.1.1.27. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg D R, Keshavan M S, O’Hearn K M, Dick E L, Bagwell W W, Seymour A B, Montrose D M, Pierri J N, Birmaher B. Arch Gen Psychiatry. 1997;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 54.Castellanos F X, Giedd J N, Marsh W L. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 55.Buchsbaum M S. Schizophr Bull. 1990;16(3):379–389. doi: 10.1093/schbul/16.3.379. [DOI] [PubMed] [Google Scholar]
- 56.Kumar A, Gottlieb G. Am J Geriatr Psychiatry. 1993;1(2):95–108. doi: 10.1097/00019442-199300120-00002. [DOI] [PubMed] [Google Scholar]
- 57.Seigel D G, Greenhouse S W. Am J Epidemiol. 1973;97:324–331. doi: 10.1093/oxfordjournals.aje.a121512. [DOI] [PubMed] [Google Scholar]
- 58.Lacro J P, Jeste D V. Biol Psychiatry. 1994;36:146–152. doi: 10.1016/0006-3223(94)91220-3. [DOI] [PubMed] [Google Scholar]
- 59.Katz I R, Streim J, Parmalee P. Biol Psychiatry. 1994;36:141–145. doi: 10.1016/0006-3223(94)91219-x. [DOI] [PubMed] [Google Scholar]
- 60.Ruegg R G, Zisook S, Swerdlow N R. Psychiatr Clin North Am. 1988;11:83–99. [PubMed] [Google Scholar]
- 61.Kessler R C, Zhao S, Blazer D G, Swartz M. J Affect Disord. 1997;45:19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- 62.Akiskal H S, Judd L L, Gillin J C, Lemmi H. J Affect Disord. 1997;45:53–63. doi: 10.1016/s0165-0327(97)00059-1. [DOI] [PubMed] [Google Scholar]
- 63.Akiskal H S. Am J Psychiatry. 1983;140:11–20. doi: 10.1176/ajp.140.1.11. [DOI] [PubMed] [Google Scholar]
- 64.Angst J, Merikangas K. J Affect Disord. 1997;45:31–40. doi: 10.1016/s0165-0327(97)00057-8. [DOI] [PubMed] [Google Scholar]
- 65.Keller M B, Hirschfeld R M A, Hanks D. J Affect Disord. 1997;45:65–73. doi: 10.1016/s0165-0327(97)00060-8. [DOI] [PubMed] [Google Scholar]
- 66.Keller M B, Lavori P W, Endicott J, Coryell W, Klerman G L. Am J Psychiatry. 1983;140:689–694. doi: 10.1176/ajp.140.6.689. [DOI] [PubMed] [Google Scholar]
- 67.Judd L L, Akiskal H S, Paulus M P. J Affect Disord. 1997;45:5–18. doi: 10.1016/s0165-0327(97)00055-4. [DOI] [PubMed] [Google Scholar]
- 68.Angst J, Merikangas K, Scheidegger P, Wicki W. J Affect Disord. 1990;19:87–98. doi: 10.1016/0165-0327(90)90013-x. [DOI] [PubMed] [Google Scholar]
- 69.Baldwin R C, Jolley D J. Br J Psychiatry. 1986;149:574–583. doi: 10.1192/bjp.149.5.574. [DOI] [PubMed] [Google Scholar]
- 70.Angst J, Hochstrasser B. J Clin Psychiatry. 1994;55:3–9. [PubMed] [Google Scholar]
- 71.Judd, L. L., Akiskal, H. S., Maser, J. D., Zeller, P. J., Endicott, J., Coryell, W., Paulus, M. P., Kunovac, J. L., Leon, A. C., Mueller, T., et al. (1998) Arch. Gen. Psychiatry, in press. [DOI] [PubMed]
- 72.Baldwin R C, Benbow S M, Marriott A, Tomenson B. Br J Psychiatry. 1993;163:82–90. doi: 10.1192/bjp.163.1.82. [DOI] [PubMed] [Google Scholar]
- 73.Devanand D P, Nobler M S, Singer T. Am J Psychiatry. 1994;151:1592–1599. doi: 10.1176/ajp.151.11.1592. [DOI] [PubMed] [Google Scholar]
- 74.Tannock C, Katona C. Drugs Aging. 1995;6:278–292. doi: 10.2165/00002512-199506040-00003. [DOI] [PubMed] [Google Scholar]
- 75.Kiloh L G, Andrews G, Neilson M. Br J Psychiatry. 1988;153:752–757. doi: 10.1192/bjp.153.6.752. [DOI] [PubMed] [Google Scholar]
- 76.Judd L L. J Affect Disord. 1997;45:109–116. doi: 10.1016/s0165-0327(97)00064-5. [DOI] [PubMed] [Google Scholar]
- 77.Murphy E. Brit J Psychiatry. 1983;142:111–119. doi: 10.1192/bjp.142.2.111. [DOI] [PubMed] [Google Scholar]
- 78.Maier W, Gansicke M, Weiffenbach O. J Affect Disord. 1997;45:41–51. doi: 10.1016/s0165-0327(97)00058-x. [DOI] [PubMed] [Google Scholar]
- 79.Gallo J J, Rabins P V, Iliffe S. Int J Psychiatry Med. 1997;27(3):185–204. doi: 10.2190/JF9W-9Q87-KV0F-YCY4. [DOI] [PubMed] [Google Scholar]
- 80.Callahan C M, Hui S L, Nienaber N A, Musick B, Tierney W M. J Am Geriatr Soc. 1994;42:833–838. doi: 10.1111/j.1532-5415.1994.tb06554.x. [DOI] [PubMed] [Google Scholar]
- 81.Gallo J J, Rabins P V, Lyketsos C G, Tien A Y, Anthony J C. J Am Geriatr Soc. 1997;45:570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 82.Alexopolous G S, Young R C, Meyers B S. Psychiatr Clin North Am. 1988;11:101–105. [PubMed] [Google Scholar]
- 83.Judd, L. L., Rapaport, M. H., Paulus, M. P. & Brown, J. L. (1994) J. Clin. Psychiatry 55, Suppl., 18–28. [PubMed]
- 84.Winokur G. J Affect Disord. 1997;45:97–108. doi: 10.1016/s0165-0327(97)00063-3. [DOI] [PubMed] [Google Scholar]