Abstract
The decrement in dopamine levels exceeds the loss of dopaminergic neurons in Parkinson’s disease (PD) patients and experimental models of PD. This discrepancy is poorly understood and may represent an important event in the pathogenesis of PD. Herein, we report that the rate-limiting enzyme in dopamine synthesis, tyrosine hydroxylase (TH), is a selective target for nitration following exposure of PC12 cells to either peroxynitrite or 1-methyl-4-phenylpyridiniun ion (MPP+). Nitration of TH also occurs in mouse striatum after MPTP administration. Nitration of tyrosine residues in TH results in loss of enzymatic activity. In the mouse striatum, tyrosine nitration-mediated loss in TH activity parallels the decline in dopamine levels whereas the levels of TH protein remain unchanged for the first 6 hr post MPTP injection. Striatal TH was not nitrated in mice overexpressing copper/zinc superoxide dismutase after MPTP administration, supporting a critical role for superoxide in TH tyrosine nitration. These results indicate that tyrosine nitration-induced TH inactivation and consequently dopamine synthesis failure, represents an early and thus far unidentified biochemical event in MPTP neurotoxic process. The resemblance of the MPTP model with PD suggests that a similar phenomenon may occur in PD, influencing the severity of parkisonian symptoms.
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by disabling motor abnormalities attributed to a profound deficit in dopamine (1). The decline in dopamine level has been thought to arise solely from the severe loss of dopaminergic neurons in the nigrostriatal pathway. However, the dopamine deficit in the affected regions of the brain significantly exceed the loss of dopaminergic neurons (2, 3), suggesting that dopamine synthesis is impaired before cellular demise. Support for this hypothesis comes from studies of experimental models of PD demonstrating that the reduction in dopamine metabolism-related markers such as tyrosine hydroxylase (TH) and dopamine transporter is far greater than the loss of neuronal cell bodies (4–6). Because the severity of PD symptoms correlates with the magnitude of dopamine deficit, elucidating mechanisms that impair dopamine synthesis and metabolism in neurons that undergo selective degeneration in PD may have important therapeutic implications.
There is experimental evidence from studies of humans and animals in support of the hypothesis that oxidative stress contributes to the pathogenesis of PD (7). Studies performed in the MPTP model of PD suggest that peroxynitrite, a reactive species formed by the nearly diffusion-limited reaction of nitric oxide with superoxide, may be a mediator of nigrostriatal damage in PD (8–10). The potential role of peroxynitrite in the pathogenesis of PD is further supported by demonstrating that exposure of the monoamine-producing PC12 cells to peroxynitrite induced a dose-dependent alteration in dopamine synthesis that was not due to cell death or the oxidation of dopamine (11).
Based on these observations, we propose that the inhibition of dopamine metabolism in PD may result from the peroxynitrite-mediated inactivation of TH, the rate limiting enzyme in dopamine synthesis. Previous work has shown that protein tyrosine residues are a major target of peroxynitrite reactivity. The nitration of the ortho position of tyrosine by peroxynitrite occurs spontaneously as well as by CO2 or low molecular mass transitional metal catalysis (12–14). The latter mechanism may be particularly relevant to PD because the free iron content in affected brain regions is markedly increased (15, 16). Protein associated or free nitrotyrosine has been detected in human postmortem specimens of patients with neurodegenerative disorders such as Alzheimer’s, multiple sclerosis, and amyotrophic lateral sclerosis, as well as in animal models of neurodegeneration (17–23).
Therefore, experiments were performed to test the hypothesis that the inactivation of dopamine synthesis is caused by nitration of TH by peroxynitrite in models of PD. Nitration of TH was examined in PC12 cells challenged with different concentrations of peroxynitrite and 1-methyl-4-phenylpyridinium ion (MPP+), the active metabolite of MPTP (24), as well as in mice treated with MPTP. Nitration of TH was detected and quantified by immunoprecipitation and reaction with affinity purified anti-nitrotyrosine antibodies and amino acid analysis. Nitrated TH was found in all models, and the extent of TH nitration correlated with loss of TH catalytic activity and decline in dopamine levels.
MATERIALS AND METHODS
Exposure of PC12 Cells and Tyrosine Hydroxylase to Peroxynitrite.
Peroxynitrite was synthesized from nitrite and hydrogen peroxide. The concentration of peroxynitrite was measured by the increase in absorbance at 302 nm (ɛ302 nm = 1,700 M−1 cm−1) in 1.2 M NaOH. The peroxynitrite was added to the samples as a small drop along the wall of the tube just above the reaction mixture and then rapidly mixed by vortexing. The pH of the buffer was the same after each addition of peroxynitrite. PC12 cells were washed with Earle’s balanced salt solution, scraped off the plates, and centrifuged at 800 × g for 5 min, and the pellet was solubilized with lysis buffer (20 mM Tris⋅HCl, pH 7.4/150 mM NaCl/4 mM EGTA/10% glycerol/1% Triton X-100) (Bio-Rad).
Immunoprecipitation of Tyrosine Hydroxylase and Nitrotyrosine Detection.
Mice brain homogenates and PC12 cell lysates were precleared with protein G-Sepharose (Pharmacia) (1 hr at 4°C) to reduce the amount of protein precipitated nonspecifically. The mixture was centrifuged for 1 min at 10,000 rpm to pellet the beads with nonspecifically bound proteins. Five microliters of anti-tyrosine hydroxylase mAbs (1 mg/ml) were incubated for 12 hr at 4°C with 500 μl of appropriately diluted samples in lysis buffer containing 1 mM PMSF, 10 mg/ml aprotinin, 0.2 mM sodium orthovanidate, and 1 mg/ml leupeptin. The immune complexes were precipitated with 30 μl of 25% wt/vol protein G-Sepharose (rotating the suspension for 1 hr and 30 min at 4°C) after which the beads were collected by centrifugation and washed three times with lysis buffer. The beads were finally suspended in 50 μl of sample buffer containing SDS and 2-mercaptoethanol and heated at >90°C for 5–10 min. The protein G-Sepharose was pelleted by centrifugation for 1 min, and supernatant was analyzed by SDS gel electrophoresis on 12% running gels. Proteins were transferred to 0.2-mm nitrocellulose membranes (Schleicher & Schull) and reacted with either a polyclonal anti-tyrosine hydroxylase 1:3,000 dilution (Eugene Tech, Ridgefield, NJ) or 1.0 μg/ml affinity purified rabbit anti-nitrotyrosine antibodies that were preconjugated overnight at 4°C with 1:3,000 dilution of horseradish peroxidase labeled-goat anti-rabbit IgG (H+L; Bio-Rad). After washing, the nitrocellulose was incubated with chemiluminescent substrate (Amersham) and then exposed to the x-ray film (AIF, Fuji).
Animals and Treatment.
Eight-week-old male C57/bl mice (25–30 g; Charles River Breeding Laboratories) were housed three per cage in a temperature-controlled room under a 12-hr light/dark cycle with free access to food and water. On the day of the experiment, mice received four i.p. injections of MPTP⋅HCl (20 mg/kg free base; Research Biochemicals) in saline at 2 hr intervals; control mice received saline only. MPTP-injected mice (4–6 per group) were killed 0, 3, and 6 hr after the last injection. Right and left striata were rapidly dissected on ice, immediately frozen on dry ice, and stored at −80°C until analysis (3). To examine the effects of copper/zinc superoxide dismutase (SOD1) activity on tyrosine nitration of the enzyme tyrosine hydroxylase, hemizygote male (aged 2–8 mo) SF-218 mice also were injected with MPTP as above. These mice carry eight copies of the wild-type human SOD1 gene, presumably in tandem array, and have ≈fourfold higher striatal and ventral midbrain SOD1 activity compared with their nontransgenic littermates (25).
Measurement of Striatal Dopamine Levels.
HPLC with electrochemical detection was used to measure striatal levels of dopamine. On the day of the assay, frozen tissue samples were sonicated in 50 vol (wt/vol) of 0.1 M perchloric acid containing 25 ng/ml dihydrobenzylamine (Sigma) as internal standard. After centrifugation (15,000 × g, 10 min, 4°C), 20 μl of supernatant was injected onto a C18-reversed phase RP-80 catecholamine column (ESA, Bedford, MA). The mobile phase consisted of 90% of a solution of 50 mM sodium phosphate, 0.2 mM EDTA, and 1.2 mM heptanesulfonic acid (pH 3.5), and 10% methanol. Flow rate was 1.0 ml/min. Peaks were detected by a Coulochem 5100A detector (E1 = −0.04 V, E2 = +0.35 V) (ESA). Data were collected and processed on a computerized Dinamax data manager (Rainin, Woburn, MA).
Tyrosine Hydroxylase Activity.
A radiometric assay based on the release of [3H]H2O from l-[ring-3,5-3H]tyrosine (NEN) was used to determine the striatal catalytic activity of tyrosine hydroxylase (26). Frozen samples were sonicated in 10 vol (wt/vol) 50 mM Mes (pH 6.1) and centrifugated (15,000 × g, 10 min, 4°C). In a total volume of 100 μl, 50 μl of supernatant were mixed with 25 μl of a mixture containing 2.5 nmol l-tyrosine, 1 mCi (1 Ci = 37 GBq) l-[ring-3,5-3H]tyrosine (specific activity 50 Ci/mmol, NEN), 0.5 mmol DTT, and 15 μl catalase (80 units/ml). The reaction was started by adding 10 μl of 10 mM 6(R)-l-erythro-5,6,7,8-tetrahydrobiopterin (BH4, Sigma); for blank, BH4 was omitted and 10 μl of Mes buffer was added instead. At the end of the incubation (20 min, 37°C), 1 ml of cold 7.5% activated charcoal (R-60, Fisher Scientific) suspension in 1 M HCl was added to each tube, vortexed, and centrifuged (2,000 × g, 5 min, 25°C). Then, 0.4 ml of the supernatant was mixed with 4 ml of Acquasol-II (NEN), and radioactivity was counted by scintillation spectrometry. Protein concentrations were determined by using the Bradford assay (Pierce).
Tyrosine Hydroxylase Protein.
An ELISA was used to measure striatal content of tyrosine hydroxylase protein according to the method reported by Reinhard, Jr. et al. (27) with minor modifications. Falcon 96-well polystyrene ELISA plates (Fisher Scientific) were coated with 10 ng/well of rabbit polyclonal anti-tyrosine hydroxylase (Gift from J. W. Haycock, Louisiana State University Medical Center, New Orleans) in 10 mM sodium phosphate buffer (pH 7.4) containing 100 mM NaCl (PBS) by overnight incubation at 4°C. The plates were then washed (PBS containing 0.05% Tween 20), blocked with 1% BSA in PBS (1 hr, 25°C), and stored at 4°C. On the day of the assay, frozen samples were homogenized (glass/glass homogenizer) in 10 vol (wt/vol) PBS containing 1 mg/ml leupeptin (Sigma). After centrifugation (15,000 × g, 10 min, 4°C), 100 μl of supernatant diluted at 1:75 in buffer were added to the well and incubated (30 min, 37°C); for blanks, 100 μl of buffer were added instead of supernatant. The plates were successively incubated with 1:16,000 mouse monoclonal anti-tyrosine hydroxylase (TH-16, Sigma), 1:400 biotinylated anti-mouse IgG (Vector, Burlingame, CA), and 1:200 horseradish peroxidase conjugated-streptavidin (Vector) in PBS; between each of these incubations (30 min, 37°C), plates were washed twice with PBS/0.05% Tween 20 at 25°C. After the last wash, 100 μl of horseradish peroxidase substrate 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS; Vector) was added to each well and the plate was incubated (20 min, 25°C) in the dark before being read at 405 nm (with reference at 450 nm) on a computerized dual microplate reader (Bio-Rad model 3550). Tyrosine hydroxylase content (μg/mg protein) was derived from a purified rat tyrosine hydroxylase standard curve with a linear concentration range of 1.25–20 ng/well.
Two-Dimentional Electropheresis.
Brain tissue was dissected and sonicated immediately in ice-cold PBS (pH 7.4), and the sonicate was centrifuged at 13,000 × g for 20 min. The supernatant obtained was either analyzed immediately or stored at −70°C; the pellet was resolubilized in 10 vol PBS and stored at −70°C. Proteins (100 μg/tube) were first separated by isoelectric focusing in capillary tubes by using pH 3–10 ampholytes (Bio-Rad). Denaturing SDS/PAGE was performed on miniature 8% polyacrylamide gels enabling separation of proteins with apparent molecular mass of 5–200 kDa. Color molecular weight markers (Sigma) were run in all gels, enabling visual verification that the membrane transfer was complete. To calibrate the pH, parallel runs were performed by using pI markers (Sigma). Before staining, blots were incubated overnight at 4°C in a 5% blocking solution (nonfat dry milk in PBS, pH 7.4). Blots were then washed three times for 5 min/wash in PBS containing 0.05% Tween-20 and a 5-min wash in PBS. Blots were incubated at 25°C in a primary antibody solution of 1 μg/ml anti-nitrotyrosine antibody in 3% blocking buffer. Blots were then washed and incubated 1 hr in biotinylated secondary antibody (goat anti-rabbit IgG; Sigma) in 3% blocking buffer. Blots were then washed and incubated 30 min in streptavidin–biotin-linked horseradish peroxidase solution and visualized with chemiluminescence as described above. Appropriate controls showed that all staining can be eliminated by either elimination of the primary antibody or by including 10 mM 3-nitrotyrosine in the initial primary antibody solution (28).
RESULTS
PC12 Cell Models.
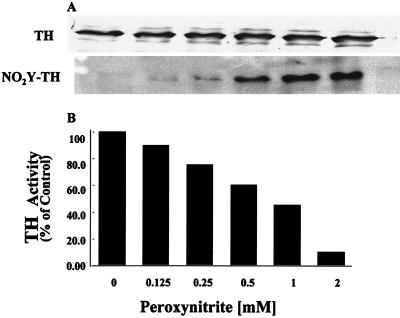
PC12 cell lysates rich in TH were treated with different concentrations of peroxynitrite, and immunoprecipitated TH was reacted with affinity purified anti-nitrotyrosine antibodies. As expected, exposure to peroxynitrite resulted in a dose-dependent nitration of TH (Fig. 1A). The degree of TH nitration correlated with the loss of enzymatic activity (Fig. 1B). TH activity was assayed under optimal conditions to insure that the decline in activity is not related to availability of substrate or cofactor but to TH modification(s). Other amino acid residues susceptible to peroxynitrite attack are tryptophan, cysteine, and methionine. The content of tryptophan in purified TH was determined by measuring the fluorescence of the indole ring (excitation λ = 325 nm and emission λ = 410 nm). Rat and human TH contains three tryptophan residues that are sufficient to permit accurate detection of fluorescence. There was no difference in the fluorescence of the indole ring of unreacted purified TH and TH reacted with up to 1 mM peroxynitrite. Amino acid analysis of purified TH before and after reaction with peroxynitrite did not reveal any other change in the recovery of amino acids including methionine. Therefore, in the absence of any other amino acid modification, tyrosine nitration is the primary reason for the inactivation of the enzyme. Moreover, amino acid analysis of purified TH exposed to peroxynitrite revealed that the same mol percentage of nitrotyrosine (0.32 ± 0.03) was present irrespective of the peroxynitrite concentration. Exposure of purified TH to 2 mM peroxynitrite results in nitration of all TH molecules, and thus under this condition, the 0.32 mol percent represents nitration of a single tyrosine residue in every TH molecule.
Figure 1.
Nitration of TH molecules results in proportional loss of activity. (A) Levels of immunoprecipitated TH in PC12 cell lysates treated with different concentrations of peroxynitirite. (B) Nitrated TH. The level of nitration was evaluated after staining with affinity purified polyclonal anti-nitrotyrosine antibodies and by amino acid analysis. (C) TH activity determined under optimal conditions.
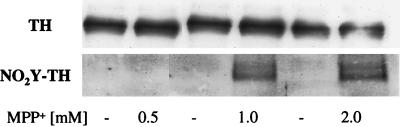
PC12 cells also were exposed for 1 hr to different concentrations of MPP+. In similar to exposure to peroxynitrite, MPP+ induced the nitration of TH (Fig. 2). It is important to note that the PC12 cells used in these experiments contain the neuronal form of nitric oxide synthase (nNOS), documented by Western blot analysis (not shown) and by the presence of nitric oxide metabolites, nitrite and nitrate. Under our experimental conditions, lysates of 1 × 106 cells contain 3.7 ± 0.4 μM nitrite plus nitrate. Moreover, 1 hr after exposure to MPP+, the cells were viable and capable of reducing 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to formazan. Control cells value for MTT reduction was 0.800 ± 0.07 absorbance at 570 nm, (n = 3, mean ± SD) and of cell treated with 1 mM MPP+, 0.979 ± 0.04 absorbance at 570 nm, (n = 3 mean ± SD). The reduction of MTT by the mitochondrial and cytosolic dehydrogenase(s) requires NADH and, therefore, reflects the availability of pyridine nucleotides and the cellular redox state.
Figure 2.
(A) Immunoprecipitation of TH after 1-hr treatment of PC12 cells with different concentrations of MPP+. (B) Nitration of tyrosine residues in the immunoprecipitated TH was evaluated by reaction with the anti-nitrotyrosine antibodies. Representative data from three independent experiments.
MPTP Murine Models.
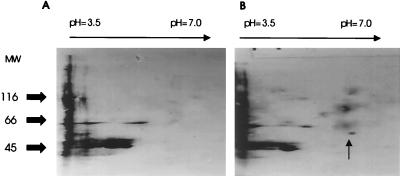
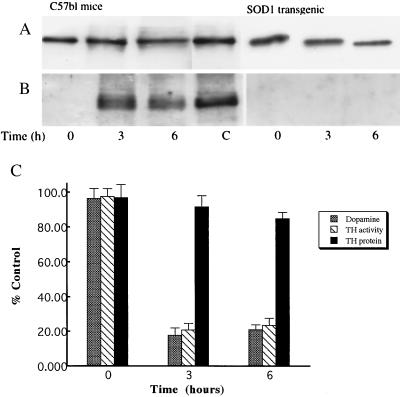
To determine whether MPTP induces nitration of TH in vivo, adult C57/bl mice were injected with MPTP and controls were injected with saline as described (8). In both striatum and ventral midbrain, increases in protein tyrosine nitration were detected as early as 3 hr after MPTP injection. Two-dimensional separation of proteins followed by immunoblotting with anti-nitrotyrosine antibody revealed that several proteins were nitrated in the striatum 3 hr after MPTP injection (Fig. 3). A prominent nitrated band was a protein with an apparent molecular weight of 58,000 and pI of 5.8, which are physical properties of tyrosine hydroxylase (29–31). To confirm that TH is the nitrated protein, TH from striatal protein extracts of MPTP-injected mice was immunoprecipitated and subsequently reacted with affinity purified anti-nitrotyrosine antibodies. Striatal TH was indeed nitrated 3 and 6 hr after MPTP injection (Fig. 4A) whereas TH immunoprecipitated from saline controls or immediately after the last injection of MPTP was not nitrated. To demonstrate the critical role of superoxide in tyrosine nitration of TH, MPTP was administrated to transgenic mice with increased SOD1 activity, the enzyme responsible for the conversion of cytosolic superoxide to hydrogen peroxide. In the transgenic mice with increased SOD1 activity, MPTP did not cause any detectable nitration of striatal TH at any time points studied (Fig. 4B). This observation is consistent with the previous reports that MPTP exposure of SOD1-transgenic mice failed to induce a dopamine deficit (25).
Figure 3.
MPTP treatment leads to protein tyrosine nitration of specific proteins in the mouse midbrain. Two-dimensional, chemiluminescence-enhanced anti-nitrotyrosine immunoblots of ventral midbrain tissue of C57/bl mice 3 hr after injection of either saline (A) or MPTP (B). MPTP injection resulted in nitration of selective proteins. Indicated by the arrow is a protein with the physical characteristics of mouse tyrosine hydroxylase listed in protein databases.
Figure 4.
(A) Immunoprecipitation of striatal TH from MPTP-exposed mice immediately after exposure (time 0), 3- and 6-hr post exposure. TH was visualized with polyclonal anti-tyrosine hydorxylase antibodies. Representative data from four different striatal preparations. (B) Nitration of tyrosine residues in the immunoprecipitated TH was evaluated by reaction with the anti-nitrotyrosine antibodies. As a positive control (C), TH was laso immunoprecipitated from peroxynitrite treated PC12 cell lysates as described in Fig. 1. (C) Time course of striatal dopamine, tyrosine hydroxylase activity and TH protein levels after MPTP administration (n = 4–6).
The present regimen of MPTP caused dramatic reductions in striatal dopamine levels in C57/bl mice similar to previously reported albeit at earlier time points (4, 8, 25). Striatal dopamine levels declined to nearly 20% of saline control 3 and 6 hr post-injection. The magnitude and the time course of the changes in striatal TH activity following MPTP administration paralleled very closely that of dopamine levels (Fig. 4C). Conversely, loss in striatal TH protein content was insignificant (only 6% as compared with zero time) during the first 6 hr post MPTP injection.
DISCUSSION
Previously we demonstrated that peroxynitrite impairs dopamine metabolism in PC12 cells (11). In the present study, the molecular mechanism for the peroxynitrite-impairment of dopamine production and the potential role of this process in the pathogenesis of MPTP model of PD was investigated. The rate-limiting enzyme in the dopamine synthesis, TH, is a target for peroxynitrite-induced tyrosine nitration and that nitration of a single tyrosine residue within this enzyme appears to be sufficient to impair its catalytic activity. Nitration of tyrosine residues in vitro results in inactivation of a vast number of mammalian proteins whose activity is dependent on tyrosine residues (32). Nitration of tyrosine results in a marked shift of the local pKa from 10.07 of the OH group of tyrosine to 7.5 of 3-nitrotyrosine that is expected to change the hydrophobicity, hydrogen bonding, and electrostatic interactions within the protein. The shift in pKa provided the biochemical explanation for the inhibition in the rate of phosphorylation by tyrosine kinases as well as for the inactivation of protein function (28). Native tyrosine 3-hydroxylase (EC 1.14.16.2) is a tetrameter of four identical monomers. Isolated monomers have catalytic activity, and by limited proteolytic digestion, the catalytic domain has been located in the carboxyl-terminal between residues Leu188 and Phe456 (29–31). The rat enzyme contains 17 tyrosine residues of 498 total residues, and 15 of these tyrosine residues are found in the catalytic domain (30, 31). The human enzyme contains 15 tyrosine residues with 14 of them in the catalytic domain. Although the site of nitration has not been identified, Tyr225 in both the rat and human TH is a likely candidate because it is located within a sequence (X-X-Glu-Tyr-Thr-Ala) that is a target for nitration by peroxynitrite. This sequence was found to be the most effective target for tyrosine nitration during a screening of peptide sequences for efficiency of nitration by peroxynitrite (H.I., unpublished results).
The pivotal role of TH in catechdamine synthesis, and consequently in PD, led to the second set of experiments aimed at assessing whether MPTP that is suspected to exert its neurotoxic effects, at least in part, through the formation of peroxynitrite, also would inactivate TH. MPTP produced a rapid and profound loss in striatal dopamine content that was closely matched by the loss in TH activity. The loss in TH activity was not caused by a decrease in striatum TH protein. In addition, negligible dopaminergic neuronal death is observed 6 hr after MPTP injection (4). Therefore, TH inactivation and dopamine synthesis failure is an early event in MPTP neurotoxic process that precedes loss in TH protein and dopaminergic neurons. Nagatsu and coworkers (33, 34) have found at least three distinct phases in TH activity and content after either MPTP or MPP+ administration. During the early phase, minutes after MPTP or MPP+ administration there is a decrease in the in vivo TH activity but not in the Vmax of the enzyme measured under optimal conditions in vitro. During the same time, the TH concentration remains the same, and as a result the homeospecific activity of TH, expressed as Vmax over TH protein, also remains the same. The loss of TH activity in vivo was attributed to either the increase in cytoplasmic levels of dopamine or to the inhibition of TH phoshorylation (34, 35). During the middle phase, such as 3 hr after continuous exposure of striatal dopaminergic neurons to MPP+ or after the last injection of MPTP, the TH activity both in vivo and in vitro was decreased significantly whereas the TH protein levels are unchanged (33, 34). As expected, the decrease in the Vmax resulted in a decrease in the homeospecific activity of TH. The mechanism for the inactivation of TH and loss of homeospecific activity during the middle phase (hours) has not been elucidated. This study provides evidence that the molecular mechanism responsible for the inactivation of TH after MPTP administration is tyrosine nitration. Previously, it was shown that MPTP stimulates nitration of free tyrosine (10) and of total proteins (22), but the current work indicates that TH is a preferential target for MPTP-induced tyrosine nitration. The late phase of MPTP or MPP+ administration resembles the late stages of PD disease in which TH activity in vivo and in vitro, TH protein content are significantly decreased (33, 34). In PD patients, a compensatory increase in TH-homeospecific activity has been reported and is thought to arise from the existence of multiple forms of TH mRNA, which are products of alternative splicing (35).
The existence of nitrated TH implies the formation of a nitrating agent after injection of MPTP. MPTP has been shown to selectively accumulate in dopaminergic neurons where it is oxidized to MPP+ by monoamine oxidase-B (24). In turn, redox cycling of MPP+ by dehydrogynases such as the complex I of the mitochondrial electron transport chain and cytosolic oxidoreductases such as the cytochrome P450 reductase and xanthine oxidase results in the formation of superoxide by the one electron reduction of oxygen (36). Thus, in both the mitochondria and cytosol compartments, the redox cycling of MPP+ results in an increase in the steady–state levels of superoxide. We hypothesize that the increase in the steady–state levels of superoxide in dopaminergic neurons allows for the formation of peroxynitrite because the second order rate constant for the reaction of superoxide with nitric oxide is 0.4–1 × 1010 M−1 s−1, which is essentially the fastest reaction known for superoxide to date (37). Nitric oxide is membrane-permeable and can diffuse into dopaminergic neurons. Thus, it is the site of elevated levels of superoxide that determines whether a cell will be affected by the reactivity of peroxynitrite. Although peroxynitrite is a strong oxidant, it reacts with most biological targets with relatively slow rates (second order rate constants range from 103 to 10 6 M−1 s−1) (38). Therefore, the concentration of the biological target and the proximity to the formation of peroxynitrite will determine which molecule will react with peroxynitrite. It is apparent from data in this study and in other models that protein tyrosine residues are selective targets for peroxynitrite resulting in nitration. In the MPTP model of PD, TH appears to be a major and critical target for peroxynitrite-mediated nitration that leads to enzymatic inactivation. As such, this finding represents the first example of in vivo protein nitration that leads to a functional deficit that is directly related with a pathogenic outcome.
Support for our working hypothesis also comes from transgenic mice with increased SOD1 activity. The decrease in the steady-state levels of superoxide and consequently of peroxynitrite was able to prevent nitration of TH and loss of enzymatic activity. Therefore the previous findings that these mice are resistant to MPTP neurotoxicity can now be biochemically explained on the basis of preventing peroxynitrite formation. Blocking the formation or effective scavenging of peroxynitrite can potentially provide a therapeutic intervention because it will eliminate nitration of TH and loss of catalytic activity preserving dopamine levels in the brain. Moreover, eliminating peroxynitrite also may be useful because this reactive species can induce apoptosis and delayed cell death independent of its nitrating ability (39–43).
Acknowledgments
We are grateful to Drs. Virginia M-Y Lee, John Q. Trojanowski, and Michael Beers (University of Pennsylvania) for discussions and reading of the manuscript, Dr. John W. Haycock (Louisiana State University) for TH antibodies and discussions, Dr. Charles Epstein (University of California, San Francisco) for the SOD1-transgenic mice, and Ms. N. Romero and Mr. Scott Coffey for technical assistance. This work was supported by grants from National Institute on Aging, 1-RO1-AG13966 (to H.I.), National Institute of Neurological Disorders and Stroke, 1-K08-NS01724 (to S.P.), and by the Allegheny Health Education Research Foundation (to J.H.), the Parkinson’s Disease Foundation (to S.P., V.J.-L., A.B.N.), Smart Foundation, and the Lowenstein Foundation (to S.P.). S.P. is a recipient of the Cotzias Award of the American Parkinson Disease Association and H.I. is an Established Investigator of the American Heart Association.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD, Parkinson’s disease; TH, tyrosine hydroxylase; MPP+, 1-methyl-4-phenylpyridiniun ion; Cu/Zn, copper/zinc; SOD, superoxide dismutase.
References
- 1.Fahn S. In: Cecil’s Textbook of Medicine. Wyngaarden J B, Smith LH Jr, editors. Philadelphia: Saunders; 1988. pp. 2143–2147. [Google Scholar]
- 2.Hornykiewicz O, Kish S J. In: Parkinson’s Disease. Yahr M, Bergmann K J, editors. New York: Raven; 1987. pp. 19–34. [Google Scholar]
- 3.Pakkenberg B, Moller A, Gundersen H J G, Mouritzen A, Pakkenberg H. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson-Lewis V, Jakowec M, Burke R E, Przedborski S. Neurodegener. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 5.Seniuk N A, Tatton W G, Greenwood C E. Brain Res. 1990;527:7–20. doi: 10.1016/0006-8993(90)91055-l. [DOI] [PubMed] [Google Scholar]
- 6.Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki D M. Neuroscience. 1995;67:631–647. doi: 10.1016/0306-4522(95)00066-r. [DOI] [PubMed] [Google Scholar]
- 7.Fahn S, Cohen G. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 8.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson V L, Dawson T M. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews R T, Beal M F. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 10.Schulz J B, Matthews R T, Muqit M K, Browne S E, Beal M F. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- 11.Ischiropoulos H, Duran D, Horwitz J. J Neurochem. 1995;65:2366–2372. doi: 10.1046/j.1471-4159.1995.65052366.x. [DOI] [PubMed] [Google Scholar]
- 12.Ischiropoulos H, Zhu L, Chen J, Tsai J-H M, Martin J C, Smith C D, Beckman J S. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 13.Beckman J S, Ischiropoulos H, Zhu L, Van der Woerd M, Smith C D, Harrison J, Martin J C, Tsai J-H M. Arch Biochem Biophys. 1992;298:438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- 14.Gow A, Duran D, Thom S R, Ischiropoulos H. Arch Biochem Biophys. 1996;333:42–48. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch E C, Brandel J-P, Galle P, Javoy-Agid F, Agid Y. J Neurochem. 1991;56:446–451. doi: 10.1111/j.1471-4159.1991.tb08170.x. [DOI] [PubMed] [Google Scholar]
- 16.Youdim M B H, Ben-Shachar D, Eshel G, Finberg J P M, Riederer P. In: Adv. Neurology. Narabayashi H, Nagatsu N, Yanagisawa N, Mizuno Y, editors. New York: Raven; 1993. pp. 259–266. [Google Scholar]
- 17.Good P F, Werner P, Hsu A, Olanow C W, Perl D P. Am J Pathol. 1997;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Smith M A, Richey-Harris P L, Sayre L M, Beckman J S, Perry G. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basarga O, Michaels F H, Zheng Y M, Borboski L E, Spitsin S V, Fu Z F, Tawadros R, Koprowski H. Proc Natl Acad Sci USA. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou S M, Wang H S, Komai K J. Chem Neuroanatomy. 1996;10:249–258. doi: 10.1016/0891-0618(96)00137-8. [DOI] [PubMed] [Google Scholar]
- 21.Brujin L I, Beal M F, Becher M W, Schulz J B, Wong P C, Price D L, Cleveland D W. Proc Natl Acad Sci USA. 1997;94:7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przedborski, S., Bandele, A. N., Jackson-Lewis, V., Kostic, V. & Trifiletti, R. R. (1997) Neurology 48, Suppl. 2, A201 (abstr.).
- 23.Ayata C, Ayata G, Hara H, Matthews R T, Beal M F, Ferrante R J, Endres M, Kim A, Christie R H, Waeber C, et al. J Neurosci. 1997;17:6908–6917. doi: 10.1523/JNEUROSCI.17-18-06908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tipton K F, Singer T P. J Neurochem. 1993;61:1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- 25.Przedborski S, Kostic V, Jackson-Lewis V, Naini A B, Simonetti S, Fahn S, Carlson E, Epstein C J, Cadet J L. J Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhard J F, Jr, O’Callaghan J P. Anal Biochem. 1991;196:296–301. doi: 10.1016/0003-2697(91)90469-a. [DOI] [PubMed] [Google Scholar]
- 27.Reinhard J F, Jr, Smith G K, Nichol C A. Life Sci. 1986;39:2185–2189. doi: 10.1016/0024-3205(86)90395-4. [DOI] [PubMed] [Google Scholar]
- 28.Gow A, Duran D, Malcolm S, Ischiropoulos H. FEBS Lett. 1996;385:63–66. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- 29.Saadat S, Stehel A D, Lamouroux A, Mallet J, Thoenen H. J Neurochem. 1988;51:572–578. doi: 10.1111/j.1471-4159.1988.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 30.Daubner S C, Lohse D L, Fitzpatrick P F. Protein Sci. 1993;2:1452–1460. doi: 10.1002/pro.5560020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker S J, Liu X, Roskoski R, Vrana K E. Biochim Biophys Acta. 1994;1206:113–119. doi: 10.1016/0167-4838(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen A T. Nitrocarbons. New York, NY: VCH Publishers; 1995. pp. 40–41. [Google Scholar]
- 33.Ozaki N, Nakahara D, Mogi M, Harada M, Kiuchi K, Kaneda N, Miura Y, Kasahara Y, Nagatsu T. Neurosci Lett. 1988;85:228–232. doi: 10.1016/0304-3940(88)90356-4. [DOI] [PubMed] [Google Scholar]
- 34.Nagatsu T. Neurochem Res. 1990;15:425–429. doi: 10.1007/BF00969928. [DOI] [PubMed] [Google Scholar]
- 35.Grima B, Lamouroux A, Boni C, Julien J-F, Javoy-Agid F, Mallet J. Nature (London) 1987;326:707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- 36.Klaidman L K, Adams J D, Jr, Leung A L, Kim S S, Cadenas E. Free Radical Biol Med. 1993;15:169–179. doi: 10.1016/0891-5849(93)90056-z. [DOI] [PubMed] [Google Scholar]
- 37.Kissner R, Nauser T, Bugnon P, Lye P G, Koppenol W H. Chem Res Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 38.Beckman J S, Koppenol W H. Am J Physiol. 1996;217:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 39.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton S A. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin K-T, Xue J-Y, Nomen M, Spur B, Wong P Y-K. J Biol Chem. 1995;270:16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- 41.Troy C M, Derossi D, Prochiantz A, Greene L A, Shelanski M L. J Neurosci. 1996;16:253–261. doi: 10.1523/JNEUROSCI.16-01-00253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estevez A G, Spear N, Manuel S M, Radi R, Hederson C E, Barbeito L, Beckman J S. J Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gow A, Thom S R, Ischiropoulos H. Am J Physiol. 1998;274:L112–L118. doi: 10.1152/ajplung.1998.274.1.L112. [DOI] [PubMed] [Google Scholar]