Abstract
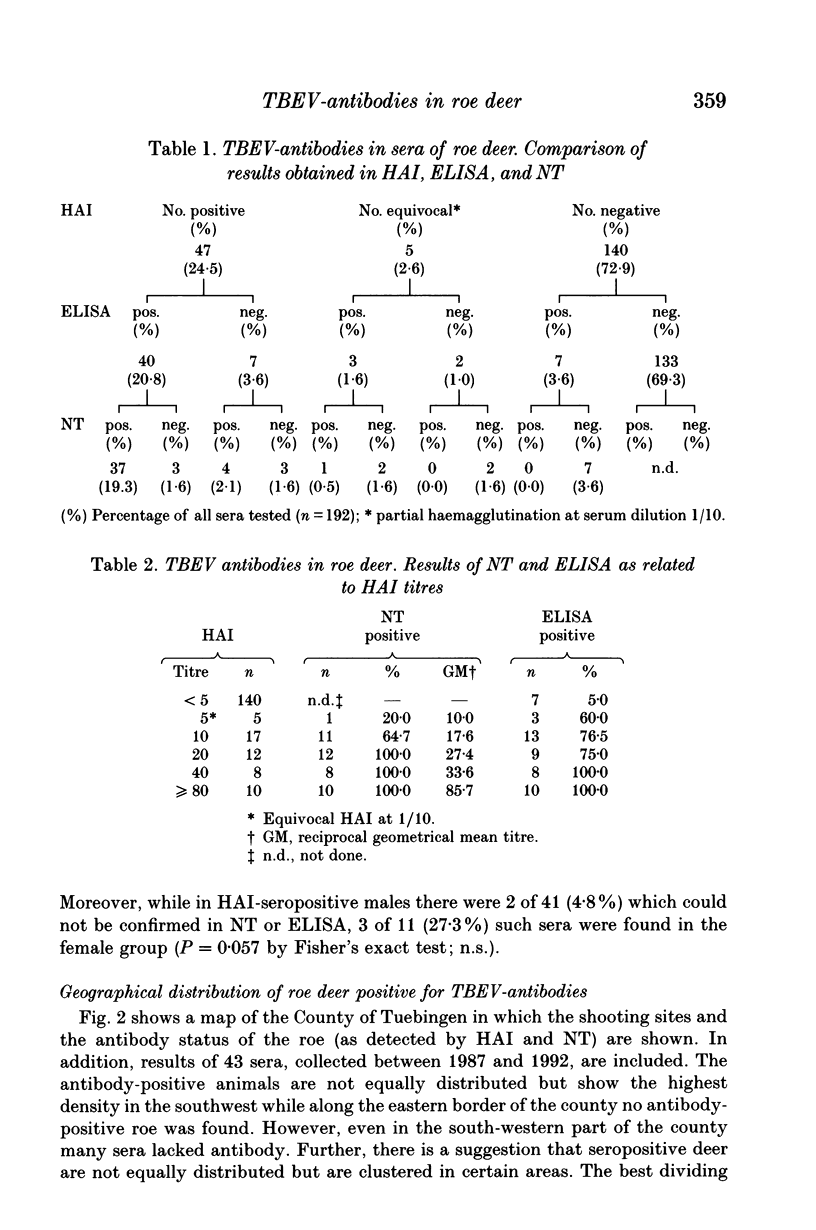
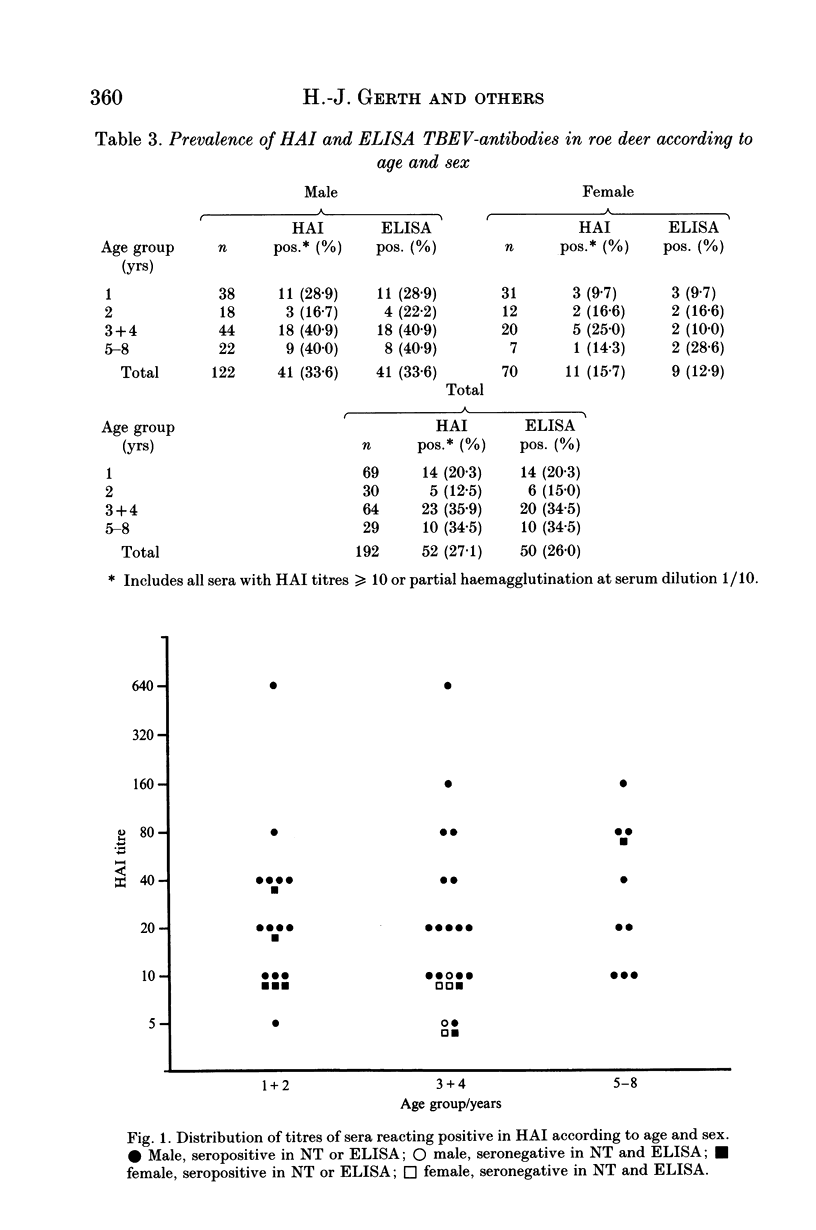
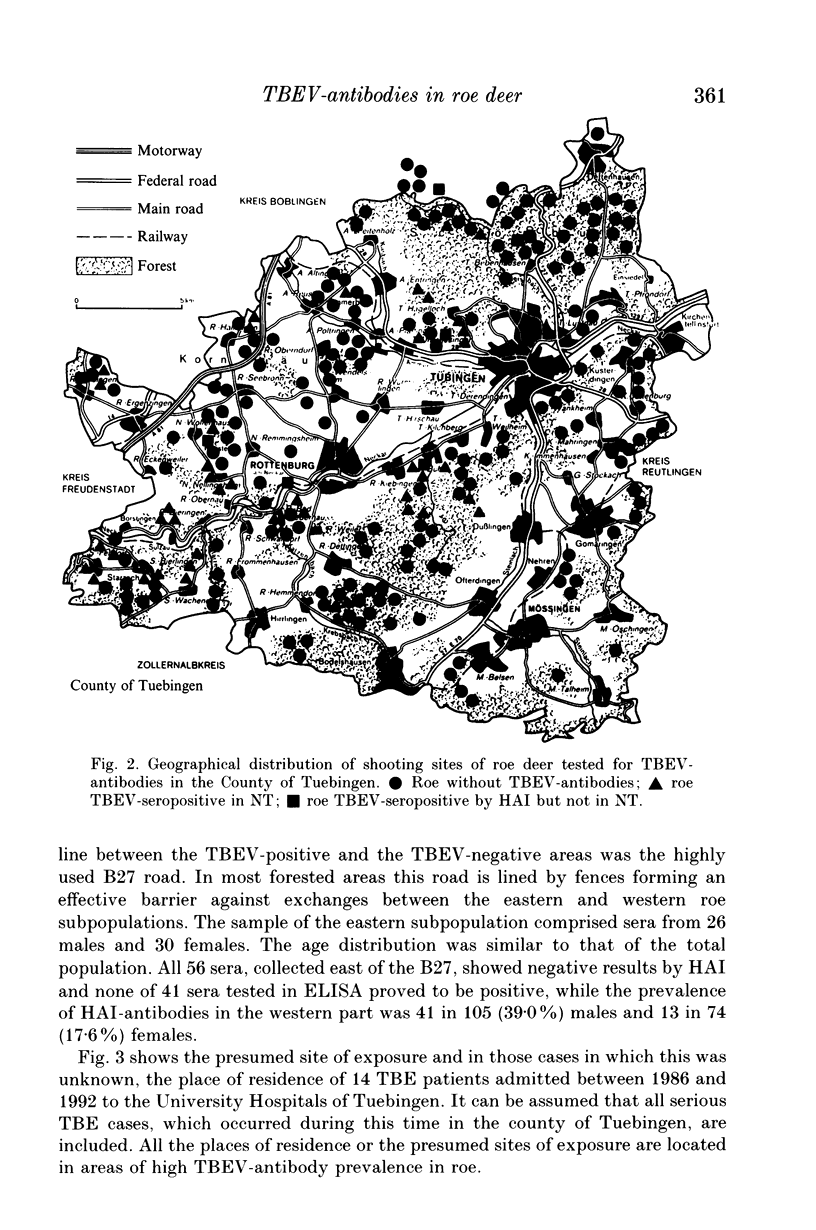
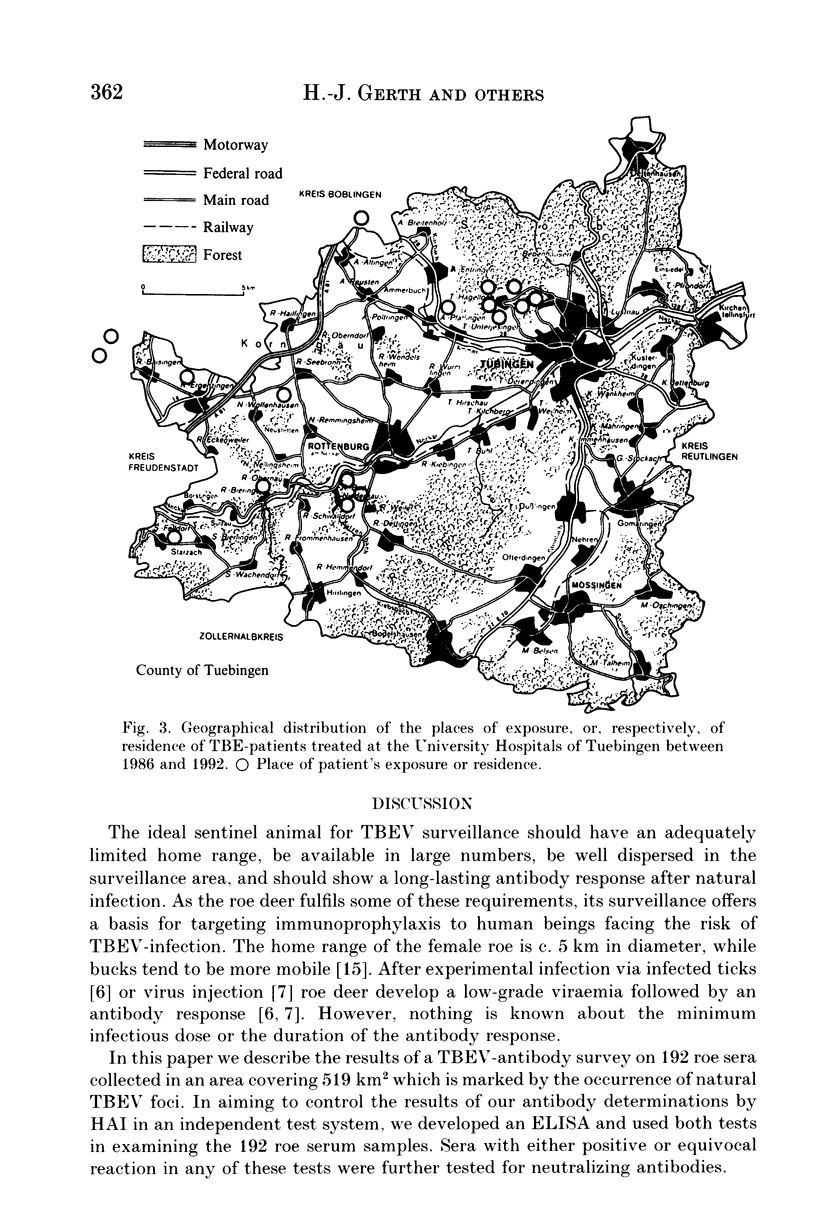
The suitability of serological surveys of roe deer (Capreolus capreolus) in determining the spread of tick-borne encephalitis virus (TBEV) was tested in a south German area with a low risk of TBEV infection to humans. Sera obtained from 192 hunted roe were screened by an haemagglutination-inhibition test (HAI) and in an ELISA developed in our laboratory. Those found positive were tested in a neutralization test (NT). Fifty (26.0%) sera reacted positive by ELISA and 43 (86.0%) of these were confirmed by HAI or NT. Forty-seven (24.5%) samples were positive by HAI, 44 (93.6%) of which were also positive in NT or ELISA. Only insignificant increase of the antibody prevalence with age (P = 0.17 for HAI antibodies) suggests that most infections occur at an early age in scattered natural foci. The antibody prevalence in females was lower than in males (OR = 0.63; P = 0.02 for HAI antibodies). In determining the distribution of seropositive roe we increased the sample size to 235 sera. No antibodies were detected in 56 (23.8%) sera collected in the eastern third of the county. The areas of high antibody prevalence in roe match those in which humans have been infected. We conclude that serosurveys of roe deer are useful in marking out areas in which humans face the risk of infection, provided that an adequate number of sera, preferably from males, is available.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann R., Rehse-Küpper B., Löser R., Scheid W. Neutralisierende Serumantikörper gegen das Virus der Zentraleuropäischen Enzephalitis bei der ländlichen Bevölkerung der Bundesrepublik Deutschland. Dtsch Med Wochenschr. 1968 Sep 13;93(37):1747–1754. doi: 10.1055/s-0028-1110820. [DOI] [PubMed] [Google Scholar]
- Asmera J., Heinz F. Delimitation of natural foci of tick-borne encephalitis in north-eastern Moravia. Folia Parasitol (Praha) 1972;19(3):263–271. [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Heinz F., Kunz C. Ein sensitives Gewebekultur-Antigen des Frühsommer-Meningoenzephalitis-Virus zur Verwendung im Hämagglutinationshemmungstest. Arch Virol. 1975;48(2):191–194. doi: 10.1007/BF01318152. [DOI] [PubMed] [Google Scholar]
- Kunz C. Tick-borne encephalitis in Europe. Acta Leiden. 1992;60(2):1–14. [PubMed] [Google Scholar]
- Miller T. J., Stone H. O. The rapid isolation of ribonuclease-free immunoglobulin G by protein A-sepharose affinity chromatography. J Immunol Methods. 1978;24(1-2):111–125. doi: 10.1016/0022-1759(78)90092-3. [DOI] [PubMed] [Google Scholar]
- Radda A., Hofmann H., Kunz C. Experimentelle Infektion einiger heimischer Saugerarten mit dem Fruhsommer-Meningo-Enzephalitis (FSME)-Virus. Zentralbl Bakteriol Orig. 1968;208(1):100–104. [PubMed] [Google Scholar]
- Radda A., Kunz C., Hofmann H. Nachweis von Antikörpern in Wildseren zur Erfassung von Herden des Virus der Frühsommer-Meningo-Enzephalitis FSME) in Niederösterreich. Zentralbl Bakteriol Orig. 1968;208(1):88–93. [PubMed] [Google Scholar]


