Abstract
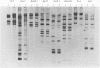
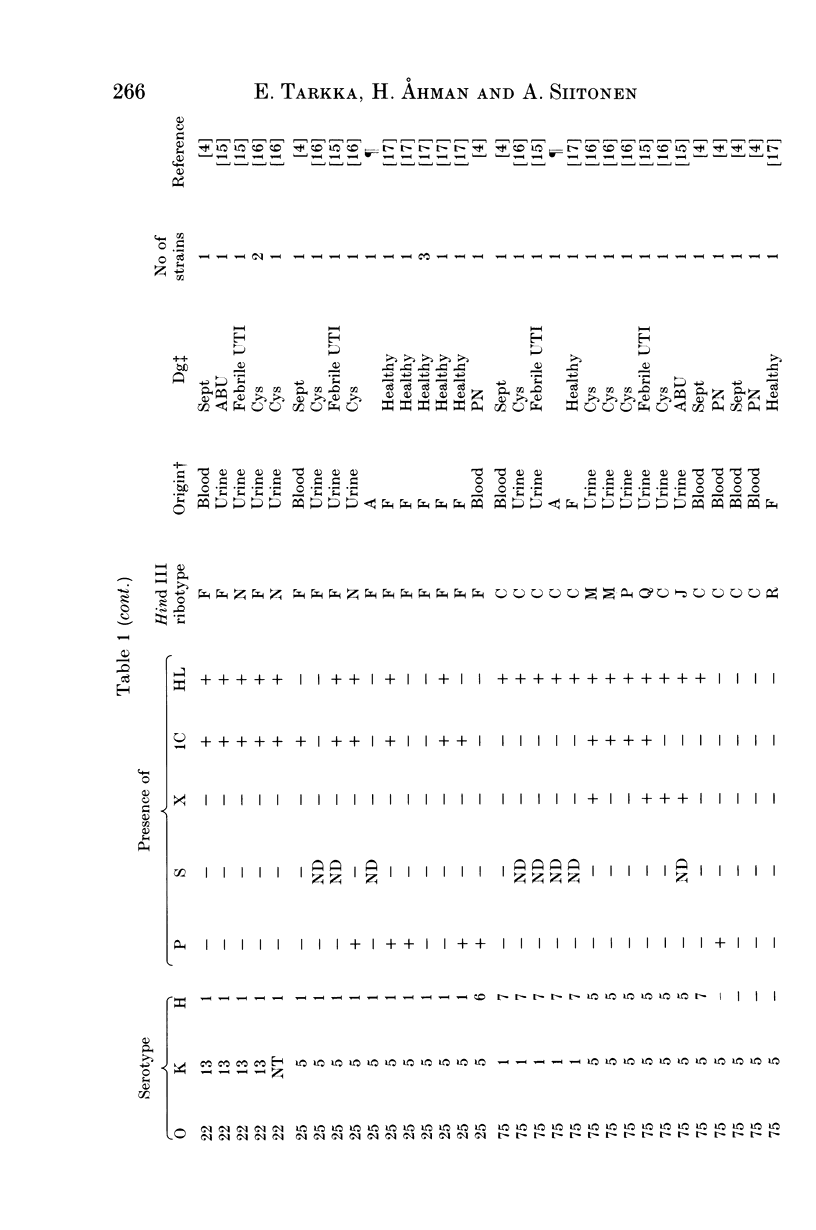
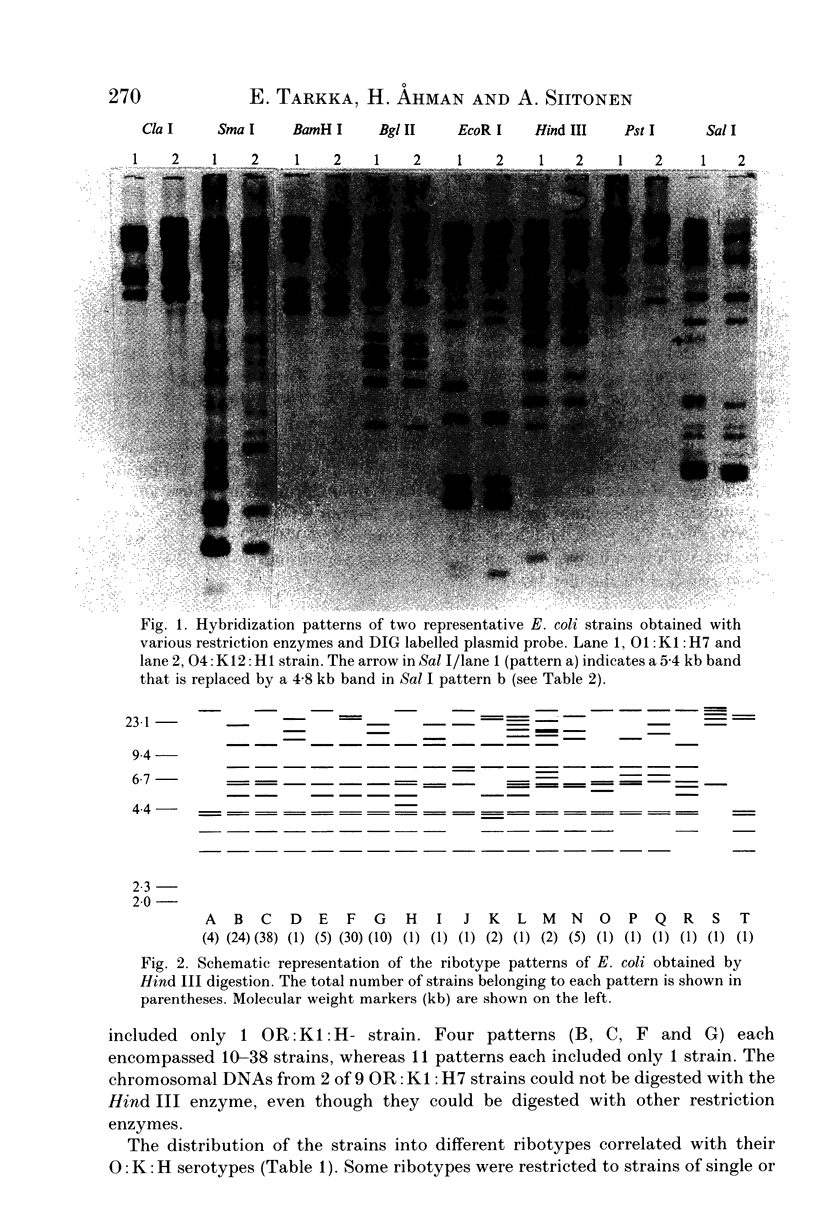
Restriction fragment length polymorphism of ribosomal RNA genes was analysed among 133 Escherichia coli strains predominantly from blood and urine, including 21 isolates from faeces of healthy persons. The strains had also been characterized for their O:K:H serotypes, for the presence of P, S and type 1C fimbriae, non-P, non-S mannose-resistant haemagglutinins and haemolysin production. Hind III-digested genomic DNA was subjected to Southern blot analysis with either plasmid pKK3535 containing E. coli rRNA operon or purified rRNA as a probe. Among the 133 strains 20 ribotypes were obtained. The distribution of strains into different ribotypes generally correlated with their O:K:H serotype. Ribotype variation within serotypes was mainly seen among strains with the K5 capsule. The origin of the strains or the presence of virulence-associated factors did not correlate with the ribotype. In conclusion, ribotyping appears to be a valuable method in epidemiologic studies especially when the serotyping methods are not available.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Arbeit R. D., Arthur M., Dunn R., Kim C., Selander R. K., Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990 Feb;161(2):230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- Arthur M., Arbeit R. D., Kim C., Beltran P., Crowe H., Steinbach S., Campanelli C., Wilson R. A., Selander R. K., Goldstein R. Restriction fragment length polymorphisms among uropathogenic Escherichia coli isolates: pap-related sequences compared with rrn operons. Infect Immun. 1990 Feb;58(2):471–479. doi: 10.1128/iai.58.2.471-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Johnson C. E., Rubin R. H., Arbeit R. D., Campanelli C., Kim C., Steinbach S., Agarwal M., Wilkinson R., Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Picard B., Goullet P., Lambert-Zechovsky N. Y., Brahimi N., Mercier J. C., Beaufils F., Elion J. Molecular epidemiology unravels the complexity of neonatal Escherichia coli acquisition in twins. J Clin Microbiol. 1992 Jul;30(7):1896–1898. doi: 10.1128/jcm.30.7.1896-1898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E., Cavé H., Aujard Y., Lambert-Zechovsky N., Desjardins P., Elion J., Denamur E. Molecular analysis of multiply recurrent meningitis due to Escherichia coli K1 in an infant. Clin Infect Dis. 1993 Jan;16(1):82–85. doi: 10.1093/clinids/16.1.82. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Ikäheimo R., Siitonen A., Kärkkäinen U., Mäkelä P. H. Virulence characteristics of Escherichia coli in nosocomial urinary tract infection. Clin Infect Dis. 1993 Jun;16(6):785–791. doi: 10.1093/clind/16.6.785. [DOI] [PubMed] [Google Scholar]
- Johnson J. R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991 Jan;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I. From the national institutes of health. Summary of a workshop on the clone concept in the epidemiology, taxonomy, and evolution of the enterobacteriaceae and other bacteria. J Infect Dis. 1983 Aug;148(2):346–357. doi: 10.1093/infdis/148.2.346. [DOI] [PubMed] [Google Scholar]
- Ott M., Bender L., Blum G., Schmittroth M., Achtman M., Tschäpe H., Hacker J. Virulence patterns and long-range genetic mapping of extraintestinal Escherichia coli K1, K5, and K100 isolates: use of pulsed-field gel electrophoresis. Infect Immun. 1991 Aug;59(8):2664–2672. doi: 10.1128/iai.59.8.2664-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Hacker J., Schmoll T., Jarchau T., Korhonen T. K., Goebel W. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect Immun. 1986 Dec;54(3):646–653. doi: 10.1128/iai.54.3.646-653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plos K., Hull S. I., Hull R. A., Levin B. R., Orskov I., Orskov F., Svanborg-Edén C. Distribution of the P-associated-pilus (pap) region among Escherichia coli from natural sources: evidence for horizontal gene transfer. Infect Immun. 1989 May;57(5):1604–1611. doi: 10.1128/iai.57.5.1604-1611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siitonen A. Escherichia coli in fecal flora of healthy adults: serotypes, P and type 1C fimbriae, non-P mannose-resistant adhesins, and hemolytic activity. J Infect Dis. 1992 Nov;166(5):1058–1065. doi: 10.1093/infdis/166.5.1058. [DOI] [PubMed] [Google Scholar]
- Siitonen A., Takala A., Ratiner Y. A., Pere A., Mäkelä P. H. Invasive Escherichia coli infections in children: bacterial characteristics in different age groups and clinical entities. Pediatr Infect Dis J. 1993 Jul;12(7):606–612. doi: 10.1097/00006454-199307000-00012. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Dowson C. G., Spratt B. G. Localized sex in bacteria. Nature. 1991 Jan 3;349(6304):29–31. doi: 10.1038/349029a0. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tullus K., Brauner A., Fryklund B., Munkhammar T., Rabsch W., Reissbrodt R., Burman L. G. Host factors versus virulence-associated bacterial characteristics in neonatal and infantile bacteraemia and meningitis caused by Escherichia coli. J Med Microbiol. 1992 Mar;36(3):203–208. doi: 10.1099/00222615-36-3-203. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]