Abstract
Non-specific and specific mechanisms of adherence have been examined in two collections of methicillin-resistant Staphylococcus aureus (MRSA). Determination of hydrophobicity by salt aggregation, hydrophobicity indices and of adherence to the extra-cellular matrix proteins fibronectin, vitronectin, laminin and collagen type 1 have failed to reveal any correlation with phage-type, plasmid profile or antibiogram. Further, the strain collections, made over a period of years in two countries, differ markedly in their adherence characteristics; MRSA are heterogeneous in this respect. Such heterogeneity may explain the polarization of views on the epidemicity or 'virulence' of MRSA. With the exception of adherence to collagen a small group of methicillin sensitive S. aureus had characteristics intermediate between the two groups of MRSA.
Full text
PDF



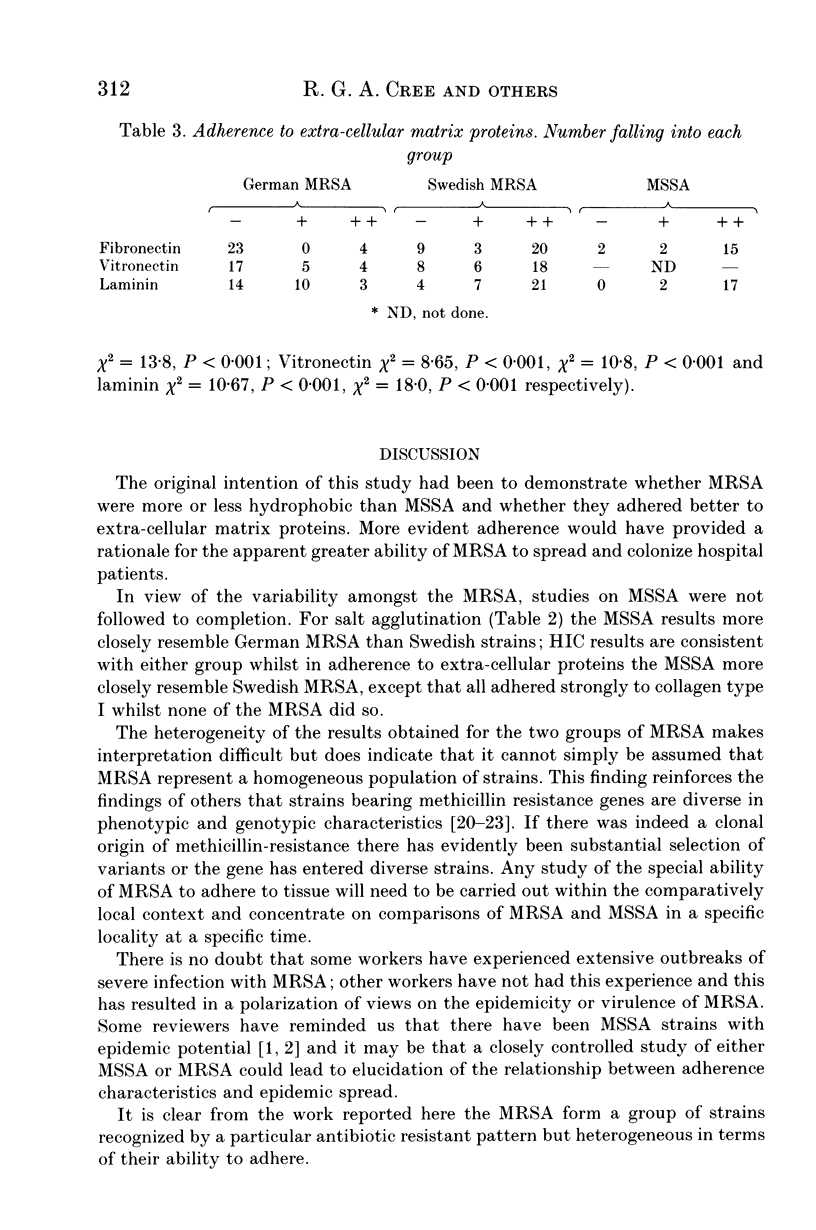


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Casewell M. W., Hill R. L. The carrier state: methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1986 Jul;18 (Suppl A):1–12. doi: 10.1093/jac/18.supplement_a.1. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Preissner K. T., Müller-Berghaus G., Blobel H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987 Aug;55(8):1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas M., Cookson B. D., Talsania H. G., Owen R. J. Numerical analysis of electrophoretic protein patterns of methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1989 Nov;27(11):2574–2581. doi: 10.1128/jcm.27.11.2574-2581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Kreiswirth B., Kornblum J., Arbeit R. D., Eisner W., Maslow J. N., McGeer A., Low D. E., Novick R. P. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993 Jan 8;259(5092):227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect Immun. 1985 Oct;50(1):77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Barr K. W., Barr V. E., Inglis T. J. Properties of methicillin-resistant Staphylococcus aureus colonizing patients in a burns unit. J Hosp Infect. 1986 Mar;7(2):137–148. doi: 10.1016/0195-6701(86)90056-3. [DOI] [PubMed] [Google Scholar]
- Lentino J. R., Hennein H., Krause S., Pappas S., Fuller G., Schaaff D., DiCostanzo M. B. A comparison of pneumonia caused by gentamicin, methicillin-resistant and gentamicin, methicillin-sensitive Staphylococcus aureus: epidemiologic and clinical studies. Infect Control. 1985 Jul;6(7):267–272. doi: 10.1017/s0195941700061737. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Ljungh A., Hjertén S., Wadström T. High surface hydrophobicity of autoaggregating Staphylococcus aureus strains isolated from human infections studied with the salt aggregation test. Infect Immun. 1985 Feb;47(2):522–526. doi: 10.1128/iai.47.2.522-526.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A. S., Paulsson M., Wadström T. Particle agglutination assays for rapid detection of fibronectin, fibrinogen, and collagen receptors on Staphylococcus aureus. J Clin Microbiol. 1988 Aug;26(8):1549–1554. doi: 10.1128/jcm.26.8.1549-1554.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Liang O. D., Ascencio F., Wadström T. Vitronectin-binding surface proteins of Staphylococcus aureus. Zentralbl Bakteriol. 1992 Jun;277(1):54–64. doi: 10.1016/s0934-8840(11)80871-6. [DOI] [PubMed] [Google Scholar]
- Prevost G., Jaulhac B., Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992 Apr;30(4):967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A. The staphylococcal fibronectin receptor: evidence for its importance in invasive infections. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S335–S340. doi: 10.1093/clinids/9.supplement_4.s355. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy P. T., Lai L. W., Drake T. A., Sande M. A. Effect of fibronectin on adherence of Staphylococcus aureus to fibrin thrombi in vitro. Infect Immun. 1985 Apr;48(1):83–86. doi: 10.1128/iai.48.1.83-86.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen K. H., Veijola J., Dagberg B., Uhlin B. E. Binding of basement-membrane laminin by Escherichia coli. Mol Microbiol. 1991 Sep;5(9):2133–2141. doi: 10.1111/j.1365-2958.1991.tb02143.x. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M. Q., Groth D. M., Mendis A. H., Sampson J., Wetherall J. D., Grubb W. B. Typing of methicillin-resistant Staphylococcus aureus with an M13 repeat probe. J Hosp Infect. 1992 Apr;20(4):233–245. doi: 10.1016/0195-6701(92)90002-4. [DOI] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Zuccarelli A. J., Roy I., Harding G. P., Couperus J. J. Diversity and stability of restriction enzyme profiles of plasmid DNA from methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1990 Jan;28(1):97–102. doi: 10.1128/jcm.28.1.97-102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


