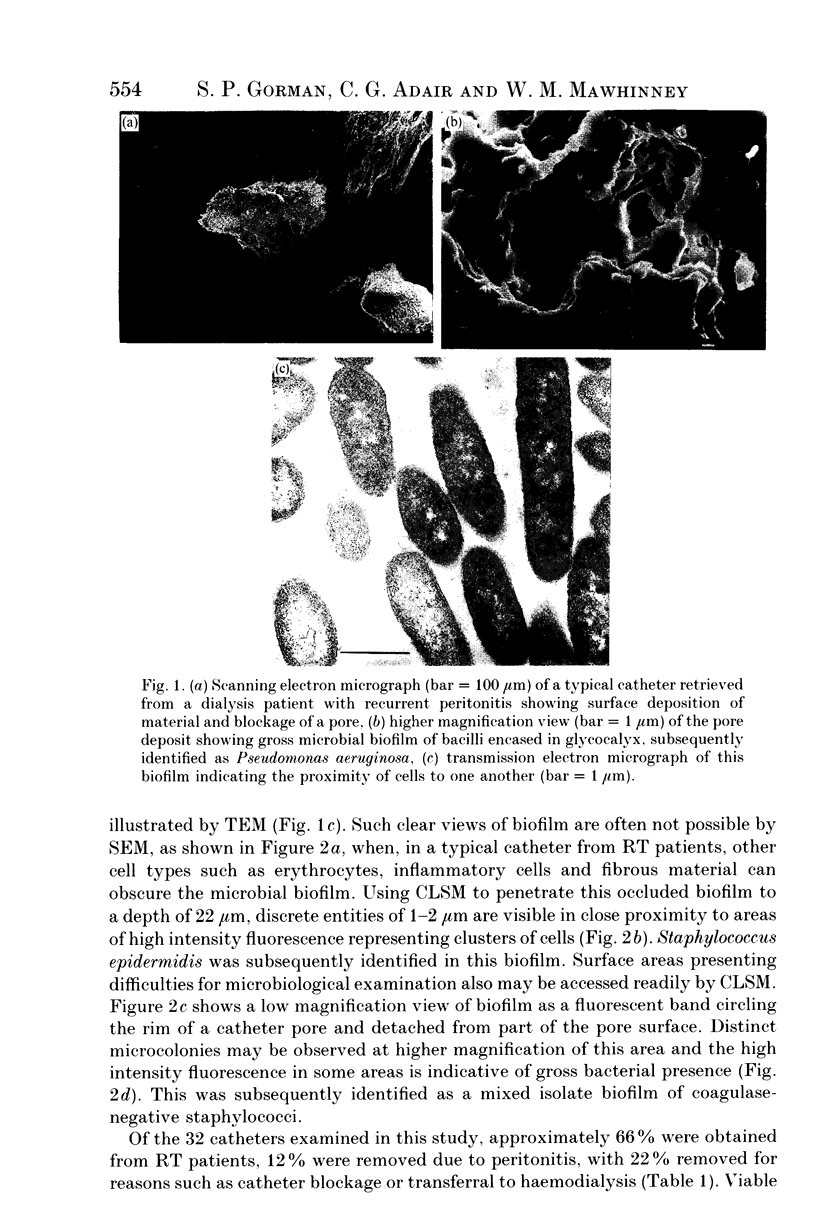
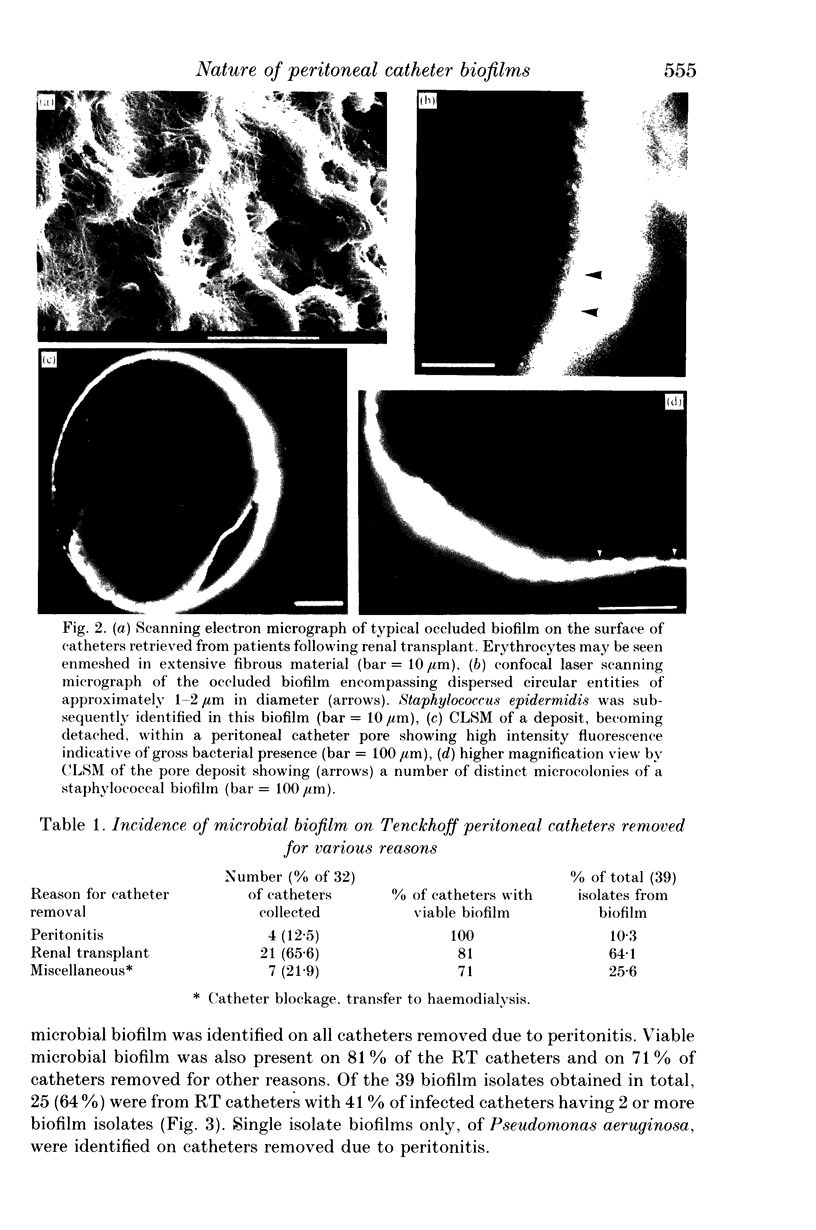
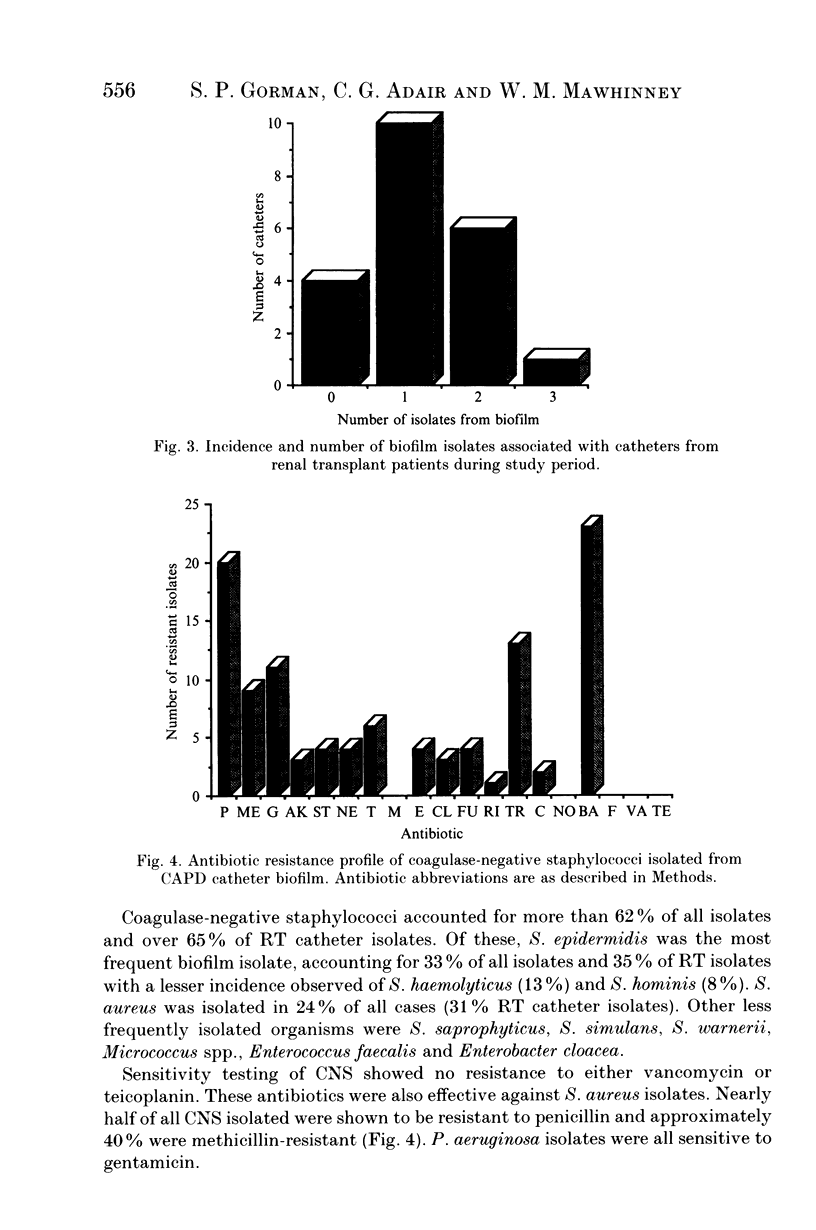
Abstract
Thirty-two Tenckhoff catheters retrieved from continuous ambulatory peritoneal dialysis patients with a history of peritonitis were examined for microbial biofilm. Confocal laser scanning microscopy was successfully employed to visualize bacteria in biofilm occluded from view by scanning electron microscopy. Occluded but viable microbial biofilm was associated with 17 (81%) catheters from patients free from infection following renal transplant. Mixed isolate biofilm with two or more isolates of coagulase-negative staphylococci or Staphylococcus aureus was found on 41% of these catheters. Clearly visible viable biofilm consisting exclusively of Pseudomonas aeruginosa occurred on all four catheters removed due to recurrent peritonitis. Five (71%) catheters retrieved from patients transferred to haemodialysis had viable biofilm. Antibiotic sensitivities of the biofilm isolates were similar in profile to those reported for non-biofilm isolates from infected dialysate. Persistence of catheter biofilm despite direct contact with therapeutic levels of antibiotics in peritoneal dialysate requires that attention be directed towards improving antibiotic efficacy against peritonitis-causing bacteria in biofilm form.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans R. C., Holmes C. J. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob Agents Chemother. 1987 Jun;31(6):889–894. doi: 10.1128/aac.31.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman S. P., Mawhinney W. M., Adair C. G., Issouckis M. Confocal laser scanning microscopy of peritoneal catheter surfaces. J Med Microbiol. 1993 Jun;38(6):411–417. doi: 10.1099/00222615-38-6-411. [DOI] [PubMed] [Google Scholar]
- Gorman S., Adair C., O'Neill F., Goldsmith C., Webb H. Influence of selective decontamination of the digestive tract on microbial biofilm formation on endotracheal tubes from artificially ventilated patients. Eur J Clin Microbiol Infect Dis. 1993 Jan;12(1):9–17. doi: 10.1007/BF01997050. [DOI] [PubMed] [Google Scholar]
- Holmes C. J., Evans R. C., Vonesh E. Application of an empirically derived growth curve model to characterize Staphylococcus epidermidis biofilm development on silicone elastomer. Biomaterials. 1989 Nov;10(9):625–629. doi: 10.1016/0142-9612(89)90118-x. [DOI] [PubMed] [Google Scholar]
- Kluge R. M. Infections of prosthetic cardiac valves and arterial grafts. Heart Lung. 1982 Mar-Apr;11(2):146–151. [PubMed] [Google Scholar]
- Lawrence J. R., Korber D. R., Hoyle B. D., Costerton J. W., Caldwell D. E. Optical sectioning of microbial biofilms. J Bacteriol. 1991 Oct;173(20):6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Noble M. A., Costerton J. W. Examination of the morphology of bacteria adhering to peritoneal dialysis catheters by scanning and transmission electron microscopy. J Clin Microbiol. 1983 Dec;18(6):1388–1398. doi: 10.1128/jcm.18.6.1388-1398.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J. C., Wright J. B., Ruseska I., Marrie T. J., Whitfield C., Costerton J. W. Antibiotic resistance of Pseudomonas aeruginosa colonizing a urinary catheter in vitro. Eur J Clin Microbiol. 1985 Apr;4(2):213–218. doi: 10.1007/BF02013600. [DOI] [PubMed] [Google Scholar]
- Spencer R. C. Infections in continuous ambulatory peritoneal dialysis. J Med Microbiol. 1988 Sep;27(1):1–9. doi: 10.1099/00222615-27-1-1. [DOI] [PubMed] [Google Scholar]
- Tenney J. H., Moody M. R., Newman K. A., Schimpff S. C., Wade J. C., Costerton J. W., Reed W. P. Adherent microorganisms on lumenal surfaces of long-term intravenous catheters. Importance of Staphylococcus epidermidis in patients with cancer. Arch Intern Med. 1986 Oct;146(10):1949–1954. [PubMed] [Google Scholar]
- Verbrugh H. A. Organisms causing peritonitis. Contrib Nephrol. 1990;85:39–48. doi: 10.1159/000419061. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]