Abstract
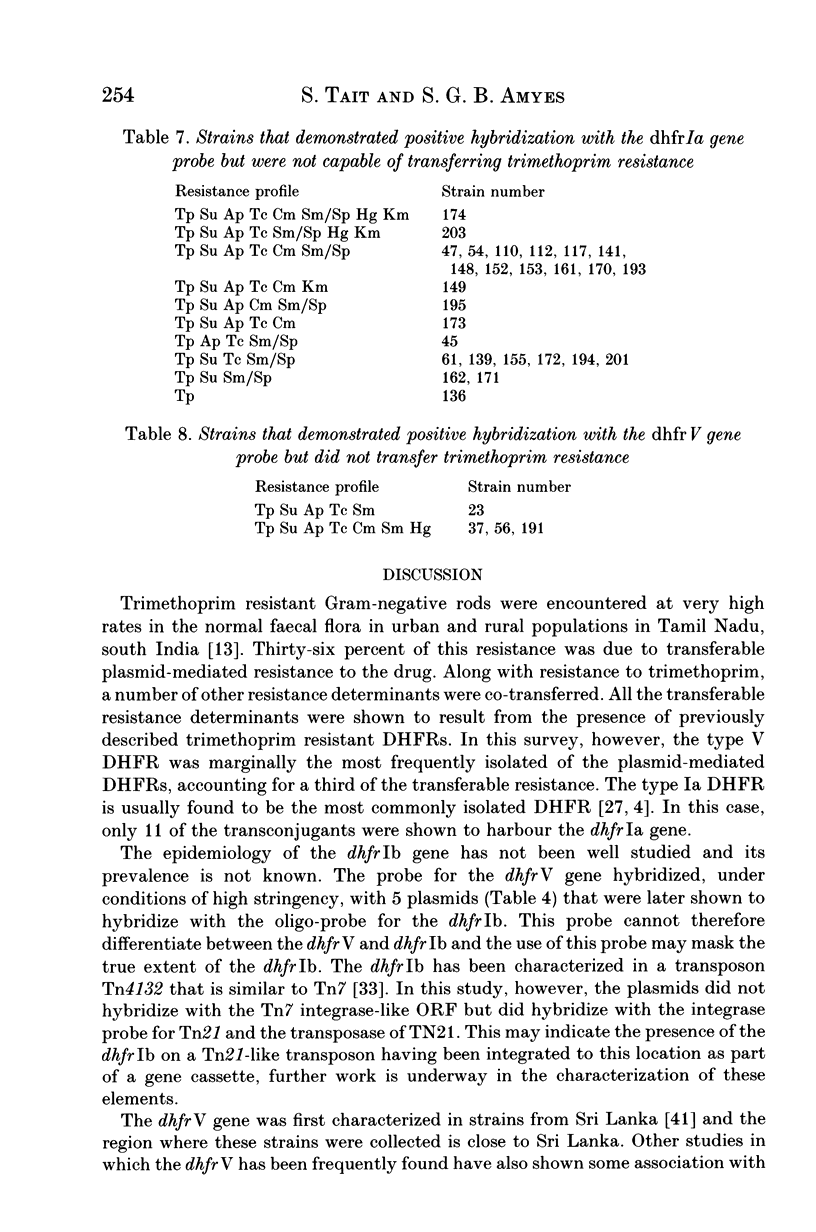
A high incidence of resistance to trimethoprim has been shown in the normal faecal flora in a population in south India. The dihydrofolate reductase (dhfr) genes mediating transferable resistance to trimethoprim have been identified. Unusually, in this study, the dhfrV was shown to be the predominant resistance gene (dhfrV 50% of transconjugants, dhfrIa 30%), the dhfrIb was also detected being distinguished from the dhfrV by an oligo-probe. However, when non-transferable resistance was considered, the dhfrIa was the most prevalent of the dhfrs identified. All those plasmids harbouring the dhfrIa were shown to possess Tn7. All the plasmids that probed positive for the dhfrV and the dhfrIb were shown to be associated with the integrase of the Tn21-like transposons, but 8 of the dhfrV genes were not associated with the Tn21 resolvase. The dhfrIV was shown to be present in all seven plasmids that produced low level trimethoprim-resistance. The dhfrV, first characterized in Sri Lanka, would seem to have a local distribution in this region of Asia but is distinguishable from the dhfrIb only by the use of an oligo-probe.
Full text
PDF


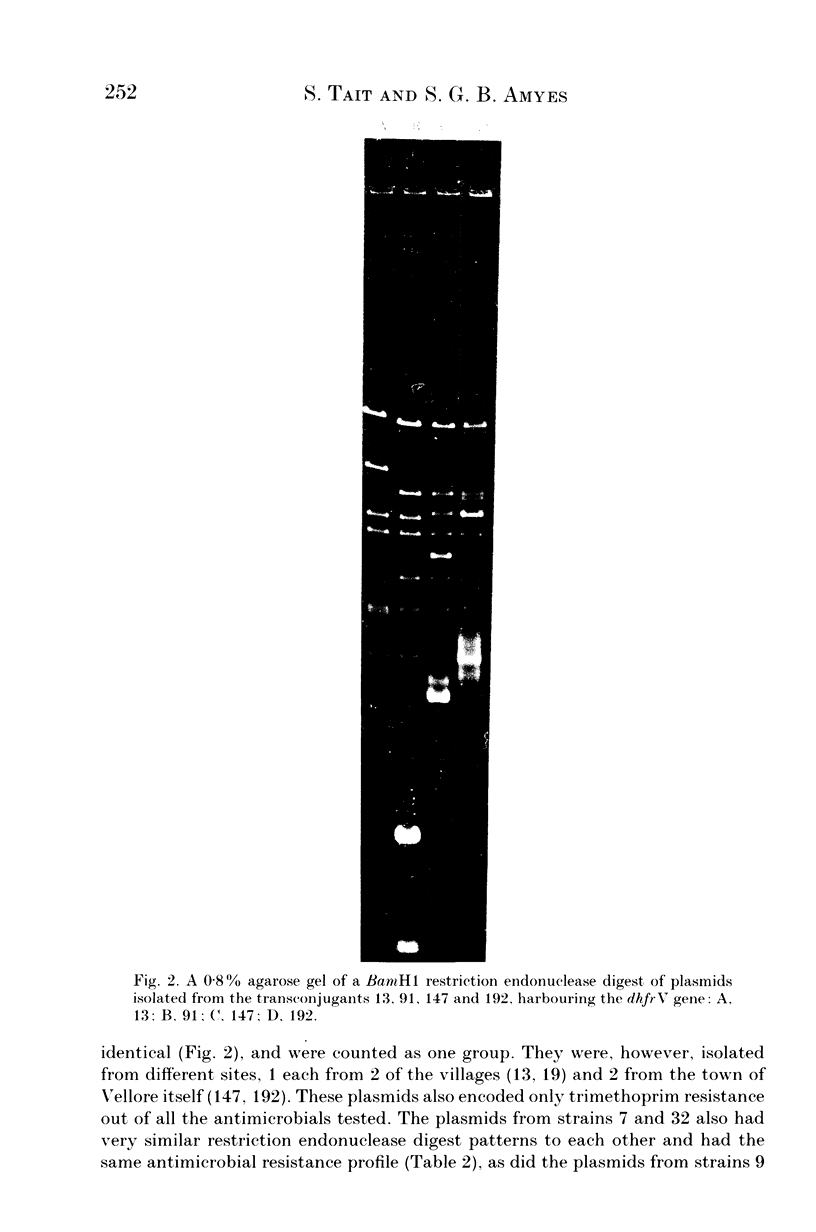





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agodi A., Jones C., Threlfall E. J., D'Angelo M., Marranzano M. Molecular characterization of trimethoprim resistance in Shigella sonnei in Sicily. Epidemiol Infect. 1990 Aug;105(1):29–40. doi: 10.1017/s0950268800047610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyes S. G., Gould I. M. Trimethoprim resistance plasmids. Ann Microbiol (Paris) 1984 Sep-Oct;135B(2):177–186. [PubMed] [Google Scholar]
- Amyes S. G., Smith J. T. R-factor trimethoprim resistance mechanism: an insusceptible target site. Biochem Biophys Res Commun. 1974 May 20;58(2):412–418. doi: 10.1016/0006-291x(74)90380-5. [DOI] [PubMed] [Google Scholar]
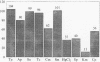
- Amyes S. G., Tait S., Thomson C. J., Payne D. J., Nandivada L. S., Jesudason M. V., Mukundan U. D., Young H. K. The incidence of antibiotic resistance in aerobic faecal flora in south India. J Antimicrob Chemother. 1992 Apr;29(4):415–425. doi: 10.1093/jac/29.4.415. [DOI] [PubMed] [Google Scholar]
- Amyes S. G., Towner K. J., Young H. K. Classification of plasmid-encoded dihydrofolate reductases conferring trimethoprim resistance. J Med Microbiol. 1992 Jan;36(1):1–3. doi: 10.1099/00222615-36-1-1. [DOI] [PubMed] [Google Scholar]
- Bonten M., Stobberingh E., Philips J., Houben A. Antibiotic resistance of Escherichia coli in fecal samples of healthy people in two different areas in an industrialized country. Infection. 1992 Sep-Oct;20(5):258–262. doi: 10.1007/BF01710790. [DOI] [PubMed] [Google Scholar]
- Bonten M., Stobberingh E., Philips J., Houben A. High prevalence of antibiotic resistant Escherichia coli in faecal samples of students in the south-east of The Netherlands. J Antimicrob Chemother. 1990 Oct;26(4):585–592. doi: 10.1093/jac/26.4.585. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Misra T. K., Winnie J. N., Schmidt A., Seiff M., Silver S. The nucleotide sequence of the mercuric resistance operons of plasmid R100 and transposon Tn501: further evidence for mer genes which enhance the activity of the mercuric ion detoxification system. Mol Gen Genet. 1986 Jan;202(1):143–151. doi: 10.1007/BF00330531. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Fling M. E., Richards C. The nucleotide sequence of the trimethoprim-resistant dihydrofolate reductase gene harbored by Tn7. Nucleic Acids Res. 1983 Aug 11;11(15):5147–5158. doi: 10.1093/nar/11.15.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost J. A., Willshaw G. A., Barclay E. A., Rowe B., Lemmens P., Vandepitte J. Plasmid characterization of drug-resistant Shigella dysenteriae 1 from an epidemic in Central Africa. J Hyg (Lond) 1985 Apr;94(2):163–172. doi: 10.1017/s0022172400061362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä E., Renkonen O. V., Sunila R., Uurasmaa P., Huovinen P. The emergence and mechanisms of trimethoprim resistance in Escherichia coli isolated from outpatients in Finland. J Antimicrob Chemother. 1990 Feb;25(2):275–283. doi: 10.1093/jac/25.2.275. [DOI] [PubMed] [Google Scholar]
- Heikkilä E., Siitonen A., Jahkola M., Fling M., Sundström L., Huovinen P. Increase of trimethoprim resistance among Shigella species, 1975-1988: analysis of resistance mechanisms. J Infect Dis. 1990 Jun;161(6):1242–1248. doi: 10.1093/infdis/161.6.1242. [DOI] [PubMed] [Google Scholar]
- Herman D. J., Gerding D. N. Antimicrobial resistance among enterococci. Antimicrob Agents Chemother. 1991 Jan;35(1):1–4. doi: 10.1128/aac.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesudason M. V., Joseph D., Koshi G. Transferable trimethoprim resistance of shigellae encountered in Vellore (south India). Indian J Med Res. 1989 Sep;89:297–299. [PubMed] [Google Scholar]
- Jesudason M. V., Joseph D., Koshi G. Transferable trimethoprim resistance of shigellae encountered in Vellore (south India). Indian J Med Res. 1989 Sep;89:297–299. [PubMed] [Google Scholar]
- Lafond M., Couture F., Vézina G., Levesque R. C. Evolutionary perspectives on multiresistance beta-lactamase transposons. J Bacteriol. 1989 Dec;171(12):6423–6429. doi: 10.1128/jb.171.12.6423-6429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein C., Brenner S. Unique insertion site of Tn7 in the E. coli chromosome. Nature. 1982 Jun 17;297(5867):601–603. doi: 10.1038/297601a0. [DOI] [PubMed] [Google Scholar]
- Mayer K. H., Fling M. E., Hopkins J. D., O'Brien T. F. Trimethoprim resistance in multiple genera of Enterobacteriaceae at a U.S. hospital: spread of the type II dihydrofolate reductase gene by a single plasmid. J Infect Dis. 1985 May;151(5):783–789. doi: 10.1093/infdis/151.5.783. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Alvarado T., Kim K. H., Vorachit M., Jayanetra P., Levine M. M., Prenzel I., Fling M., Elwell L., McCracken G. H. Increasing resistance to trimethoprim-sulfamethoxazole among isolates of Escherichia coli in developing countries. J Infect Dis. 1985 Dec;152(6):1107–1113. doi: 10.1093/infdis/152.6.1107. [DOI] [PubMed] [Google Scholar]
- Shahid N. S., Rahaman M. M., Haider K., Banu H., Rahman N. Changing pattern of resistant Shiga bacillus (Shigella dysenteriae type 1) and Shigella flexneri in Bangladesh. J Infect Dis. 1985 Dec;152(6):1114–1119. doi: 10.1093/infdis/152.6.1114. [DOI] [PubMed] [Google Scholar]
- Shanahan P. M., Wylie B. A., Adrian P. V., Koornhof H. J., Thomson C. J., Amyes S. G. The prevalence of antimicrobial resistance in human faecal flora in South Africa. Epidemiol Infect. 1993 Oct;111(2):221–228. doi: 10.1017/s0950268800056922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears P., Suliman G., Hart C. A. Occurrence of multiple antibiotic resistance and R plasmids in Enterobacteriaceae isolated from children in the Sudan. Epidemiol Infect. 1988 Feb;100(1):73–81. doi: 10.1017/s0950268800065572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. V., Reves R. R., Pickering L. K., Murray B. E. Identification by DNA sequence analysis of a new plasmid-encoded trimethoprim resistance gene in fecal Escherichia coli isolates from children in day-care centers. Antimicrob Agents Chemother. 1992 Aug;36(8):1720–1726. doi: 10.1128/aac.36.8.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steen R., Sköld O. Plasmid-borne or chromosomally mediated resistance by Tn7 is the most common response to ubiquitous use of trimethoprim. Antimicrob Agents Chemother. 1985 Jun;27(6):933–937. doi: 10.1128/aac.27.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström L., Roy P. H., Sköld O. Site-specific insertion of three structural gene cassettes in transposon Tn7. J Bacteriol. 1991 May;173(9):3025–3028. doi: 10.1128/jb.173.9.3025-3028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström L., Rådström P., Swedberg G., Sköld O. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet. 1988 Aug;213(2-3):191–201. doi: 10.1007/BF00339581. [DOI] [PubMed] [Google Scholar]
- Sundström L., Swedberg G., Sköld O. Characterization of transposon Tn5086, carrying the site-specifically inserted gene dhfrVII mediating trimethoprim resistance. J Bacteriol. 1993 Mar;175(6):1796–1805. doi: 10.1128/jb.175.6.1796-1805.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C. J., Young H. K., Amyes S. G. The role of thymine starvation in the expression of type IV plasmid-encoded trimethoprim-resistant dihydrofolate reductase. J Med Microbiol. 1993 Apr;38(4):250–255. doi: 10.1099/00222615-38-4-250. [DOI] [PubMed] [Google Scholar]
- Towner K. J., Carter G. I. Cloning of the type VII trimethoprim-resistant dihydrofolate reductase gene and identification of a specific DNA probe. FEMS Microbiol Lett. 1990 Jun 15;58(1):19–22. doi: 10.1016/0378-1097(90)90095-8. [DOI] [PubMed] [Google Scholar]
- Towner K. J., Carter G. I., Young H. K., Amyes S. G. Detection of novel trimethoprim resistance determinants in the United Kingdom using biotin-labelled DNA probes. Epidemiol Infect. 1991 Feb;106(1):63–70. doi: 10.1017/s0950268800056442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner K. J., Young H. K., Amyes S. G. Biotinylated DNA probes for trimethoprim-resistant dihydrofolate reductases types IV and V. J Antimicrob Chemother. 1988 Sep;22(3):285–291. doi: 10.1093/jac/22.3.285. [DOI] [PubMed] [Google Scholar]
- Urbina R., Prado V., Canelo E. Trimethoprim resistance in enterobacteria isolated in Chile. J Antimicrob Chemother. 1989 Jan;23(1):143–149. doi: 10.1093/jac/23.1.143. [DOI] [PubMed] [Google Scholar]
- Wohlleben W., Arnold W., Bissonnette L., Pelletier A., Tanguay A., Roy P. H., Gamboa G. C., Barry G. F., Aubert E., Davies J. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I(AAC(3)-I), another member of the Tn21-based expression cassette. Mol Gen Genet. 1989 Jun;217(2-3):202–208. doi: 10.1007/BF02464882. [DOI] [PubMed] [Google Scholar]
- Wylie B. A., Koornhof H. J. Nucleotide sequence of dihydrofolate reductase type VI. J Med Microbiol. 1991 Oct;35(4):214–218. doi: 10.1099/00222615-35-4-214. [DOI] [PubMed] [Google Scholar]
- Young H. K., Amyes S. G. A new mechanism of plasmid trimethoprim resistance. Characterization of an inducible dihydrofolate reductase. J Biol Chem. 1986 Feb 25;261(6):2503–2505. [PubMed] [Google Scholar]
- Young H. K., Amyes S. G. Characterisation of a new transposon-mediated trimethoprim-resistant dihydrofolate reductase. Biochem Pharmacol. 1985 Dec 15;34(24):4334–4337. doi: 10.1016/0006-2952(85)90296-5. [DOI] [PubMed] [Google Scholar]
- Young H. K., Jesudason M. V., Koshi G., Amyes S. G. Unusual expression of new low-level-trimethoprim-resistance plasmids. J Clin Microbiol. 1986 Jul;24(1):61–64. doi: 10.1128/jcm.24.1.61-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]