Abstract
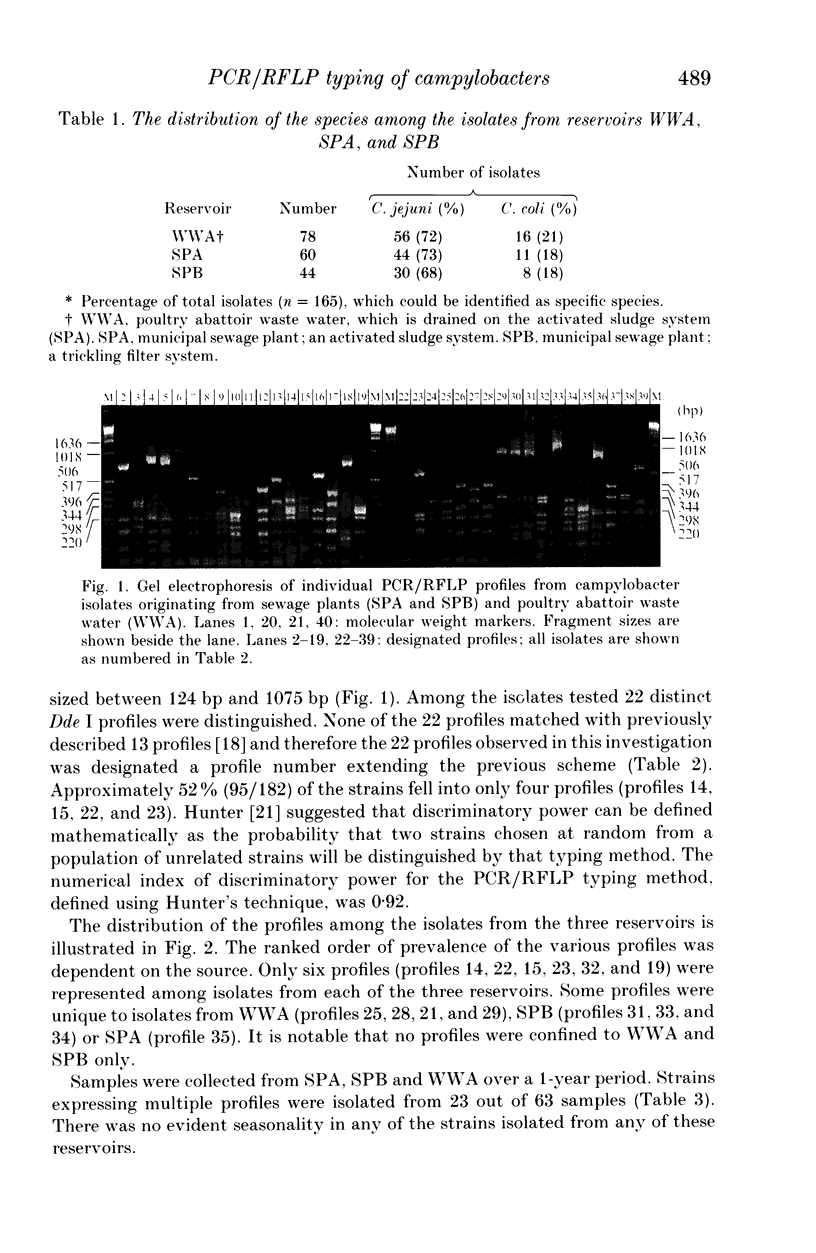
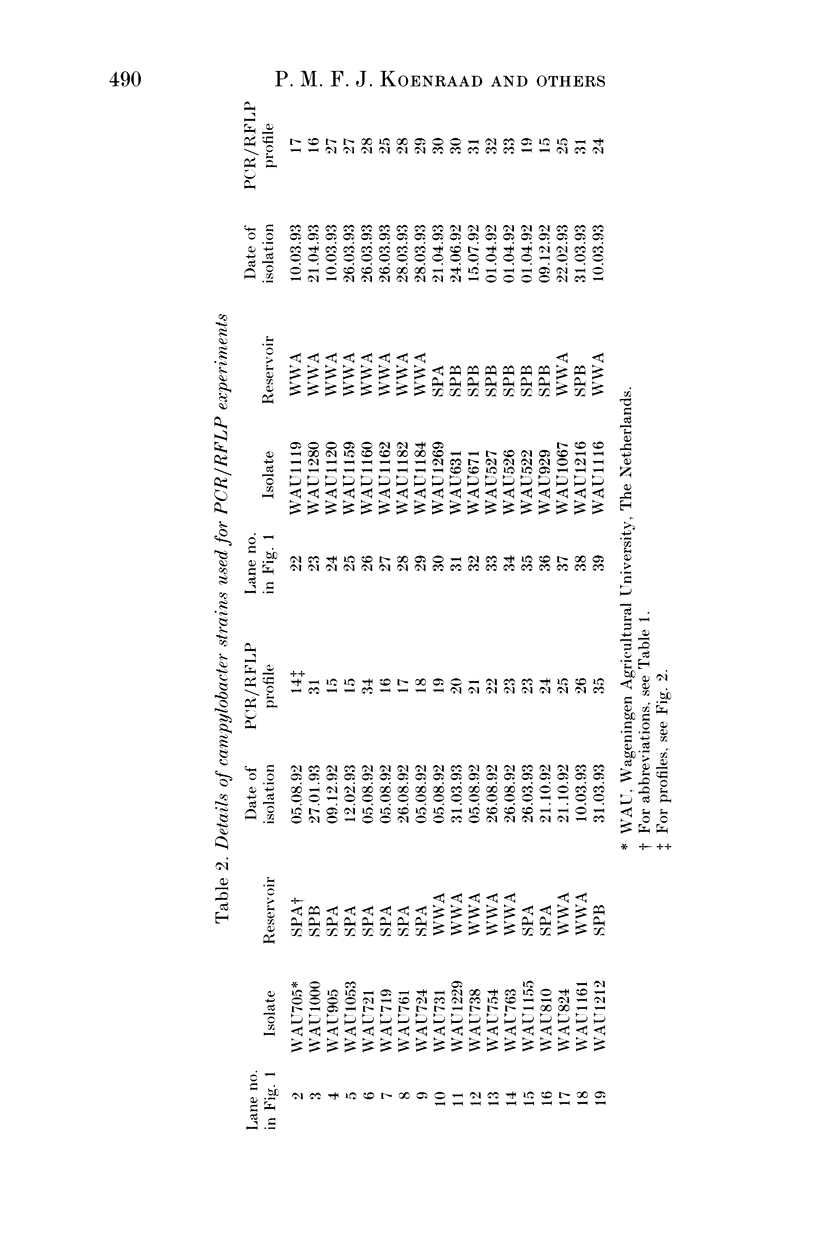
In this study the distribution of phenotypes of campylobacter strains in sewage and surface waters was investigated by subtyping and by speciation of isolates from various aquatic environments. These environments included two municipal sewage plants (SPA and SPB) and waste water from a poultry abattoir (WWA). Both the sewage plants SPA and SPB collected domestic and industrial waste, and SPA received drain water from WWA. SPB received no waste water from any meat-processing plant. The isolates were speciated by PCR and subtyped by PCR/RFLP based on the flagellin PCR products. From all three reservoirs, no Campylobacter lari was isolated, and approximately 80% of the isolates could be identified as C. jejuni and the rest belonged to the C. coli species. The PCR/RFLP typing technique has a high discrimination level and was reproducible between two separate laboratories. The 182 isolates tested yielded 22 distinct Dde I profiles. The results indicate that strains with profiles found in poultry are also detectable in waste water presumed to be solely from domestic and human sources. In addition some strains were unique to the known poultry-related sources, suggesting that avian-specific strains, non-pathogenic to man, may exist in the environment. In contrast some strains were unique to human waste indicating the potential importance of non-poultry sources of infection. No seasonality was observed in the profile distribution. So, at least in the Netherlands, it is unlikely that infections caused by contaminated surface waters contribute to the seasonality of human campylobacteriosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm R. A., Guerry P., Trust T. J. Distribution and polymorphism of the flagellin genes from isolates of Campylobacter coli and Campylobacter jejuni. J Bacteriol. 1993 May;175(10):3051–3057. doi: 10.1128/jb.175.10.3051-3057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hardesty H. L., Powers B., Wang W. L. Survival of Campylobacter fetus subsp. jejuni in biological milieus. J Clin Microbiol. 1980 Apr;11(4):309–313. doi: 10.1128/jcm.11.4.309-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton F. J., Coates D., Hinchliffe P. M., Robertson L. Comparison of selective media for isolation of Campylobacter jejuni/coli. J Clin Pathol. 1983 Jan;36(1):78–83. doi: 10.1136/jcp.36.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyers M., Chapelle S., Van Camp G., Goossens H., De Wachter R. Discrimination among thermophilic Campylobacter species by polymerase chain reaction amplification of 23S rRNA gene fragments. J Clin Microbiol. 1993 Dec;31(12):3340–3343. doi: 10.1128/jcm.31.12.3340-3343.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. R. Reproducibility and indices of discriminatory power of microbial typing methods. J Clin Microbiol. 1990 Sep;28(9):1903–1905. doi: 10.1128/jcm.28.9.1903-1905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Bindemann U., Stelzer W. Characterization of thermophilic campylobacters originated from a high-rate sewage treatment plant. Zentralbl Hyg Umweltmed. 1991 Sep;192(1):14–24. [PubMed] [Google Scholar]
- Jacobs-Reitsma W. F., Bolder N. M., Mulder R. W. Cecal carriage of Campylobacter and Salmonella in Dutch broiler flocks at slaughter: a one-year study. Poult Sci. 1994 Aug;73(8):1260–1266. doi: 10.3382/ps.0731260. [DOI] [PubMed] [Google Scholar]
- Jacobs-Reitsma W. F., Koenraad P. M., Bolder N. M., Mulder R. W. In vitro susceptibility of Campylobacter and Salmonella isolates from broilers to quinolones, ampicillin, tetracycline, and erythromycin. Vet Q. 1994 Dec;16(4):206–208. doi: 10.1080/01652176.1994.9694450. [DOI] [PubMed] [Google Scholar]
- Jones K., Betaieb M., Telford D. R. Correlation between environmental monitoring of thermophilic campylobacters in sewage effluent and the incidence of Campylobacter infection in the community. J Appl Bacteriol. 1990 Aug;69(2):235–240. doi: 10.1111/j.1365-2672.1990.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Korhonen L. K., Martikainen P. J. Comparison of the survival of Campylobacter jejuni and Campylobacter coli in culturable form in surface water. Can J Microbiol. 1991 Jul;37(7):530–533. doi: 10.1139/m91-089. [DOI] [PubMed] [Google Scholar]
- Lior H. New, extended biotyping scheme for Campylobacter jejuni, Campylobacter coli, and "Campylobacter laridis". J Clin Microbiol. 1984 Oct;20(4):636–640. doi: 10.1128/jcm.20.4.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lior H., Woodward D. L., Edgar J. A., Laroche L. J., Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 1982 May;15(5):761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I., Bohachick K., Patton C. M. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J Clin Microbiol. 1993 Jun;31(6):1531–1536. doi: 10.1128/jcm.31.6.1531-1536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Saunders F., Dehele Y., Pearson A. D. The virulence of clinical and environmental isolates of Campylobacter jejuni. J Hyg (Lond) 1985 Feb;94(1):45–54. doi: 10.1017/s0022172400061118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L. K., Stiles M. E., Taylor D. E. Inhibition of Campylobacter coli and Campylobacter jejuni by antibiotics used in selective growth media. J Clin Microbiol. 1985 Oct;22(4):510–514. doi: 10.1128/jcm.22.4.510-514.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notermans S., Hoogenboom-Verdegaal A. Existing and emerging foodborne diseases. Int J Food Microbiol. 1992 Mar-Apr;15(3-4):197–205. doi: 10.1016/0168-1605(92)90049-9. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Fayos A., Hernandez J., Lastovica A. PCR-based restriction fragment length polymorphism analysis of DNA sequence diversity of flagellin genes of Campylobacter jejuni and allied species. Mol Cell Probes. 1993 Dec;7(6):471–480. doi: 10.1006/mcpr.1993.1070. [DOI] [PubMed] [Google Scholar]
- Penner J. L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988 Apr;1(2):157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B., Benjamin J. Differentiation of enteropathogenic Campylobacter. J Clin Pathol. 1980 Nov;33(11):1122–1122. doi: 10.1136/jcp.33.11.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer W., Jacob J., Schulze E. Environmental aspects of Campylobacter infections. Zentralbl Mikrobiol. 1991;146(1):3–15. [PubMed] [Google Scholar]
- Taylor D. N., McDermott K. T., Little J. R., Wells J. G., Blaser M. J. Campylobacter enteritis from untreated water in the Rocky Mountains. Ann Intern Med. 1983 Jul;99(1):38–40. doi: 10.7326/0003-4819-99-1-38. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Patton C. M., Tenover F. C., Barrett T. J., Stamm W. E., Steigerwalt A. G., Lin J. Y., Holmes K. K., Brenner D. J. Prevalence and characterization of hippurate-negative Campylobacter jejuni in King County, Washington. J Clin Microbiol. 1987 Sep;25(9):1747–1752. doi: 10.1128/jcm.25.9.1747-1752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



