Abstract
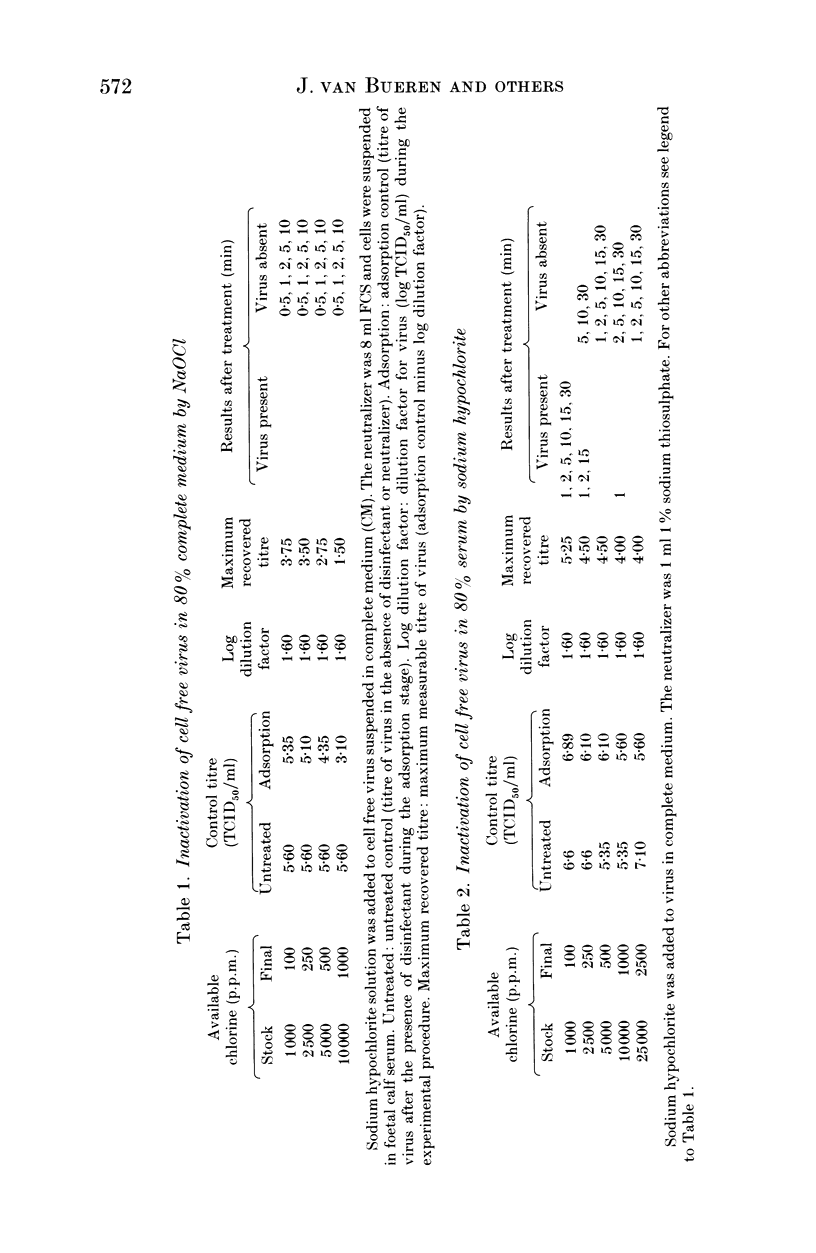
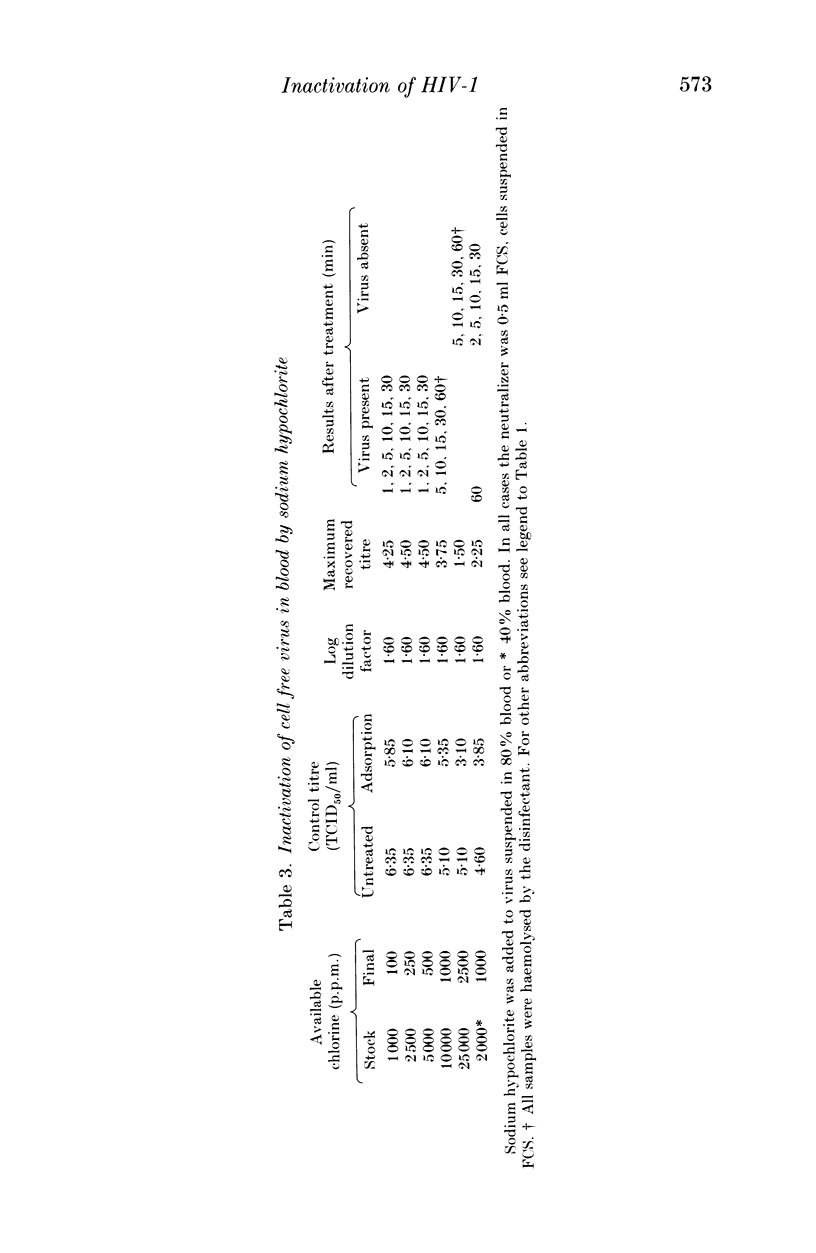
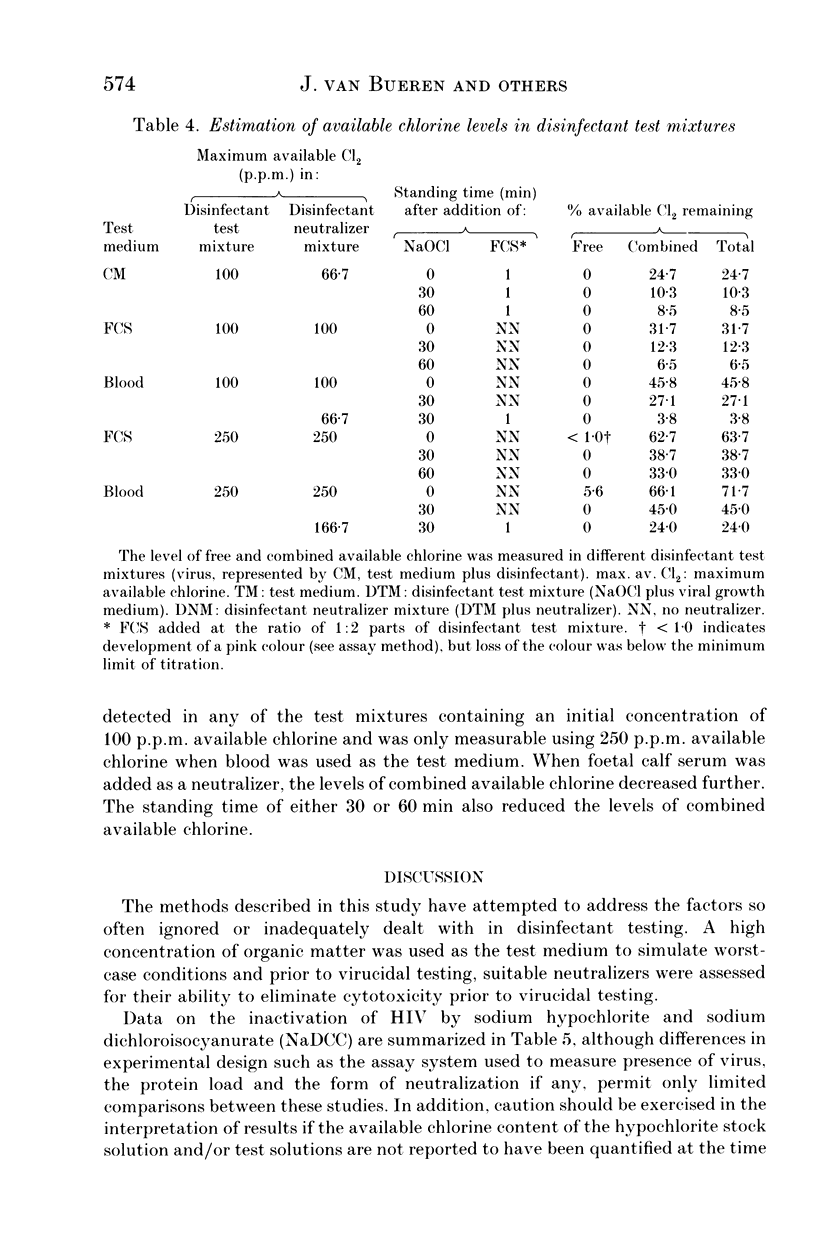
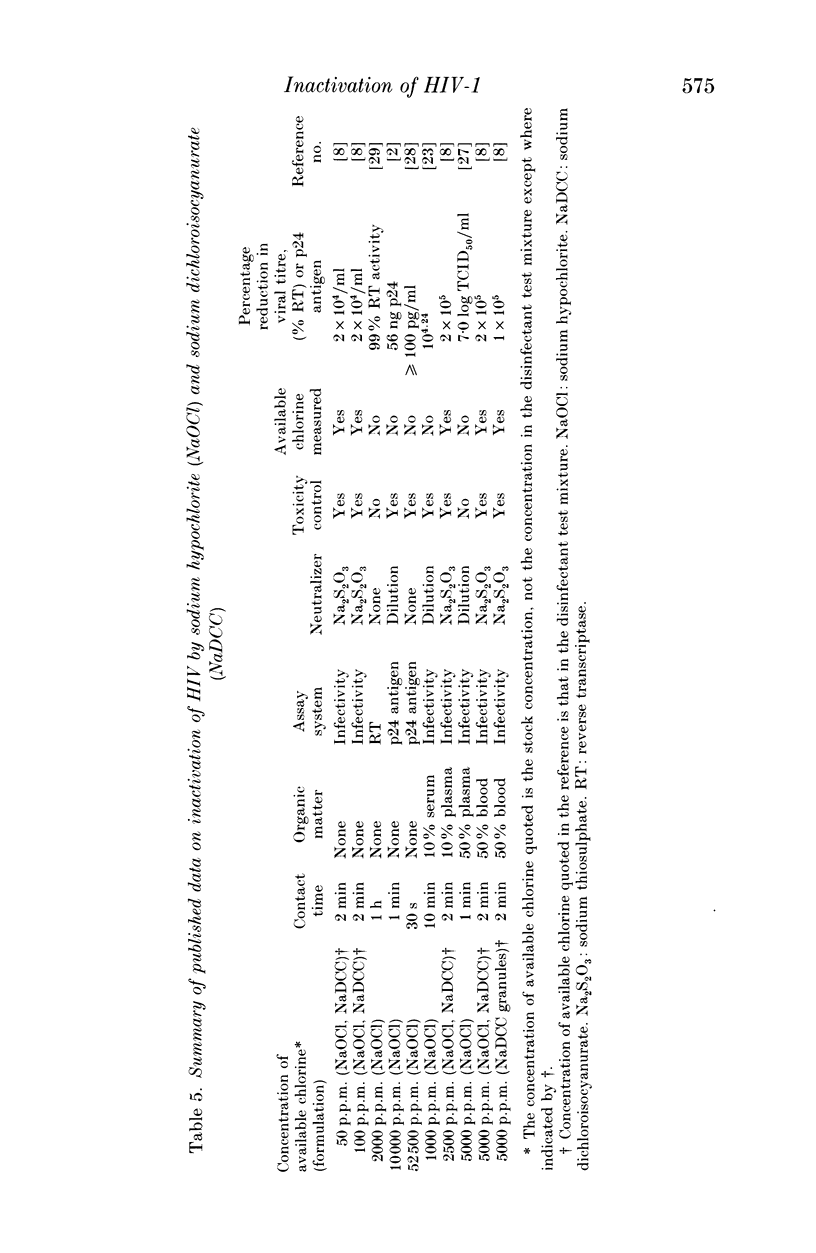
The efficacy of sodium hypochlorite was assessed against human immunodeficiency virus type 1 suspended in low (8% v/v) or high (80% v/v) concentrations of serum or in a high (80%) concentration of blood. In the presence of 8% serum, 100 p.p.m. available chlorine in the disinfectant test mixture inactivated 3.75 log TCID50 HIV/ml within 30 s. When the test mixture contained 80% serum, 500 p.p.m. available chlorine inactivated more than 4 log TCID50 HIV/ml in 1-2 min. Lower concentrations of available chlorine were unable to inactivate the virus completely. In the presence of 80% blood, 1000 p.p.m. available chlorine in the disinfectant test mixture was unable to inactivate 3.75 log TCID50 HIV/ml, although 2500 p.p.m. available chlorine was able to inactivate at least 1.5 log TCID50 HIV/ml. In all test mixtures, the chlorine rapidly became combined and thus less active. Our results emphasise the importance of cleaning prior to disinfection with sodium hypochlorite since it may prove to be ineffective in the presence of high levels of organic matter. In cases where prior cleaning is impossible, care must be taken to use the higher recommended concentration (a minimum of 10,000 p.p.m. available chlorine).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranda-Anzaldo A., Viza D., Busnel R. G. Chemical inactivation of human immunodeficiency virus in vitro. J Virol Methods. 1992 Apr;37(1):71–81. doi: 10.1016/0166-0934(92)90021-5. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Jesson W. J., Mortimer P. P., McLean K. A., Evans B. A. Cultivation of human immunodeficiency virus from whole blood: effect of anticoagulant and inoculum size on virus growth. J Med Virol. 1990 Jun;31(2):161–164. doi: 10.1002/jmv.1890310215. [DOI] [PubMed] [Google Scholar]
- Benarde M. A., Israel B. M., Olivieri V. P., Granstrom M. L. Efficiency of chlorine dioxide as a bactericide. Appl Microbiol. 1965 Sep;13(5):776–780. doi: 10.1128/am.13.5.776-780.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarde M. A., Snow W. B., Olivieri V. P. Chlorine dioxide disinfection temperature effects. J Appl Bacteriol. 1967 Apr;30(1):159–167. doi: 10.1111/j.1365-2672.1967.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Benarde M. A., Snow W. B., Olivieri V. P., Davidson B. Kinetics and mechanism of bacterial disinfection by chlorine dioxide. Appl Microbiol. 1967 Mar;15(2):257–265. doi: 10.1128/am.15.2.257-265.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield S. F., Miller E. A. A comparison of hypochlorite and phenolic disinfectants for disinfection of clean and soiled surfaces and blood spillages. J Hosp Infect. 1989 Apr;13(3):231–239. doi: 10.1016/0195-6701(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Bloomfield S. F., Smith-Burchnell C. A., Dalgleish A. G. Evaluation of hypochlorite-releasing disinfectants against the human immunodeficiency virus (HIV). J Hosp Infect. 1990 Apr;15(3):273–278. doi: 10.1016/0195-6701(90)90035-m. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Recommendations for prevention of HIV transmission in health-care settings. MMWR Suppl. 1987 Aug 21;36(2):1S–18S. [PubMed] [Google Scholar]
- Coates D., Wilson M. Powders, composed of chlorine-releasing agent acrylic resin mixtures or based on peroxygen compounds, for spills of body fluids. J Hosp Infect. 1992 Aug;21(4):241–252. doi: 10.1016/0195-6701(92)90135-9. [DOI] [PubMed] [Google Scholar]
- Coates D., Wilson M. Use of sodium dichloroisocyanurate granules for spills of body fluids. J Hosp Infect. 1989 Apr;13(3):241–251. doi: 10.1016/0195-6701(89)90004-2. [DOI] [PubMed] [Google Scholar]
- Cousins C. M., Allan C. D. Sporicidal properties of some halogens. J Appl Bacteriol. 1967 Apr;30(1):168–174. doi: 10.1111/j.1365-2672.1967.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Fabian T. M., Walker S. E. Stability of sodium hypochlorite solutions. Am J Hosp Pharm. 1982 Jun;39(6):1016–1017. [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Martin L. S., McDougal J. S., Loskoski S. L. Disinfection and inactivation of the human T lymphotropic virus type III/Lymphadenopathy-associated virus. J Infect Dis. 1985 Aug;152(2):400–403. doi: 10.1093/infdis/152.2.400. [DOI] [PubMed] [Google Scholar]
- Masquelier B., Faivre R., Paty M. C., Fleury H. J. HIV1 quantitation in infected patients: a comparison of cell viraemia, plasma viraemia and R-HEV. Res Virol. 1992 Jan-Feb;143(1):17–22. doi: 10.1016/s0923-2516(06)80072-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Resnick L., Veren K., Salahuddin S. Z., Tondreau S., Markham P. D. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA. 1986 Apr 11;255(14):1887–1891. [PubMed] [Google Scholar]
- Spire B., Barré-Sinoussi F., Montagnier L., Chermann J. C. Inactivation of lymphadenopathy associated virus by chemical disinfectants. Lancet. 1984 Oct 20;2(8408):899–901. doi: 10.1016/s0140-6736(84)90657-3. [DOI] [PubMed] [Google Scholar]
- Weber G. R., Levine M. Factors Affecting Germicidal Efficiency of Chlorine and Chloramine. Am J Public Health Nations Health. 1944 Jul;34(7):719–728. doi: 10.2105/ajph.34.7.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bueren J., Simpson R. A., Jacobs P., Cookson B. D. Survival of human immunodeficiency virus in suspension and dried onto surfaces. J Clin Microbiol. 1994 Feb;32(2):571–574. doi: 10.1128/jcm.32.2.571-574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


