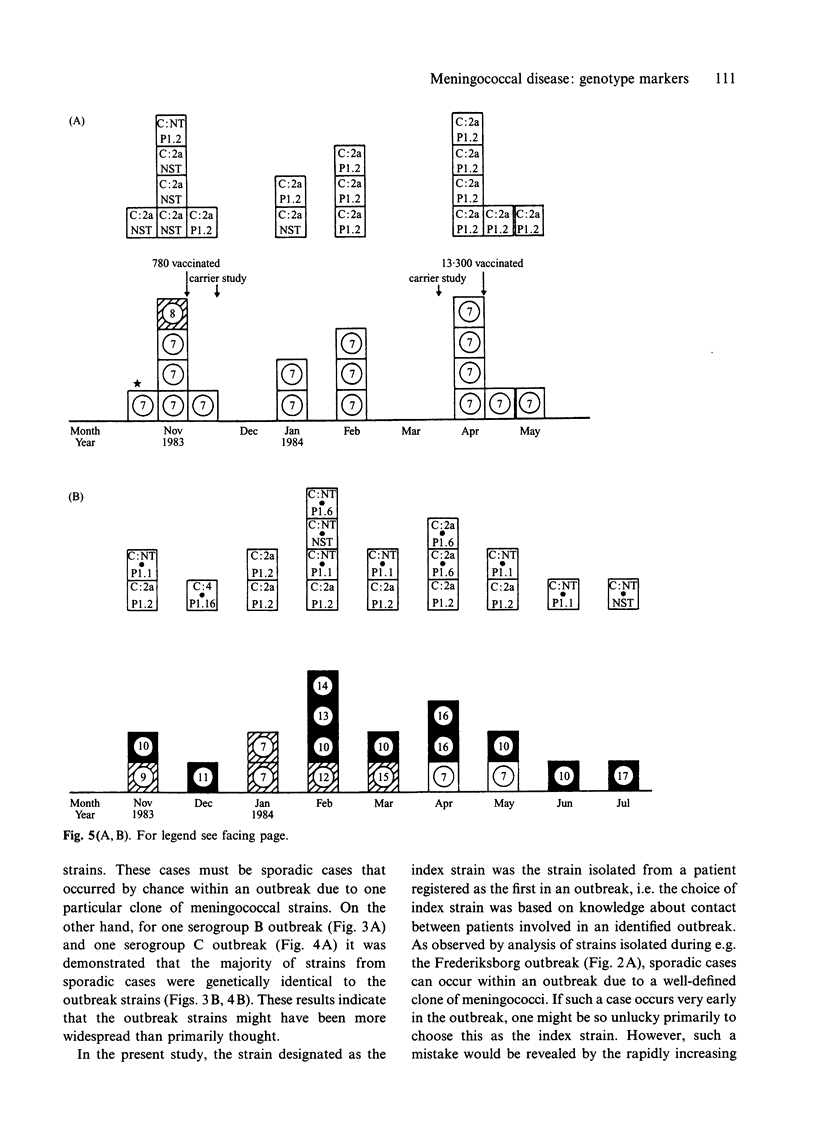
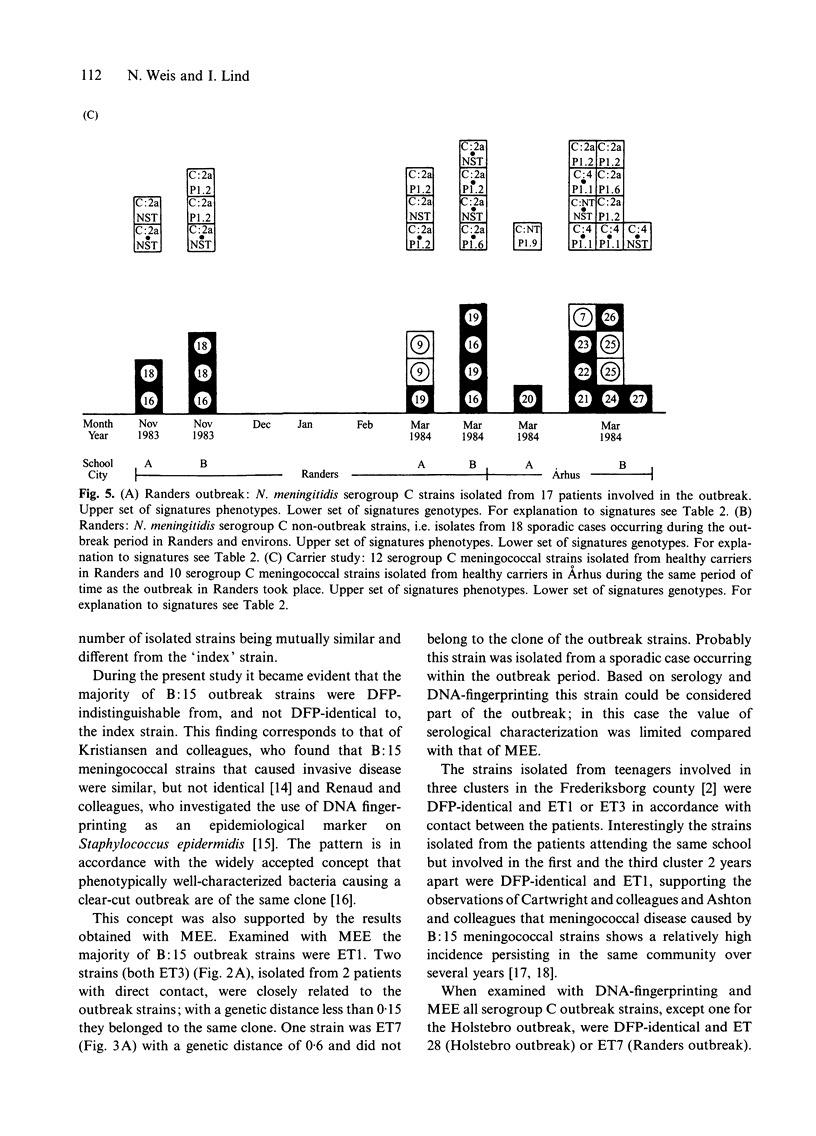
Abstract
The objective of the study was to assess whether genotypic characterization by means of DNA-fingerprinting pattern (DFP) and multilocus enzyme electrophoresis (MEE) profile as compared to phenotypic characterization would improve the differentiation of Neisseria meningitidis strains associated with outbreaks from strains associated with sporadic cases of meningococcal disease. In addition, the differentiation of serogroup C carrier strains from those associated with an outbreak of serogroup C meningococcal disease was investigated. A total of 118 N. meningitidis strains were available for the study: 59 from patients involved in outbreaks of meningococcal disease (2 serogroup B and 2 serogroup C), 37 patients considered to be sporadic cases and 22 serogroup C carrier strains. Among the 59 strains from patients involved in outbreaks the 4 strains isolated from the patient registered as the first in each outbreak were designated the index strains. Among the remaining 55 outbreak strains 52 were either DFP-identical or DFP-indistinguishable when compared with the one relevant out of the 4 index strains. This was only the case for 17 of the 37 strains isolated from sporadic cases caused by the same serogroup of meningococci during the outbreak periods, and 5 of the 22 meningococcal strains isolated from healthy carriers. Among the 56 (52 + 4) DFP-identical or DFP-indistinguishable outbreak strains 5 different electrophoretic types were identified by MEE. Among 59 assumed outbreak strains a total of 4 were identified as genotypically distinct. Among the 37 mainly DFP-indistinguishable or DFP-different strains from sporadic cases 17 different ETs were identified, and among the 22 mainly DFP-different carrier strains 13 different ETs were identified. Two strains among those selected from sporadic cases were identical to the outbreak strain. None of the local serogroup C carrier strains isolated during the outbreak of serogroup C disease were identical to the outbreak strain. Both DNA-fingerprinting and MEE improved the differentiation of meningococci when compared with phenotypic characterization. The results indicate that tracing a virulent strain within a open group of contacts is irrelevant.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton F. E., Mancino L., Ryan A. J., Poolman J. T., Abdillahi H., Zollinger W. D. Serotypes and subtypes of Neisseria meningitidis serogroup B strains associated with meningococcal disease in Canada, 1977-1989. Can J Microbiol. 1991 Aug;37(8):613–617. doi: 10.1139/m91-104. [DOI] [PubMed] [Google Scholar]
- Bygraves J. A., Maiden M. C. Analysis of the clonal relationships between strains of Neisseria meningitidis by pulsed field gel electrophoresis. J Gen Microbiol. 1992 Mar;138(3):523–531. doi: 10.1099/00221287-138-3-523. [DOI] [PubMed] [Google Scholar]
- Cartwright K. A., Stuart J. M., Jones D. M., Noah N. D. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987 Dec;99(3):591–601. doi: 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Frøholm L. O., Bøvre K., Holten E., Frasch C. E., Mocca L. F., Zollinger W. D., Selander R. K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Kristiansen B. E., Frøholm L. O., Bøvre K., Selander R. K. Clonal diversity of Neisseria meningitidis from a population of asymptomatic carriers. Infect Immun. 1988 Aug;56(8):2060–2068. doi: 10.1128/iai.56.8.2060-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facinelli B., Biavasco F., Varaldo P. E. Use of DNA fingerprinting in an epidemiologic study of outbreak-specific and non-specific strains of group C Neisseria meningitidis. Eur J Epidemiol. 1990 Mar;6(1):80–83. doi: 10.1007/BF00155555. [DOI] [PubMed] [Google Scholar]
- Fox A. J., Jones D. M., Gray S. J., Caugant D. A., Saunders N. A. An epidemiologically valuable typing method for Neisseria meningitidis by analysis of restriction fragment length polymorphisms. J Med Microbiol. 1991 May;34(5):265–270. doi: 10.1099/00222615-34-5-265. [DOI] [PubMed] [Google Scholar]
- Jewes L., Norman P., McKendrick M. W. Value of throat swabs in meningococcal meningitis. J Clin Pathol. 1989 Nov;42(11):1229–1229. doi: 10.1136/jcp.42.11.1229-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen B. E., Bjorvatn B., Lund V., Lindqvist B., Holten E. Differentiation of B15 strains of Neisseria meningitidis by DNA restriction endonuclease fingerprinting. J Infect Dis. 1984 Nov;150(5):672–677. doi: 10.1093/infdis/150.5.672. [DOI] [PubMed] [Google Scholar]
- Kronvall G. A rapid slide-agglutination method for typing pneumococci by means of specific antibody adsorbed to protein A-containing staphylococci. J Med Microbiol. 1973 May;6(2):187–190. doi: 10.1099/00222615-6-2-187. [DOI] [PubMed] [Google Scholar]
- Maiden M. C., Feavers I. M. Meningococcal typing. J Med Microbiol. 1994 Mar;40(3):157–158. doi: 10.1099/00222615-40-3-157. [DOI] [PubMed] [Google Scholar]
- Mastrantonio P., Congiu M. E., Occhionero M., Stroffolini T. Genomic fingerprints and phenotypic characteristics of strains of Neisseria meningitidis causing disease in Italy. Microbiologica. 1990 Apr;13(2):109–113. [PubMed] [Google Scholar]
- Orskov F., Orskov I. From the national institutes of health. Summary of a workshop on the clone concept in the epidemiology, taxonomy, and evolution of the enterobacteriaceae and other bacteria. J Infect Dis. 1983 Aug;148(2):346–357. doi: 10.1093/infdis/148.2.346. [DOI] [PubMed] [Google Scholar]
- Pitt T. L. Bacterial typing systems: the way ahead. J Med Microbiol. 1994 Jan;40(1):1–2. doi: 10.1099/00222615-40-1-1. [DOI] [PubMed] [Google Scholar]
- Renaud F., Freney J., Etienne J., Bes M., Brun Y., Barsotti O., Andre S., Fleurette J. Restriction endonuclease analysis of Staphylococcus epidermidis DNA may be a useful epidemiological marker. J Clin Microbiol. 1988 Sep;26(9):1729–1734. doi: 10.1128/jcm.26.9.1729-1734.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønne T., Berthelsen L., Buhl L. H., Lind I. Comparative studies on pharyngeal carriage of Neisseria meningitidis during a localized outbreak of serogroup C meningococcal disease. Scand J Infect Dis. 1993;25(3):331–339. doi: 10.3109/00365549309008507. [DOI] [PubMed] [Google Scholar]
- Samuelsson S., Ege P., Berthelsen L., Lind I. An outbreak of serogroup B:15:P1.16 meningococcal disease, Frederiksborg County, Denmark, 1987-9. Epidemiol Infect. 1992 Feb;108(1):19–30. doi: 10.1017/s0950268800049463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson S., Gustavsen S., Rønne T. Epidemiology of meningococcal disease in Denmark 1980-1988. Scand J Infect Dis. 1991;23(6):723–730. doi: 10.3109/00365549109024300. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins L. S., Troup N. J., Woods T., Bibb W., McKinney R. M. Molecular epidemiology of Legionella species by restriction endonuclease and alloenzyme analysis. J Clin Microbiol. 1987 Oct;25(10):1875–1880. doi: 10.1128/jcm.25.10.1875-1880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]