Abstract
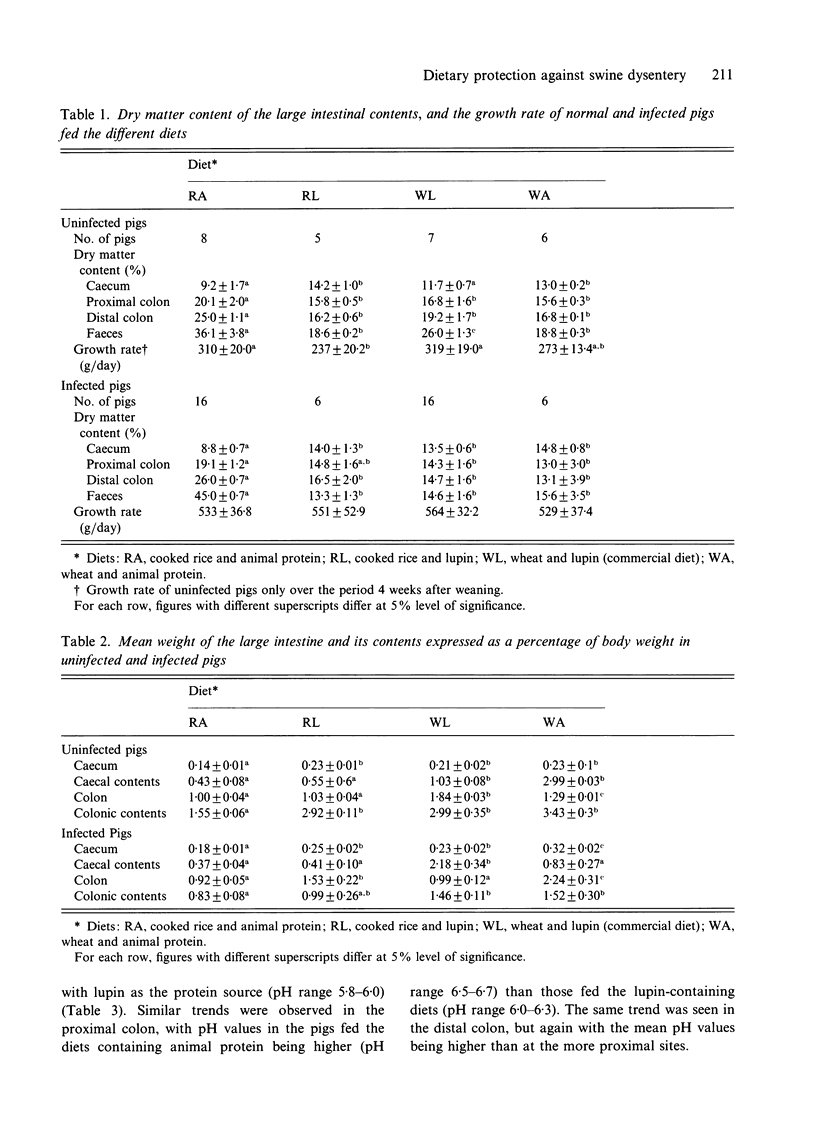
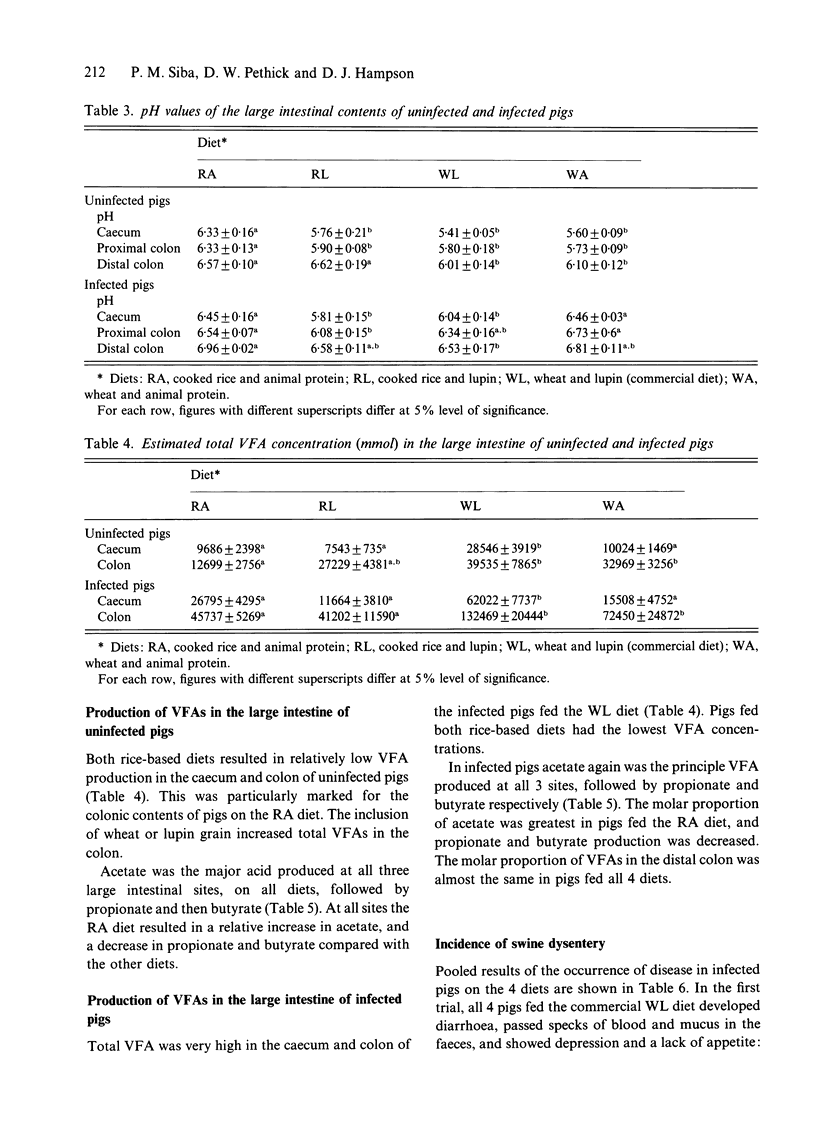
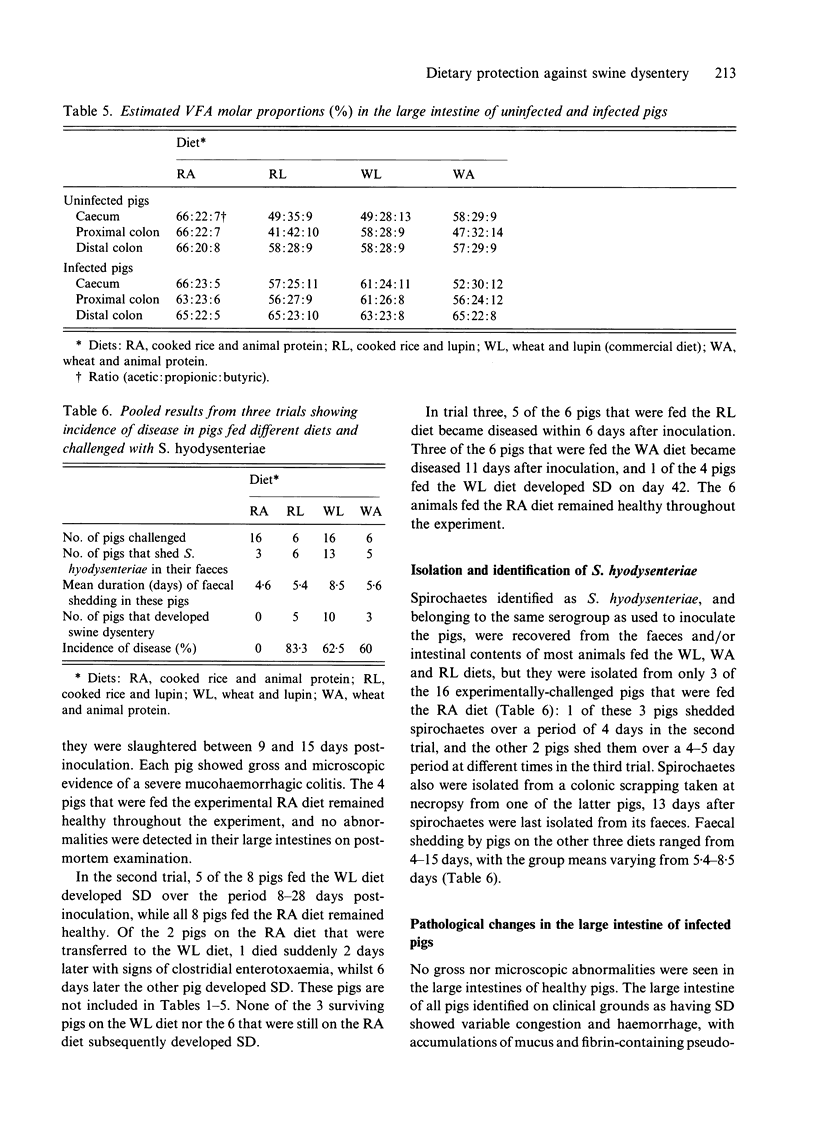
Weaner pigs (n = 72) were fed 1 of 4 diets. These were based on either cooked rice and animal protein, cooked rice and lupin, wheat and lupin, or wheat and animal protein. Twenty-six of the pigs were slaughtered after 1 month. Those fed the highly digestible cooked rice and animal protein diet had drier colonic contents and faeces, lighter large intestines, and the contents of their large intestines had increased pH values and decreased total VFA concentrations. The other 46 were orally challenged with broth cultures of Serpulina hyodysenteriae, and were monitored for faecal excretion of the spirochaetes, and for the development of swine dysentery (SD). None of 18 pigs fed the cooked rice and animal protein diet developed colonic changes or disease, whereas most pigs on the other diets developed mucohaemorrhagic colitis and dysentery. The reduced fermentation that occurred in the large intestines of pigs fed cooked rice and animal protein was associated with a subsequent failure of colonization by S. hyodysenteriae, and resultant protection against SD.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argenzio R. A., Southworth M. Sites of organic acid production and absorption in gastrointestinal tract of the pig. Am J Physiol. 1975 Feb;228(2):454–460. doi: 10.1152/ajplegacy.1975.228.2.454. [DOI] [PubMed] [Google Scholar]
- Bach Knudsen K. E., Jensen B. B., Andersen J. O., Hansen I. Gastrointestinal implications in pigs of wheat and oat fractions. 2. Microbial activity in the gastrointestinal tract. Br J Nutr. 1991 Mar;65(2):233–248. doi: 10.1079/bjn19910083. [DOI] [PubMed] [Google Scholar]
- Clemens E. T., Stevens C. E., Southworth M. Sites of organic acid production and pattern of digesta movement in the gastrointestinal tract of swine. J Nutr. 1975 Jun;105(6):759–768. doi: 10.1093/jn/105.6.759. [DOI] [PubMed] [Google Scholar]
- Cranwell P. D. Microbial fermentation in the alimentary tract of the pig. Nutr Abstr Rev. 1968 Jul;38(3):721–730. [PubMed] [Google Scholar]
- Hampson D. J., Cutler R., Lee B. J. Virulent Serpulina hyodysenteriae from a pig in a herd free of clinical swine dysentery. Vet Rec. 1992 Oct 3;131(14):318–319. doi: 10.1136/vr.131.14.318. [DOI] [PubMed] [Google Scholar]
- Hampson D. J. Slide-agglutination for rapid serological typing of Treponema hyodysenteriae. Epidemiol Infect. 1991 Jun;106(3):541–547. doi: 10.1017/s0950268800067601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. L., Alexander T. J., Whipp S. C., Robinson I. M., Glock R. D., Matthews P. J. Swine dysentery: studies of gnotobiotic pigs inoculated with Treponema hyodysenteriae, Bacteroides vulgatus, and Fusobacterium necrophorum. J Am Vet Med Assoc. 1978 Feb 15;172(4):468–471. [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Hughes R., Olander H. J., Williams C. B. Swine dysentery: pathogenicity of Treponema hyodysenteriae. Am J Vet Res. 1975 Jul;36(7):971–977. [PubMed] [Google Scholar]
- Imoto S., Namioka S. VFA production in the pig large intestine. J Anim Sci. 1978 Aug;47(2):467–478. doi: 10.2527/jas1978.472467x. [DOI] [PubMed] [Google Scholar]
- Jenkinson S. R., Wingar C. R. Selective medium for the isolation of Treponema hyodysenteriae. Vet Rec. 1981 Oct 24;109(17):384–385. doi: 10.1136/vr.109.17.384. [DOI] [PubMed] [Google Scholar]
- Jensen B. B., Jørgensen H. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl Environ Microbiol. 1994 Jun;60(6):1897–1904. doi: 10.1128/aem.60.6.1897-1904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. E., Jensen B. B., Hansen I. Oat bran but not a beta-glucan-enriched oat fraction enhances butyrate production in the large intestine of pigs. J Nutr. 1993 Jul;123(7):1235–1247. doi: 10.1093/jn/123.7.1235. [DOI] [PubMed] [Google Scholar]
- Kunkle R. A., Harris D. L., Kinyon J. M. Autoclaved liquid medium for propagation of Treponema hyodysenteriae. J Clin Microbiol. 1986 Oct;24(4):669–671. doi: 10.1128/jcm.24.4.669-671.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. C., Simon J., Byerly C. S. The etiology of swine dysentery. III. The role of selected gram-negative obligate anaerobes. Vet Pathol. 1975;12(1):46–54. doi: 10.1177/030098587501200107. [DOI] [PubMed] [Google Scholar]
- Meyer R. C. Swine dysentery: a perspective. Adv Vet Sci Comp Med. 1978;22:133–158. [PubMed] [Google Scholar]
- Mhoma J. R., Hampson D. J., Robertson I. D. A serological survey to determine the prevalence of infection with Treponema hyodysenteriae in Western Australia. Aust Vet J. 1992 Apr;69(4):81–84. doi: 10.1111/j.1751-0813.1992.tb15555.x. [DOI] [PubMed] [Google Scholar]
- Milner J. A., Sellwood R. Chemotactic response to mucin by Serpulina hyodysenteriae and other porcine spirochetes: potential role in intestinal colonization. Infect Immun. 1994 Sep;62(9):4095–4099. doi: 10.1128/iai.62.9.4095-4099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethick D. W., Lindsay D. B., Barker P. J., Northrop A. J. Acetate supply and utilization by the tissues of sheep in vivo. Br J Nutr. 1981 Jul;46(1):97–110. doi: 10.1079/bjn19810013. [DOI] [PubMed] [Google Scholar]
- Prohászka L. Comparative studies on the antibacterial defence mechanism of volatile fatty acids in five animal species. Acta Vet Hung. 1988;36(3-4):165–171. [PubMed] [Google Scholar]
- Prohászka L., Lukács K. Influence of the diet on the antibacterial effect of volatile fatty acids and on the development of swine dysentery. Zentralbl Veterinarmed B. 1984 Dec;31(10):779–785. doi: 10.1111/j.1439-0450.1984.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Sharma R., Schumacher U., Ronaasen V., Coates M. Rat intestinal mucosal responses to a microbial flora and different diets. Gut. 1995 Feb;36(2):209–214. doi: 10.1136/gut.36.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S., Casey T. A., Tordoff L. A., Dewhirst F. E., Paster B. J. Reclassification of Treponema hyodysenteriae and Treponema innocens in a new genus, Serpula gen. nov., as Serpula hyodysenteriae comb. nov. and Serpula innocens comb. nov. Int J Syst Bacteriol. 1991 Jan;41(1):50–58. doi: 10.1099/00207713-41-1-50. [DOI] [PubMed] [Google Scholar]
- Stanton T. B. Proposal to change the genus designation Serpula to Serpulina gen. nov. containing the species Serpulina hyodysenteriae comb. nov. and Serpulina innocens comb. nov. Int J Syst Bacteriol. 1992 Jan;42(1):189–190. doi: 10.1099/00207713-42-1-189. [DOI] [PubMed] [Google Scholar]
- Stephen A. M. Whole grains--impact of consuming whole grains on physiological effects of dietary fiber and starch. Crit Rev Food Sci Nutr. 1994;34(5-6):499–511. doi: 10.1080/10408399409527677. [DOI] [PubMed] [Google Scholar]
- Suenaga I., Yamazaki T. Eliminating organisms against Treponema hyodysenteriae in the gut of mice. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 May;261(3):322–329. doi: 10.1016/s0176-6724(86)80049-9. [DOI] [PubMed] [Google Scholar]
- Trowell H., Southgate D. A., Wolever T. M., Leeds A. R., Gassull M. A., Jenkins D. J. Letter: Dietary fibre redefined. Lancet. 1976 May 1;1(7966):967–967. doi: 10.1016/s0140-6736(76)92750-1. [DOI] [PubMed] [Google Scholar]
- Varel V. H. Activity of fiber-degrading microorganisms in the pig large intestine. J Anim Sci. 1987 Aug;65(2):488–496. doi: 10.2527/jas1987.652488x. [DOI] [PubMed] [Google Scholar]
- Whipp S. C., Robinson I. M., Harris D. L., Glock R. D., Matthews P. J., Alexander T. J. Pathogenic synergism between Treponema hyodysenteriae and other selected anaerobes in gnotobiotic pigs. Infect Immun. 1979 Dec;26(3):1042–1047. doi: 10.1128/iai.26.3.1042-1047.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


